Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(5):1096-1103. doi:10.7150/ijms.52433 This issue Cite
Research Paper
Microvascular Reactivity Measured by Dynamic Near-infrared Spectroscopy Following Induction of General Anesthesia in Healthy Patients: Observation of Age-related Change
1. Department of Anesthesia and Pain Medicine, Medical Research Institute, Pusan National University Hospital, Busan, Republic of Korea.
2. Department of Anesthesia and Pain Medicine, Pusan National University, School of Medicine, Yangsan, Republic of Korea
Received 2020-8-26; Accepted 2020-12-18; Published 2021-1-1
Abstract
Background: The purpose of this study was to investigate the effect of general anesthesia on microvascular reactivity and tissue oxygen saturation (StO2) using near-infrared spectroscopy in conjunction with vascular occlusion tests (VOT). Age-related changes of microvascular reactivity, that is, the capacity of capillary recruitment, were examined.
Methods: This prospective observational study was performed on 60 patients without comorbidities who underwent elective surgery under general anesthesia. Baseline StO2 on thenar eminence, hemodynamics, and laboratory profile were monitored before (T0) and 30 min after general anesthesia (T1). During VOT, occlusion slope representing oxygen consumption of muscle and recovery slope representing microvascular reactivity were also collected at T0 and T1.
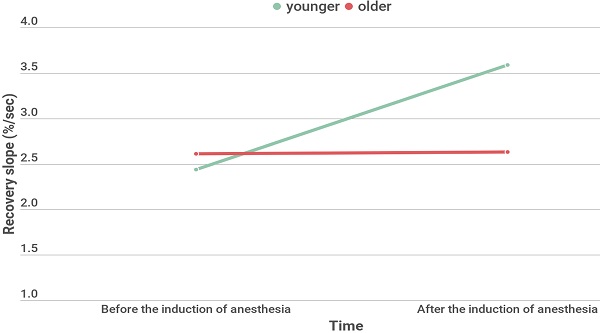
Results: Baseline StO2 and minimum / maximum StO2 during VOT increased under general anesthesia. Occlusion slope decreased while the recovery slope increased under general anesthesia. To observe aging effect, Receiver operating characteristic analysis was performed and age less than 65 years old showed a fair performance in predicting the increase of microvascular reactivity after the induction of anesthesia (AUC 0.733, 95% CI 0.594-0.845, P= 0.003). For age-related analyses, 27 patients of younger group (< 65 years) and 26 patients of older group (≥ 65 years) were divided. Recovery slope significantly increased under general anesthesia in younger group (2.44 [1.91-2.81] % ∙ sec-1 at T0 and 3.59 [2.58-3.51] % ∙ sec-1 at T1, P <0.001), but not in older group (2.61 [2.21-3.20] % ∙ sec-1 at T0, 2.63 [1.90-3.60] % ∙ sec-1 at T1, P = 0.949).
Conclusions: General anesthesia could improve StO2 through increase of microvascular reactivity and decrease of tissue metabolism. However, microvascular reactivity to capillary recruitment under general anesthesia significantly improves in younger patients, not in older patients.
Keywords: Aging, cardiovascular physiology, anesthesia, inhalation, microcirculation, spectroscopy, near-infrared
Introduction
Microcirculatory dysfunction and impaired tissue perfusion may persist even if systemic hemodynamic variables are optimized and within the therapeutic target [1,2]. This suggests that clinicians should identify therapeutic targets for functional microcirculatory restoration to prevent organ failure [3]. Microcirculatory function is impaired in a number of pathologies, including sepsis [4], diabetes mellitus [5], shock [6,7], aging [8], and smoking [9]. However, research on the effects of anesthetics on microcirculatory function has begun only recently, and the results are still inconsistent [10,11]. One study reported that general anesthesia has a negative impact on reperfusion reserve [10]. Another study showed that general anesthesia increases the recovery slope, suggesting improvement of tissue microvascular reactivity and peripheral vasodilation [11]. As patients with microcirculatory dysfunction due to various underlying diseases were included in previous studies, inconsistent outcomes may have occurred. To accurately determine the effect of anesthetic agents on microvascular reactivity, patients without comorbidities must be studied. Moreover, age could also be an important consideration in studies on microcirculatory function, even in healthy subjects [12].
Owing to near-infrared spectroscopy (NIRS) technology, the detection of microcirculatory dysfunction has become easier and non-invasive. Many studies have shown that the dynamic NIRS parameters that can be measured with a brief ischemic challenge are clinically more useful than static tissue oxygen saturation (StO2) values [10,13-15]. A vascular occlusion test (VOT) is a method of observing the change in StO2 after applying a tourniquet with a higher pressure than the patient's systolic blood pressure. The StO2 reduction rate (occlusion slope) during the ischemic period reflects oxygen extraction. The rate of StO2 increase (recovery slope) during reperfusion after the release of the vascular occlusion reflects microvascular reactivity [10,13,14].
The purpose of this study was to investigate the effect of anesthetics on microvascular reactivity and StO2 using near-infrared spectroscopy in conjunction with VOT. Age-related changes of microvascular reactivity, which means the capacity of capillary recruitment, were focused.
Methods
Patients
This study was approved by the Institutional Review Board of Pusan National University Hospital (IRB no. 1607-012-054, Busan, South Korea) and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrolment at clinicaltrials.gov (NCT03060798, Hyeon-Jeong Lee: February 19, 2017). This manuscript adheres to the applicable STROBE statements.
Sixty adult patients who were referred for elective surgery under general anesthesia participated in this study. Exclusion criteria were an American Society of Anesthesiologists (ASA) physical status classification > II, age < 18 or > 80 years, body mass index > 30 kg m-2, disorders likely to influence microcirculation (uncontrolled hypertension, diabetes, peripheral vascular disease, or chronic venous insufficiency), pregnancy, smoking, sleep apnea, history of chronic obstructive and restrictive pulmonary disease, contraindications for anesthetic agents, or refusal to participate in the investigation.
General anesthesia
In the operating room, standard monitoring including electrocardiography, non-invasive blood pressure (NIBP) monitors, and pulse oximetry were used. Skin temperature probes (400 series, GE Healthcare, Helsinki, Finland) were placed on the right palm. The operating room temperature was maintained at 22-24°C. Skin temperature was measured before and after induction of anesthesia. General anesthesia was induced with propofol 1.5 - 2 mg ∙ kg-1 and a continuous infusion of remifentanil 0.2 μg ∙ kg-1 ∙ min-1. After injection of rocuronium 0.8 mg ∙ kg-1, tracheal intubation was performed and mechanical ventilation was applied with an inspired oxygen fraction of 0.5, a tidal volume of 8 ml ∙ kg-1, and a respiratory rate adjusted to maintain end-tidal CO2 of 30-35 mmHg. General anesthesia was maintained with desflurane and remifentanil to maintain a bispectral index (BIS; Bispectral Index™, Aspect Medical System, Norwood, MA, USA) of 40-50 and hemodynamic parameters within 20% of the baseline values. Hartman's solution was infused during the study period at the rate of 5 ml ∙ kg-1 ∙ h-1. Under general anesthesia, blood samples were collected to measure the serum lactate level.
Vascular occlusion test (VOT)
VOT was performed twice for each patient, before (T0) and 30 min after the induction of general anesthesia (T1). Before induction, a NIRS sensor (INVOSTM 5100C Cerebral/Somatic Oximeter; Medtronic, Minneapolis, MN, USA) was placed on the thenar eminence and an automated tourniquet (A.T.S® 3000 Automatic Tourniquet System; Zimmer Inc., Warsaw, IL, USA) was placed around the ipsilateral upper arm. A NIBP was placed around the contralateral upper arm and the baseline blood pressure was measured. When the baseline StO2 was stabilized, the automatic tourniquet was inflated to 50 mmHg over the patient's baseline systolic blood pressure and maintained for 5 min. After the 5 min ischemic period, the tourniquet was rapidly deflated to 0 mmHg. StO2 data were continuously recorded during the VOT procedure. Baseline StO2, minimum StO2 during the 5 min inflation of the tourniquet, time to minimum StO2, maximum StO2 during deflation of the tourniquet, and time to maximum StO2 were obtained. The occlusion slope and recovery slope, which are VOT-derived dynamic parameters related to microcirculatory reactivity, were calculated based on the measured StO2 data. The occlusion slope was defined as the slope of StO2 descent to the lowest value. The recovery slope was calculated from deflation of the tourniquet until the recovery of StO2 to the highest value.
Data collection
Preoperative hemoglobin concentration was obtained. Mean blood pressure (MBP), heart rate (HR), pulsed oxygen saturation (SpO2), skin temperature, and VOT-derived measurements, including baseline StO2, occlusion slope, minimum StO2, time to minimum StO2, recovery slope, maximum StO2, and time to maximum StO2 were recorded at T0 and T1. Serum lactate level was measured at T1.
Statistical analysis
The primary outcome of this study was the difference of the recovery slope of the VOT after the induction of general anesthesia. According to our pilot study performed prior to this investigation, mean difference of recovery slope of 0.28 % ∙ sec-1 with SD of 0.72 % ∙ sec-1 was obtained. A sample size of 54 patients was necessary to gain a power of 80%, by using a Wilcoxon signed rank test with a two-sided significance level of five. Considering a drop-out rate of 10%, a total of 60 patients were required.
Data are expressed as number (proportion), median [IQR], or mean (SD). All continuous variables were tested for normality assumption with a Q-Q plot and Kolmogorov-Smirnov test. StO2 values during VOT at T0 and T1 were compared with repeated measures ANOVA with a Bonferroni post hoc test. A paired t-test or Wilcoxon signed rank test was used to analyze differences in the hemodynamic variables and VOT-derived measurements between T0 and T1.
Patients were divided by an increase of recovery slope under general anesthesia and an unpaired student t test or Mann-Whitney U test was used to analyze differences in the patient's characteristics and hemodynamic variables between the groups. The significantly different variables were evaluated their ability to predict the increase of recovery slope of VOT under general anesthesia using a receiver operating characteristics (ROC) curves analysis with 95% confidence interval (CI). The optimal cut-off was selected to maximize the Youden index and age less than 65 years old showed a fair performance in predicting the increase of microvascular reactivity after the induction of anesthesia. For age-related analyses, patients were divided at 65 years old; 27 patients of younger group (< 65 years) and 26 patients of older group (≥ 65 years). An unpaired student t test or Mann-Whitney U test was used to analyze differences in the hemodynamic variables and VOT-derived measurements between the age groups.
A P-value < 0.05 was considered significant. All statistical analyses were performed using PASW Statistics for Windows, Version 18.0 (SPSS Inc., Chicago, IL, USA) and MedCalc for Windows, version 13.2 (MedCalc Software, Ostend, Belgium).
Results
A total of 60 patients were enrolled in this study. Seven patients were excluded due to protocol violation. Demographic and intraoperative characteristics are summarized in Table 1.
Receiver operating characteristic analysis to predict the increase of recovery slope under general anesthesia
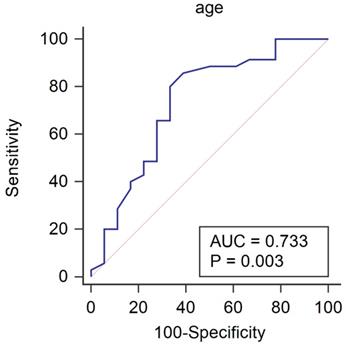
Patient's characteristics and hemodynamic variables of patients with and without the increase of recovery slope under general anesthesia are shown in Table 1. Patients whose recovery slope increased under general anesthesia were significantly younger than those whose recovery slope did not increase (P=0.013). The area under the ROC curve for age to predict the increase of recovery slope after the induction of anesthesia was 0.733 (Fig. 1, P = 0.003; 95% CI 0.594 - 0.845). If age was lower than 65 years old, the increase of recovery slope under general anesthesia was predicted with a sensitivity of 85.7% and a specificity of 61.1%.
Effects of general anesthesia on VOT-derived parameters and hemodynamic variables
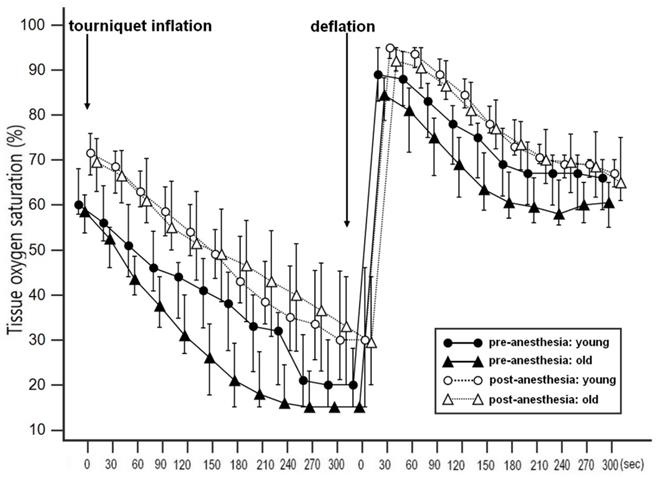
Demographic and intraoperative characteristics of two age groups divided by age of 65 years old are shown in Table 2. The type of operation and hemoglobin showed significantly different between the groups. The changes in StO2 during VOT before and after the induction of anesthesia in two age groups are shown in Fig. 2. Repeated measures of ANOVA revealed significantly different StO2 values before and after the induction of anesthesia in both groups (all p < 0.001); the Bonferroni post hoc test revealed significant differences at all time points. The changes in microcirculatory and hemodynamic parameters during VOT at T0 and T1 depending on the age are shown in Table 3. At T0, baseline StO2 was significantly higher in the younger group compared to the older group (P = 0.025). After the induction of anesthesia, recovery slope were significantly higher in the younger group compared to the older group (3.59 [2.58-3.51] % ∙ sec-1 in the younger group and 2.63 [1.90-3.60] % ∙ sec-1 in the older group, P = 0.047). MBP significantly increased and HR and skin temperature significantly decreased under general anesthesia in both groups. However, there were no differences in MBP, HR, SpO2, and skin temperatures between two age groups.
Patient's characteristics and hemodynamic variables between patients with or without the increase of recovery slope under general anesthesia
| All patients (n=53) | Patients without the increase of recovery slope under general anesthesia (n=18) | Patients with the increase of recovery slope under general anesthesia (n=35) | P value | |
|---|---|---|---|---|
| Age; years | 56.5 (14.5) | 63.3 (14.4) | 53.0 (13.5) | 0.013 |
| Sex | 0.145 | |||
| Male | 24 (45.3) | 10 (55.6) | 14 (40.0) | |
| Female | 29 (54.7) | 8 (44.4) | 21 (60.0) | |
| Body mass index; kg.m-2 | 24.0 (3.2) | 23.8 (3.4) | 24.1 (3.1) | 0.803 |
| Weight; kg | 63.1 (12.0) | 62.8 (10.5) | 63.2 (12.9) | 0.906 |
| Height; cm | 161.6 (9.1) | 162.3 (8.8) | 161.3 (9.3) | 0.713 |
| ASA class | 0.065 | |||
| I | 26 (49.1) | 10 (55.6) 8 (44.4)15 (55.5) | 17 (48.6) | |
| II | 27 (50.9) | 8 (44.4) | 18 (51.4) | |
| Type of operation | 0.301 | |||
| Laparoscopic cholecystectomy | 18 (34.0) | 8 (44.4) | 10 (28.6) | |
| Thyroidectomy | 18 (34.0) | 5 (27.8) | 13 (37.1) | |
| Endoscopic sinus surgery | 17 (32.1) | 5 (27.8) | 12 (34.3) | |
| Lactate; mg.l-1 | 0.99 [0.78-1.48] | 0.79 [0.65-1.62] | 1.02 [0.84-1.34] | 0.296 |
| Hemoglobin; g.dl-1 | 13.5 (1.5) | 13.6 (1.4) | 13.5 (1.6) | 0.837 |
| Fluid administration; ml | 170.7 (29.7) | 163.3 (28.9) | 174.6 (29.8) | 0.194 |
| Mean blood pressure; mmHg | 96.0 [86.5-112.5] | 95.0 [89.3-104.5] | 0.954 | |
| SpO2; % | 99.0 [97.0-100.0] | 99.0 [97.0-100.0] | 99.0 [96.0-100.0] | 0.512 |
| Heart rate; beats.min-1 | 74.0 [64.0-86.0] | 71.0 [60.0-76.0] | 76.0 [66.0-88.0] | 0.292 |
| Skin temperature; °C | 32.3 (2.3) | 31.7 (1.7) | 32.9 (1.9) | 0.245 |
Data are number (proportion), mean (SD) or median [IQR].
Patients' characteristics of the age groups
| Younger (< 65 years) (n = 27) | Older (≥ 65 years) (n = 26) | P value | |
|---|---|---|---|
| Age; years | 45.1 (10.7) | 68.3 (5.8) | <0.001 |
| Sex | 0.502 | ||
| Male | 11 (40.7) | 13 (50.0) | |
| Female | 16 (59.2) | 13 (50.0) | |
| Body mass index; kg.m-2 | 24.1 (3.3) | 24.0 (3.1) | 0.887 |
| Weight; kg | 64.2 (13.6) | 62.0 (10.4) | 0.521 |
| Height; cm | 162.7 (10.0) | 160.5 (8.1) | 0.389 |
| ASA class | 0.339 | ||
| I | 15 (55.5) | 11 (42.3) | |
| II | 12 (44.4) | 15 (57.7) | |
| Type of operation | 0.004 | ||
| Laparoscopic cholecystectomy | 5 (18.5) | 13 (50.0) | |
| Thyroidectomy | 8 (29.6) | 10 (38.5) | |
| Endoscopic sinus surgery | 14 (51.9) | 3 (11.5) | |
| Lactate; mg.l-1 | 0.87 [0.78-1.18] | 1.18 [0.78-1.58] | 0.296 |
| Hemoglobin; g.dl-1 | 13.9 (1.6) | 13.0 (1.2) | 0.031 |
| Fluid administration; ml | 176.4 (31.2) | 165.4 (27.8) | 0.278 |
Data are number (proportion), mean (SD) or median [IQR].
Receiver operating characteristic curve for age to predict the increase of recovery slope under general anesthesia. If age was lower than 65 years old, the increase of recovery slope under general anesthesia was predicted with a sensitivity of 85.7% and a specificity of 61.1%.
Discussion
Our study showed that general anesthesia improves StO2 in healthy adult patients through an increase of microvascular reactivity and reduced metabolic demand. However, the important finding of our study was that the recovery slope significantly increased after the induction of general anesthesia only in the younger patients. This result suggests that general anesthesia may increase the microvascular reactivity and capillary recruitment only in the young, healthy patients [11,14]. Concordantly, age could predict the increase of recovery slope after the induction of anesthesia, showing an area under the ROC curve of 0.733.
The recovery slope during VOT reflects the capacity of microvascular reactivity to recruit the capillary network [16]. At rest, only 30% of capillaries are normally perfused, and 70% are not [17]. These blood vessels are reserves that can be opened under stress conditions through relaxation of the precapillary sphincter and arteriolar vasodilation [18]. Even in elderly patients without comorbidities in daily life, the limit of physiologic reserve can be seen during general anesthesia. This limited physiologic reserve is one of the causes of microcirculation changes during aging [19]. In aged organs, dynamic control of the precapillary sphincter to regulate blood flow does not work well, and reserved vessels are barely recruited [20,21]. A variety of mechanisms are involved, in which aging is associated with endothelial dysfunction, impaired vasodilatory and vasoconstrictive responses, increased vascular stiffness, and decreased vascular density and impaired vascular organization [8,22,23].
Changes in tissue oxygen saturation (StO2) during vascular occlusion test (VOT) before and after induction of general anesthesia in the younger and the older groups. A repeated measures ANOVA revealed significantly different StO2 values between before and after the induction of anesthesia, with a significant Bonferroni post hoc test for the differences at all the time points.
Microcirculatory and hemodynamic parameters of the age groups.
| All patients (n=53) | Younger (< 65 yrs) (n = 27) | Older (≥ 65 yrs) (n = 26) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T0 | T1 | P value | T0 | T1 | P value | T0 | T1 | P value | |
| Baseline StO2; % | 60.0 [56.0-68.0] | 70.0 [63.0-78.0] | <0.001 | 66.0 [60.0-68.8] | 71.0 [62.5-77.8] | 0.002 | 58.0* [53.0-65.0] | 70.0 [63.0-78.0] | < 0.001 |
| Occlusion slope; %.sec-1 | 0.19 [0.15-0.25] | 0.15 [0.11-0.18] | <0.001 | 0.19 [0.16-0.25] | 0.15 [0.12-0.18] | <0.001 | 0.21 [0.14-0.28] | 0.12 [0.10-0.20] | < 0.001 |
| Minimum StO2; % | 15.0 [15.0-30.0] | 29.0 [15.0-47.0] | <0.001 | 15.0 [15.0-31.5] | 29.0 [15.0-46.0] | <0.001 | 15.0 [15.0-28.0] | 27.5 [15.0-50.0] | <0.001 |
| Time to minimum StO2; sec | 271.0 [210.8-300.0] | 300.0 [299.3-300.0] | <0.001 | 270.0 [214.3-300.0] | 300.0 [297.8-300.0] | 0.004 | 285.5 [210.0-300.0] | 300.0 [300-300.0] | < 0.001 |
| Recovery slope; %.sec-1 | 2.50 [1.99-2.97] | 2.77 [2.24-3.98] | 0.004 | 2.44 [1.91-2.81] | 3.59 [2.58-3.51] | <0.001 | 2.61 [2.21-3.20] | 2.63* [1.90-3.60] | 0.949 |
| Maximum StO2; % | 87.0 [80.8-95.0] | 95.0 [89.8-95.0] | <0.001 | 90.0 [82.5-95.0] | 95.0 [90.5-95.0] | 0.008 | 86.5 [79.0-91.0] | 93.5 [89.0-95.0] | <0.001 |
| Time to maximum StO2; sec | 27.0 [23.8-30.5] | 22.0 [16.8-28.3] | 0.202 | 26.0 [20.5-34.3] | 24.0 [15.5-32.5] | 0.559 | 28.0 [23.0-34.0] | 22.0 [18.0-30.0] | 0.264 |
| Mean blood pressure; mmHg | 95.5 [87.0-107.0] | 89.0 [78.0-100.0] | <0.001 | 94.5 [87.0-104.0] | 88.0 [77.0-99.0] | 0.003 | 97.5 [88.0-108.5] | 89.0 [78.5-103.0] | 0.003 |
| SpO2; % | 99.0 [97.0-100.0] | 100.0 [98.0-100.0] | 0.004 | 99.0 [97.0-100.0] | 99.5 [98.0-100.0] | 0.469 | 99.0 [96.0-100.0] | 100.0 [98.0-100.0] | 0.001 |
| Heart rate; beats.min-1 | 74.0 [64.0-86.0] | 82.0 [75.3-93.0] | <0.001 | 78.0 [66.3-87.8] | 85.5 [77.0-98.0] | 0.029 | 72.0 [59.5-78.5] | 82.0 [75.0-90.3] | 0.013 |
| Skin temperature; °C | 32.3 (2.3) | 33.5 (1.99) | <0.001 | 31.9 (1.8) | 33.4 (2.1) | <0.001 | 32.3 (1.9) | 33.6 (1.6) | 0.041 |
Data are mean (SD) or median [IQR]. T0 = before the induction of anesthesia; T1 = after the induction of anesthesia; StO2 = tissue oxygen saturation. *p < 0.05 compared to the younger group.
Vasodilation occurs in a complex way through endothelium dependent vasodilation (EDV) mediated by endothelium or endothelium independent vasodilation (EIV) mediated by smooth muscle of the vessel wall [12]. EDV is known to decrease with age [12,24] and mechanisms include decreased NO sensitivity and bioavailability, and an increased oxidative stress [25,26]. On the contrary, EIV is maintained even in aged vessel [27]. Recovery slope of VOT has been known as a useful clinical tool for evaluating EDV [28,29]. In our study, the baseline StO2 is much lower in elderly patients, however, other VOT-derived parameters were not significantly different at T0. Assuming EIV is age independent, EDV may be also preserved during VOT in older patients under non-stress condition. Inhalational anesthetic agents inhibit EDV but promote EIV via reduction of intracellular Ca2+ availability and sensitivity to contractile proteins [30,31]. The net effect of these 2 opposing effects is generally vasodilation. Propofol and opioids decreases systemic vascular resistance in a predominantly EIV and partly mediated by EDV [32-34]. Considering VOT at T1 was performed 30 min after the injection of propofol and remifentanil, mainly desflurane affected the microcirculation. Our results showed opposite results that desflurane promoted EDV in younger patients. The reason of the contradictory results is not elucidative, but may not be consistent with the in vitro results because numerous factors affect the microvascular system in vivo.
VOT parameters after the induction of anesthesia are not consistent among previous studies that showed a reduced or an increased recovery slope after the induction of anesthesia [10,11,35]. We assume that the reason for these differences is that the previous studies included patients with various comorbidities who required cardiac surgery; our study included only patients without comorbidities. Recent studies showed that reactive hyperemia is significantly decreased in elderly patients with heart failure with preserved ejection fraction or hypertension compared to healthy age-matched controls, indicating endothelial dysfunction in these patient groups [36,37]. Also, they demonstrated that vasodilation under loading condition, such as exercise, was blunted in these patients [36]. Considering these results, patients who required cardiac surgery in the previous studies would have significant microvascular dysfunction and decreased capacity of reactive vasodilation. And microvascular dysfunction appears to be more severe in the cardiac patients than in heathy elderly patients.
There are numerous clinical variables that could contribute to regulate microcirculation in human. Therefore, it is difficult to confirm that the improvement of StO2 at T1 was solely due to general anesthesia. However, other potential factors, such as body temperature, stress hormone caused by intubation, fluid administration, and decreased MBP are estimated to have little influence on our results. Body temperature within normal range does not seem to influence StO2 [38]. Previous study showed that stress hormones increased slightly in response to laryngoscopy and intubation, all returning to or below baseline 5 min later in normotensive patients [39]. Moreover, 161 ml of Hartman's solution was infused in our study, which is not the amount that causes clinically significant hemodilution in people with normal hemoglobin. MBP was significantly decreased under general anesthesia where the lowest value was 66 mmHg which might provide enough perfusion pressure to allow peripheral autoregulation.
There are some limitations to the interpretation of the results of our study. First, VOT is not yet standardized, and the duration of peripheral ischemia is an area of strong debate. There are two methods to advocate peripheral ischemia: time-targeted and StO2-targeted VOT. We used 5-minute time-targeted VOT because a smaller minimum StO2 value after ischemia results in a more defined recovery slope [40,41]. Second, it is difficult to confirm that older participants are completely healthy as younger patients. Because we enrolled only the patients undergoing minor surgery, more advanced hemodynamic monitoring or tests related to cardiovascular disease could not be performed. However, in clinical practice, completely healthy elderly is rare and most of the elderly have the compensated comorbidities in their daily life and more severe microcirculatory dysfunction, which more prominent results might be seen in real world. Finally, the effects of FiO2 on microcirculation are in debate. Previous study showed that normobaric hyperoxia reduced capillary perfusion and oxygen consumption and increased heterogeneity of the perfusion, while there was no change in recovery slope of VOT in healthy subjects [42]. In the same line, high FiO2 had only a relatively small increase in mean (SD) StO2 of 5.3 (7.1) % and this effect reached a plateau when FiO2 was between 30% and 40% [43]. When previous studies that investigated the effect of anesthetics on StO2 in cardiac surgery, StO2 under general anesthesia seems more likely affected by anesthetic agents, rather than FiO2 [9,11,35]. In their studies, despite of increasing FiO2 to 0.5-0.6 under general anesthesia, only sevoflurane significantly increased StO2 and propofol did not significantly change StO2. Further research on the comparisons of effects of different types of general anesthetics on microcirculation of patients without underlying disease is needed.
General anesthesia is known to reduce cardiovascular function, which would ultimately reduce organ perfusion. However, our study shows that general anesthesia would compensate tissue hypoperfusion by improving microvascular reactivity representing the ability to recruit capillaries and reducing metabolic demands. However, improved microvascular reactivity by general anesthesia was only apparent in the younger patients without accompanying diseases, not in the older patients, even those without comorbidities. These findings suggest that general anesthesia could have different effects on microvascular reactivity depending on the status of the microvasculature. Additional studies on the effects of general anesthesia on microvascular reactivity in certain diseases are necessary.
Abbreviations
ASA: American Society of Anesthesiologists; BIS: Bispectral index; CI: Confidence interval; EDV: Endothelium dependent vasodilation; EIV: Endothelium independent vasodilation; HR: Heart rate; MBP: Mean blood pressure; NIRS: Near-infrared spectroscopy; SpO2: Pulsed oxygen saturation; ROC: Receiver operating characteristics; StO2: Tissue oxygen saturation; VOT: Vascular occlusion test.
Acknowledgements
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. NRF-2018R1C1B5040928) and clinical research grant in 2020 from Pusan National University Hospital.
Ethics approval
This study was approved by the Institutional Review Board of Pusan National University Hospital (number H-1607-012-054) and registered at www.clinicaltrials.gov (NCT03060798).
Author Contributions
AC helped study design/planning, study conduct, data analysis, and writing and revising paper. HL helped study design/planning, study conduct, data analysis, and writing and revising paper. HjK helped study design/planning and revising paper. WD helped study conduct and revising paper. SJ helped study conduct and revising paper. SB helped study design/planning and revising paper. EK helped study conduct and revising paper. JK helped data analysis and revising paper. HkK helped data analysis and revising paper.
Competing Interests
The authors have declared that no competing interest exists.
References
1. De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. American Journal of Respiratory and Critical Care Medicine. 2002;166:98-104
2. Kara A, Akin S, Ince C. Monitoring microcirculation in critical illness. Current Opinion in Critical Care. 2016;22:444-52
3. Moore JP, Dyson A, Singer M, Fraser J. Microcirculatory dysfunction and resuscitation: why, when, and how. British Journal of Anaesthesia. 2015;115:366-75
4. Lush CW, Kvietys PR. Microvascular dysfunction in sepsis. Microcirculation (New York, N.Y.: 1994). 2000;7:83-101
5. Muris DM, Houben AJ, Schram MT, Stehouwer CD. Microvascular dysfunction is associated with a higher incidence of type 2 diabetes mellitus: a systematic review and meta-analysis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:3082-94
6. Fang X, Tang W, Sun S, Huang L, Chang YT, Castillo C, Weil MH. Comparison of buccal microcirculation between septic and hemorrhagic shock. Critical Care Medicine. 2006;34:S447-53
7. Kirschenbaum LA, Astiz ME, Rackow EC, Saha DC, Lin R. Microvascular response in patients with cardiogenic shock. Critical Care Medicine. 2000;28:1290-4
8. Bentov I, Reed MJ. The effect of aging on the cutaneous microvasculature. Microvascular Research. 2015;100:25-31
9. Rossi M, Pistelli F, Pesce M, Aquilini F, Franzoni F, Santoro G, Carrozzi L. Impact of long-term exposure to cigarette smoking on skin microvascular function. Microvascular Research. 2014;93:46-51
10. Bernet C, Desebbe O, Bordon S. et al. The impact of induction of general anesthesia and a vascular occlusion test on tissue oxygen saturation derived parameters in high-risk surgical patients. Journal of Clinical Monitoring and Computing. 2011;25:237-44
11. Kim TK, Cho YJ, Min JJ, Murkin JM, Bahk JH, Hong DM, Jeon Y. Tissue microcirculation measured by vascular occlusion test during anesthesia induction. Journal of Clinical Monitoring and Computing. 2016;30:41-50
12. Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension (Dallas, Tex.: 1979). 1996;27:849-53
13. Butler E, Chin M, Aneman A. Peripheral Near-Infrared Spectroscopy: Methodologic Aspects and a Systematic Review in Post-Cardiac Surgical Patients. Journal of Cardiothoracic and Vascular Anesthesia. 2016
14. Futier E, Christophe S, Robin E. et al. Use of near-infrared spectroscopy during a vascular occlusion test to assess the microcirculatory response during fluid challenge. Critical Care (London, England). 2011;15:R214
15. Shapiro NI, Arnold R, Sherwin R. et al. The association of near-infrared spectroscopy-derived tissue oxygenation measurements with sepsis syndromes, organ dysfunction and mortality in emergency department patients with sepsis. Critical Care (London, England). 2011;15:R223
16. Moens AL, Goovaerts I, Claeys MJ, Vrints CJ. Flow-mediated vasodilation: a diagnostic instrument, or an experimental tool? Chest. 2005;127:2254-63
17. Chade AR. Renal vascular structure and rarefaction. Comprehensive Physiology. 2013;3:817-31
18. Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118:968-76
19. Montagna W, Carlisle K. Structural changes in ageing skin. The British Journal of Dermatology. 1990;122(Suppl 35):61-70
20. Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. The Journal of Physiology. 2010;588:2269-82
21. Scheeren TW. Journal of Clinical Monitoring and Computing 2015 end of year summary: tissue oxygenation and microcirculation. Journal of Clinical Monitoring and Computing. 2016;30:141-6
22. Muller-Delp JM. Aging-induced adaptations of microvascular reactivity. Microcirculation (New York, N.Y.: 1994). 2006;13:301-14
23. Sweat RS, Sloas DC, Stewart SA. et al. Aging is associated with impaired angiogenesis, but normal microvascular network structure, in the rat mesentery. American Journal of Physiology.Heart and Circulatory Physiology. 2017;312:H275-84
24. DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. The Journal of Physiology. 2002;542:255-62
25. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. American Journal of Physiology.Heart and Circulatory Physiology. 2002;283:H1662-72
26. Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension (Dallas, Tex.: 1979). 2001;38:274-9
27. Sinkler SY, Segal SS. Aging alters reactivity of microvascular resistance networks in mouse gluteus maximus muscle. American Journal of Physiology.Heart and Circulatory Physiology. 2014;307:H830-9
28. Patel S, Celermajer DS. Assessment of vascular disease using arterial flow mediated dilatation. Pharmacological Reports: PR. 2006;58(Suppl):3-7
29. Le Brocq M, Leslie SJ, Milliken P, Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxidants & Redox Signaling. 2008;10:1631-74
30. Akata T. General anesthetics and vascular smooth muscle: direct actions of general anesthetics on cellular mechanisms regulating vascular tone. Anesthesiology. 2007;106:365-91
31. Villeneuve E, Blaise G, Sill JC, Guerard MJ, Buluran J, Girard D. Halothane 1.5 MAC, isoflurane 1.5 MAC, and the contractile responses of coronary arteries obtained from human hearts. Anesthesia and Analgesia. 1991;72:454-61
32. Robinson BJ, Ebert TJ, O'Brien TJ, Colinco MD, Muzi M. Mechanisms whereby propofol mediates peripheral vasodilation in humans. Sympathoinhibition or direct vascular relaxation? Anesthesiology. 1997;86:64-72
33. Duman A, Saide Sahin A, Esra Atalik K, oZtin ogun C, Basri Ulusoy H, Durgut K, oKesli S. The in vitro effects of remifentanil and fentanyl on isolated human right atria and saphenous veins. Journal of Cardiothoracic and Vascular Anesthesia. 2003;17:465-9
34. Aguirre JA, Lucchinetti E, Clanachan AS, Plane F, Zaugg M. Unraveling Interactions Between Anesthetics and the Endothelium: Update and Novel Insights. Anesthesia and Analgesia. 2016;122:330-48
35. Vandenbulcke L, Lapage KG, Vanderstraeten KV, De Somer FM, De Hert SG, Moerman AT. Microvascular reactivity monitored with near-infrared spectroscopy is impaired after induction of anaesthesia in cardiac surgery patients: An observational study. European Journal of Anaesthesiology. 2017;34:688-94
36. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. Journal of the American College of Cardiology. 2010;56:845-54
37. Lee JF, Barrett-O'Keefe Z, Garten RS. et al. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart (British Cardiac Society). 2016;102:278-84
38. Cho YJ, Lee SY, Kim TK, Hong DM, Jeon Y. Effect of Prewarming during Induction of Anesthesia on Microvascular Reactivity in Patients Undergoing Off-Pump Coronary Artery Bypass Surgery: A Randomized Clinical Trial. PloS One. 2016;11:e0159772
39. Kayhan Z, Aldemir D, Mutlu H, Ogus E. Which is responsible for the haemodynamic response due to laryngoscopy and endotracheal intubation? Catecholamines, vasopressin or angiotensin? European Journal of Anaesthesiology. 2005;22:780-5
40. Lipcsey M, Eastwood GM, Woinarski NC, Bellomo R. Near-infrared spectroscopy of the thenar eminence: comparison of dynamic testing protocols. Critical Care and Resuscitation: Journal of the Australasian Academy of Critical Care Medicine. 2012;14:142-7
41. Iannetta D, Inglis EC, Soares RN, McLay KM, Pogliaghi S, Murias JM, CAPES scholarship holder. Reliability of microvascular responsiveness measures derived from near-infrared spectroscopy across a variety of ischemic periods in young and older individuals. Microvascular Research. 2019;122:117-24
42. Orbegozo Cortes D, Puflea F, Donadello K. et al. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvascular Research. 2015;98:23-8
43. Kyle B, Litton E, Ho KM. Effect of hyperoxia and vascular occlusion on tissue oxygenation measured by near infra-red spectroscopy (InSpectra): a volunteer study. Anaesthesia. 2012;67:1237-41
Author contact
![]() Corresponding author: Hyeon-Jeong Lee, Department of Anesthesia and Pain Medicine and Medical Research Institute, Pusan National University Hospital, Gudeok-ro 179, Seo-gu, Busan, 49241, Republic of Korea. Tel: 82-51-240-7399; Fax: 82-51-242-7466; E-Mail: lhjkskac.kr
Corresponding author: Hyeon-Jeong Lee, Department of Anesthesia and Pain Medicine and Medical Research Institute, Pusan National University Hospital, Gudeok-ro 179, Seo-gu, Busan, 49241, Republic of Korea. Tel: 82-51-240-7399; Fax: 82-51-242-7466; E-Mail: lhjkskac.kr

 Global reach, higher impact
Global reach, higher impact