3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(4):1007-1014. doi:10.7150/ijms.47912 This issue Cite
Research Paper
Intraocular pressure-lowering effect of Cordyceps cicadae mycelia extract in a glaucoma rat model
1. Department of Ophthalmology, Fooyin University Hospital, Pingtung, Taiwan
2. Department of Pharmacy and Master Program, Tajen University, Pingtung, Taiwan
3. Grape King Bio Ltd, Zhong-Li Dist., Taoyuan City, Taiwan
*These authors contributed equally to this work.
Received 2020-5-8; Accepted 2020-11-25; Published 2021-1-1
Abstract
Glaucoma is a leading cause of irreversible blindness worldwide. This study evaluates the reduction of intraocular pressure (IOP) induced by C. cicadae mycelia extract in a steroid-induced rat model of glaucoma. Cordyceps cicadae mycelia is a well-known and valued traditional Chinese herbal medicine. C. cicadae mycelia were cultured using a liquid fermentation technique. The harvested C. cicadae mycelia were then lyophilized and extracted with two solvents, water and ethanol. The aqueous extract (CCM-DW) and ethanolic extract (CCM-EtOH) of the mycelia were obtained through lyophilization. Sprague Dawley rats were randomly divided into four groups (n = 6 in each group): a normal group, a control group, and experimental groups treated with CCM-DW, or CCM-EtOH (both at 50 mg/kg/body weight). Except for those in the normal group, all rats received a subconjunctival injection of betamethasone to induce high IOP. The rats in the experimental groups received a daily administration of CCM by oral gavage for four consecutive weeks. IOP reduction is the known treatment for glaucoma. The results revealed that steroid treatment caused a significant increase in the animals' IOP (control group). Elevated IOP decreased significantly after treatment with CCM-DW and CCM-EtOH (p < 0.01), and CCM-DW was more effective than CCM-EtOH. CCM-DW and CCM-EtOH were capable of causing significant decreases in high IOP-induced lesions in pathological studies in which it was shown that the efficacy of CCM-DW surpassed that of CCM-EtOH. After CCM-DW administration for 28 days, there were significant decreases in malondialdehyde and lactate dehydrogenase levels and significant increases in catalase, superoxide dismutase, and glutathione peroxidase levels. In summary, C. cicadae mycelia may be beneficial for preventing or treating glaucoma due to its significant IOP-lowering and antioxidant activities.
Keywords: Cordyceps cicadae mycelia, Glaucoma, Intraocular pressure, Antioxidant
Introduction
Glaucoma is one of the leading causes of vision loss worldwide [1], and elevated intraocular pressure (IOP) is a known risk factor for glaucoma and optic nerve damage [2]. In 2013, an estimated 64.3 million people worldwide were affected by glaucoma and were at risk for vision loss, including blindness. This number may increase to 76.0 million in 2020 and 111.8 million by 2040 [3]. High IOP results from the accumulation of aqueous humor in the anterior chamber, which is primarily due to the eye's lack of capabilities to drain aqueous fluid sufficiently. Blood flowing through the arteries of the ciliary body is the primary source of aqueous humor. The aqueous humor in the posterior chamber is secreted by the ciliary body between the iris and lens. The humor then flows from the posterior to the anterior chamber between the cornea and the iris before being drained from the eye at the iridocorneal junction. In a normal eye, the amount of aqueous humor produced is equal to the amount draining out. However, when this mechanism is compromised, elevated IOP occurs [4]. Increased IOP is considered a major risk factor for progressive loss of retinal ganglion cells (RGCs) in the retina [5]. Research indicates that a longer the duration of IOP elevation correlates with more severe effects on the optic nerve [6]. Common treatment options for glaucoma include medications, surgery, and laser therapy [7]. Ocular hypotensive drugs can act by decreasing aqueous humor production or improving trabecular meshwork, Schlemm's canal, or uveoscleral outflow [8]. However, most clinical drugs have potential side effects, and natural plant extracts could provide alternative sources of medicine.
Herbal medicines have become a prominent global field of research for health care. C. cicadae has long been used as a Chinese herbal medicine since ancient times. C. cicadae belongs to the Clavicipitaceae family, and is also known as cicadae flower or Chan-hua. It parasitizes the larvae or nymphs of cicadas (Cicada flammate) and forms a biological complex of larva and fungus [9-11]. The Compendium of Materia Medica notes that C. cicadae is a valuable traditional Chinese herbal medicine for the treatment of epilepsy in children, palpitations, eye diseases, and nighttime crying. Several studies have also reported that C. cicadae has a wide variety of pharmaceutical properties and applications, including lipid metabolism regulation, immune regulation, renal function and vision improvements, as well as antitumor, anti-fatigue, anti-inflammatory, and antidiabetes properties [11-19]. In addition, the varous constitents of C. cicadae include polysaccharides, nucleosides, cordycepins, N6-(2-hydroxyethyl) adenosine (HEA), cyclic heptapeptide, mannitol, and ergosterol [16]. Based on the diversity of its composition and efficacy, C. cicadae has become a focus in recent research. Thus far, however, relatively little scientific literature has addressed the beneficial effects of C. cicadae on patients with glaucoma. Therefore, this study evaluates the effects of C. cicadae mycelia on IOP in a rat model of glaucoma.
Materials and methods
C. cicadae mycelia were cultured in a Grape King Bio Ltd. factory according to the following procedure. C. cicadae mycelia grown on potato dextrose agar were transferred to 1 L of basal medium (2% glucose, 1% yeast extract, and 1% yellow bean; pH 6.0) in a 2-L flask and shaken at 120 rpm and 25 °C. Three days later, the mycelial culture was inoculated into a 500-L fermenter containing 400 L of basal medium and stored at 25°C for three days. The submerged mycelial culture was heated at 100 °C for 3 h. Subsequently, C. cicadae mycelia powder was obtained through lyophilization. The freeze-dried powder was further extracted with distilled water and ethanol. The extracted solutions were then filtered, concentrated, lyophilized, and ground to obtain extract powder (referred to as “CCM” hereafter).
Phytochemical and antioxidant analysis of C. cicadae mycelia extract
A phytochemical analysis was carried out on CCM (CCM-DW and CCM-EtOH), including HEA (C12H17N5O5), polyphenols, and polysaccharides. HEA was quantified according to a previous study [17]. The content of polyphenols in CCM was measured spectrophotometrically using Folin-Ciocalteu reagent based on a colorimetric oxidation/reduction reaction and was expressed as micrograms of gallic acid equivalent (GAE) [20, 21]. The polysaccharide content in CCM was estimated using the phenol-sulfuric acid method and expressed as galactose equivalents (GE) [22, 23]. The antioxidant activity of CCM was analyzed using the 2, 2-azinobis (3-ethylbenzthiazoline-6-sulphonate (ABTS) radical scavenging assay [24, 25]. The antioxidant activity of CCM was calculated as the ABTS radical scavenging activity (%) based on cation decolorization of the ABTS radical in the presence of CCM.
Glaucoma model in rats
Twenty-four male Sprague Dawley (SD) rats at seven weeks of age were purchased from BioLASCO Taiwan Co., Ltd. (Yilan, Taiwan). The rats were maintained in 12-h dark/light cycles and housed at 22 ± 2 °C for one week prior to the experiment. The animals were provided with clean water ad libitum and a rodent diet. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Tajen University (IACUC Approval No. 106-04) and conducted according to the guidelines of The Association for Research in Vision and Ophthalmology (ARVO). The SD rats used in the study were divided into four groups (n = 6 in each group): a normal group, control group, a group that received CCM-DW and a group that received CCM-EtOH. In all animals, the right eyes were used as the experimental eyes, and the rats' tails were marked with a felt-tipped pen. Except for those in the normal group, all rats received a subconjunctival injection of betamethasone disodium phosphate (10 mg/mL, 0.03 mL) to induce high IOP, which was monitored every 2-3 days using an AccuPen handheld tonometer (Accutome, USA). After injection of steroids, 1-2 drops of an antibiotic, 0.15% moxifloxacin hydrochloride (Vigmox; Alcon, Inc. Bayer, USA), were administered to prevent eye infection. After about 7 days, the elevated IOP varied within the range of 30-40 mmHg in the experimental SD rats, indicating glaucoma. The experimental groups were administered CCM-DW (50 mg/kg/bw/day) and CCM-EtOH (50 mg/kg/bw/day) by gastric gavage. Doses with no toxic effects were selected based on previous studies [26, 27]. IOP in the right eyes of the rats was then measured weekly. Four weeks after completion of the experiments, all the rats were sacrificed and subjected to biopsies of all dissected right eyes. Serum biochemical parameters for malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), GSH peroxidase (GPx), hemoglobin (ABTS), and lactate dehydrogenase (LDH) were determined.
Histopathological evaluation
All eye tissue specimens were immersed in Davidson's Fluid and fixed after 48 h. The fixative was replaced with 10% formalin and subsequently embedded in hydroxyethyl methacrylate. We obtained a series of sections at intervals of 5 μm, which were all stained with hematoxylin and eosin. Images were captured under a light microscope (Nikon, Japan) for histopathological evaluation.
Statistical analysis
All data are expressed as the mean ± standard deviation (n = 6). Changes in body weight before and after treatment, IOP, and serum biochemical parameters were analyzed by one-way analysis of variance (ANOVA). Differences were evaluated using ANOVA followed by Dunnett's multiple comparison tests, and p < 0.05 was considered statistically significant.
Results
Phytochemical and antioxidant analysis of C. cicadae mycelia extract
The results of HEA, phytochemical, and antioxidant analyses of CCM are shown in Table 1. The HEA content was 1.6 and 1.2 mg/g in CCM-DW and CCM-EtOH, respectively. The phytochemical compositions for polyphenols in CCM-DW and CCM-EtOH were 45.7 ± 1.0 and 31.6 ± 0.7 mg GAE/g, and the polysaccharide contents were 330.0 ± 2.9 and 50.9 ± 0.9 mg GE/g, respectively. According to the antioxidant analysis, CCM has significant ABTS radical scavenging activity. The ABTS radical scavenging percentages of CCM-DW and CCM-EtOH were 74.5% and 49.0% at a concentration of 2.5 mg/ml, respectively.
Effect of C. cicadae mycelia extract on body weight in the glaucoma animal model
As shown in Table 2, significant average weight loss occurred in the groups with high IOP at the time of glaucoma induction, but not the normal group. Loss of appetite may have led to weight loss in animal groups with high IOP. Compared with the control group, a trend toward an increase in average body weight was observed for the groups that received CCM for four weeks.
Phytochemical and antioxidant analysis of C. cicadae mycelia extract (CCM).
| C. cicadae mycelia extract | HEA (mg/g) | Polyphenols (mg GAE/g) | Polysaccharides (mg GE/g) | ABTS radical scavenging activity (%) at 2.5 mg/mL |
|---|---|---|---|---|
| CCM-DW | 1.65 ± 0.02 | 45.7 ± 1.0 | 330.0 ± 2.9 | 74.5 ± 1.0 |
| CCM-EtOH | 1.21 ± 0.03 | 31.6 ± 0.7 | 50.9 ± 0.9 | 49.0 ± 2.5 |
CCM-DW (C. cicadae mycelia aqueous extract); CCM-EtOH (C. cicadae mycelia ethanolic extract); GAE: gallic acid equivalent; GE: galactose equivalent.
Effect of C. cicadae mycelia extract on body weight in the glaucoma animal model.
| Groups | Body weight (g) at the beginning | Body weight (g) at time of glaucoma induction | Body weight (g) after treatment for 4 weeks |
|---|---|---|---|
| Normal | 239.6 ± 4.9 | 306.5 ± 7.4 | 416.8 ± 14.6 |
| Control | 251.7 ± 8.7 | 198.8 ± 11.8# | 204.5 ± 40.4# |
| CCM-DW | 243.1 ± 11.7 | 193.1 ± 12.2 | 238.4 ± 53.9 |
| CCM-EtOH | 243.5 ± 9.7 | 188.3 ± 17.6 | 221.9 ± 45.8 |
Data are reported as mean ± standard deviation (n = 6). CCM-DW (C. cicadae mycelia aqueous extract, 50mg/kg/bw/day); CCM-EtOH (C. cicadae mycelia ethanolic extract, 50 mg/kg/bw/day.
Effects of C. cicadae mycelia extract on IOP in the glaucoma animal model
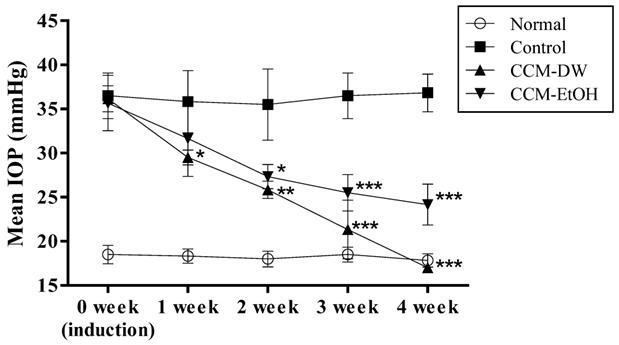
The effects of CCM on IOP in the glaucoma animal model are shown in Fig. 1. The IOP of the normal and the control groups at 0 week were 18.2 ± 0.3 and 36.9 ± 1.0 mmHg, respectively. The results revealed that the steroid-treated groups experienced significantly increased IOP at 0 weeks after steroid induction compared with the normal group, indicating that the glaucoma animal model with high IOP was successfully induced. The IOP levels after oral administration of CCM-DW and CCM-EtOH for four consecutive weeks were 17.5 ± 0.5 and 24.2 ± 2.3 mmHg, respectively. The experimental results demonstrate that the CCM-DW and CCM-EtOH groups experienced significant reductions in elevated IOP induced by steroids (p < 0.01) in a time-dependent manner over the course of four weeks, and CCM-DW exhibited superior efficacy for IOP reduction. Notably, IOP decreased to its normal range in the CCM-DW group after four weeks of treatment.
Effects of C. cicadae mycelia extract on serum biological parameters in the glaucoma animal model
The effects of the extracts on serum biological parameters in the glaucoma animal model are shown in Table 3. In the control group, the seum levels of MDA and LDH significantly increased (p < 0.05), whereas those of CAT, SOD, and GPx significantly decreased (p < 0.05) compared with the normal group. CCM-DW led to significant decreases in MDA and LDH levels and significant increases in CAT, GPx, and SOD levels after oral administration of CCM-DW for four weeks (p < 0.05). CCM-EtOH exhibited a similar trend in results as those of CCM-DW but not at significant levels. The experimental results indicated that CCM-DW was capable of enhancing the antioxidant capacity in the glaucoma rats.
Effects of C. cicadae mycelia extract on optical pathological observations in the glaucoma animal model
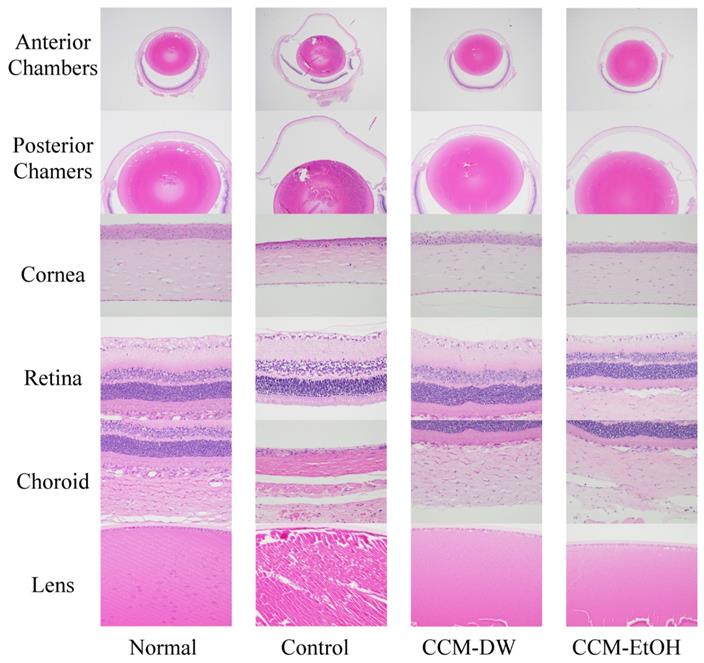
The histopathological findings for normal and chronic ocular hypertension eyes are presented in Fig. 2. The normal group exhibited whole eyes with normal anterior and posterior chambers, vitreous humor, cornea, retina, choroid, and lens. Glaucoma eyes exhibited moderate dilation in the anterior and posterior chambers with vitreous humor dilation, retinal detachment, and thin cornea, retina, and choroid, in addition to lens degeneration in the control group. The CCM-DW group exhibited whole eyes with normal anterior and posterior chambers and vitreous humor, cornea, retina, choroid, and lens. Whole eyes with slight anterior and posterior chambers with vitreous humor, normal cornea, retina, choroid, and lens were observed in the CCM-EtOH group. Regarding the micro-findings of each group, lesions were graded from 1 to 5 for severity: (1) minimal (<1%), (2) slight (1%-25%), (3) moderate (26%-50%), (4) moderate-severe (51%-75%), and (5) severe or high (76%-100%). As shown in Table 4, the normal group showed no lesions in the anterior and posterior chambers, vitreous humor, cornea, choroid or sclera, as well as no retinal detachment, no retinal thinning, and no ciliary bodies. This group had a score of 0 ± 0. In the control group, these items yielded lesion scores of 1.3-4.5, indicating that high IOP caused slight to severe damage (p < 0.01). No obvious lesions were observed in the CCM-DW group, which scored 0.0 ± 0.0, whereas the CCM-EtOH group experienced minimal lesions in the anterior and posterior chambers with scores of 0.7 ± 1.0. These results indicate that CCM-DW and CCM-EtOH were capable of significantly minimizing lesions caused by high IOP in glaucoma rats according to pathological observations (p < 0.01), and the efficacy of CCM-DW was superior to that of CCM- EtOH.
Effects of C. cicadae mycelia on hepatic and renal serum biological parameters in the glaucoma rat model.
| Groups | CAT (U/mg) | GSH (U/mg) | GPx (U/mg) | ABTS (mg/mL) | MDA (nmol/mg) | SOD (U/mg) | LDH (U/L) |
|---|---|---|---|---|---|---|---|
| Normal | 57.92±12.66 | 1.34±0.03 | 1.05±0.32 | 19.27±1.88 | 0.83±0.02 | 107.14±8.61 | 1495.7±320.9 |
| Control | 19.71±11.45# | 1.32±0.07 | 0.64±0.27# | 19.19±2.74 | 1.31±0.03# | 80.85±9.16# | 2167.0±334.1# |
| CCM-DW | 40.52±14.96* | 1.55±0.03 | 1.05±0.37* | 21.55±1.67 | 1.01±0.01* | 112.61±9.67* | 1294.8±815.1* |
| CCM-EtOH | 27.06±16.26 | 1.38±0.03 | 0.75±0.29 | 21.18±2.09 | 1.26±0.03 | 82.79±2.36 | 1739.2±798.7 |
Data are reported as mean ± standard deviation (n = 6). #p < 0.05 compared with the normal group; *p < 0.05 compared with the control group. CAT, catalase; GSH, glutathione; GPx, glutathione peroxidase; ABTS, 2,2'-Azinobis-(3-ethylbenzthiazoline-6-sulphonate); MDA, malondialdehyde; SOD, superoxide dismutase; LDH, lactic dehydrogenase. CCM-DW (C. cicadae mycelia aqueous extract, 50mg/kg/bw/day); CCM-EtOH (C. cicadae mycelia ethanolic extract, 50 mg/kg/bw/day).
Effect of C. cicadae mycelia extract on IOP in the glaucoma model. Data are expressed as mean ± standard deviation (n = 6). *p < 0.05, **p < 0.01, and ***p < 0.001 indicate a significant difference from the control group. CCM-DW (C. cicadae mycelia aqueous extract, 50mg/kg/bw/day); CCM-EtOH (C. cicadae mycelia ethanolic extract, 50 mg/kg/bw/day).
Histopathological findings for eyes in the chronic intraocular hypertension model. Anterior and posterior chambers with vitreous humor (20×, 40×), cornea (400×), retina (400×), choroid (400×), and lens (400×). Haematoxylin and eosin stain.
Effects of C. cicadae mycelia on micropathological observations in the glaucoma model.
| Groups | anterior chamber, posterior chamber | Vitreous humor | Cornea | Choroid and sclera | Retinal detachment | Retinal thinning | ciliary body |
|---|---|---|---|---|---|---|---|
| Normal | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 | 0±0 |
| Control | 3.2±0.8## | 3.5±0.5## | 2.7±0.8## | 3.5±0.8## | 4.5±1.2## | 1.7±0.8## | 1.3±1.0## |
| CCM-DW | 0±0** | 0±0** | 0±0** | 0±0** | 0±0** | 0±0** | 0±0** |
| CCM-EtOH | 0.7±1.0** | 0±0** | 0±0** | 0±0** | 0±0** | 0±0** | 0±0** |
Data are reported as mean ± standard error of the mean (n = 6). ##p < 0.01 compared with the normal group. **p < 0.01 compared with the control group. Lesions were graded from 1 to 5 depending on severity: 1 = minimal (<1%), 2 = slight (1%-25%), 3 = moderate (26%-50%), 4 = moderate-severe (51%-75%), 5 = severe-high (76%-100%).
Discussion
Glaucoma is the second most common cause of blindness worldwide and is characterized by progressive degeneration of the optic nerve and RGCs [28]. Although other factors have been proposed as playing roles in glaucoma, high IOP remains the primary documented risk factor for glaucomatous optic nerve damage [29]. For the treatment of eye disease, following the administration of benzalkonium chloride, fermented mycelia extracts of C. cicadae reportedly enhanced corneal resilience and the maintenance of conjunctival goblet cells, thus causing an improvement in dry-eye symptoms [27]. In the present study, we first evaluated the IOP-lowering effects of C. cicadae mycelia extract in a steroid-induced glaucoma rat model for glaucoma disease treatment.
The results revealed that steroid treatment-induced glaucoma led to a significant increase in IOP and decrease in the average weight of rats. Steroid-induced elevated IOP is reportedly dose-dependent and followed by known systemic toxic effects, including weight loss [30]. Even though the common side effects of glucocorticoid in humans include weight gain [31], the development of stomach irritation is also commonly observed, which could lead to the loss of appetite and weight in rats. However, body weight loss improved slightly compared with the control group after the administration of CCM for four weeks. Compared with the normal group, IOP significantly increased by approximately 20 mmHg in the groups given steroid (subconjunctival betamethasone injection), indicating that the glaucoma animal model was successfully induced. The experimental results revealed that CCM-DW and CCM-EtOH were capable of significantly lowering IOP in glaucoma rats (p < 0.01) in a week-dependent manner, and the effectiveness of CCM-DW was higher than that of CCM-EtOH. In the CCM-DW group, IOP was restored to its normal range after four weeks of treatment compared with the normal group. Pathological observations revealed significant lesions for the anterior and posterior chambers, vitreous humor, cornea, choroid, and sclera in addition to retinal detachment, retinal thinning, and ciliary bodies in the control group. No evident lesions were observed in the micropathological findings of the CCM-DW group, whereas the CCM-EtOH group exhibited minimal-grade lesions in the anterior and posterior chambers. The results indicated that CCM-DW and CCM-EtOH caused a significant decrease in IOP-induced lesions in glaucoma rats according to histopathological findings, and the efficacy of CCM-DW was superior to that of CCM-ETOH. The control group had significantly increased MDA and LDH levels and significantly decreased GPx, CAT, and SOD levels, indicating that the glaucoma rats had high oxidative stress levels. In the experimental groups, CCM-DW caused a significant decrease in MDA and LDH levels and a significant increase in GPx, CAT, and SOD levels after four weeks of treatment. CCM-EtOH yielded similar trend results. The experimental results indicate that C. cicadae mycelia extract enhanced the intrinsic antioxidant capabilities of the glaucoma rats.
Currently, medical therapy to lower IOP remains the most common initial treatment method in addition to surgical procedures and laser treatment for glaucoma. Medical therapy involves two mechanisms to control IOP: enhancing outflow drainage and hindering the production of aqueous humor [32]. Among the many drugs available for glaucoma treatment, rho-associated protein kinase (ROCK) inhibitors have commonly been used to alter the shape of trabecular meshwork cells, allowing for enhanced aqueous humor outflow to lower IOP [33]. However, according to our unpublished data, the IOP-lowering effects of CCM-DW and CCM-EtOH are not mediated through the ROCK/myosin light chain phosphorylation (MLC) pathway, suggesting that there may be other pathways.
Adenosine and different adenosine receptor subtype agonists (A1, A2, and A3) have also been proposed as potential IOP lowering drugs [34]. Previous studies have shown that topical treatment with an adenosine A1 receptor agonist can significantly decrease IOP in monkeys [35], rabbits [36], and mice [37]. Moreover, A2 and A3 adenosine receptor agonists are currently being evaluated in larger populations in the clinical trial phase with the expectation of lowering IOP [33]. The proposed mechanism of these adenosine receptor agonists is via the induction of the level of matrix metalloproteinases (MMPs), which leads to remodeling of the extracellular matrix in the trabecular meshwork and a consequent increase in aqueous humor outflow. As a derivative of adenosine, HEA may exert pharmacological activity by acting on adenosine receptors. Nevertheless, further studies are needed to clarify this point.
Antioxidant pretreatment reportedly results in a marked reduction of the effect of oxidative stress on the trabecular meshwork [38]. C. cicadae mycelia has been reported to have a high clearance rate of 2',2'-diphenyl-1-picrahydrazyl (DPPH) free radicals [39]. C. cicadae has also been reported to have antioxidant components, including polysaccharides, cordycepin, HEA, and ergosterol [16]. Intracellular polysaccharides and extracted exopolysaccharides from C. cicadae exhibit higher antioxidant potential with significant ABTS and DPPH radical-scavenging activities, reducing power, and iron chelating activity [40]. In addition, HEA isolated from C. cicadae has antihyperglycemic, anti-inflammatory, and antioxidant effects in diabetic rats and the results suggested that HEA attenuates inflammation and oxidative stress in kidney tissue [41]. HEA is an antioxidant compound from C. cicadae and has beneficial effects on oxidative stress-related diseases, including diabetes and kidney disease. Our phytochemical analysis results showed that CCM-DW and CCM-EtOH contains HEA, polyphenol, and polysaccharide components. The amounts of HEA, polysaccharides, and polyphenol contents in CCM-DW were significantly higher than those in CCM-EtOH, suggesting a reason why the effectiveness of CCM-DW was higher than that of CCM-EtOH. In addition, both CCM-DW and CCM-EtOH had higher contents of polysaccharides than polyphenols. Notable, that the polysaccharide content was approximately seven times higher than that of polyphenols in CCM-DW. The results indicate that polysaccharides were the abundant constituent in CCM-DW. The ABTS radical-scavenging assay has been widely used to compare the antioxidant ability of plant extracts. In the case of antioxidant activity, the ABTS radical-scavenging activity of CCM-DW was significantly higher than that of CCM-EtOH. The results indicate that when compared with polyphenols, polysaccharides as the main components of CCM-DW dominate the antioxidant activity of CCM. An increasing amount of evidence shows that reactive oxygen species play a key role in the pathogenesis of primary open-angle glaucoma [42]. The correlation among the content of antioxidant components, in vitro/in vivo antioxidant capabilities, and IOP-lowering activity suggest that oxidative stress plays a critical role in the development of IOP increase and glaucoma-associated lesions. Theoretically, based on our overall results, CCM and its antioxidant activity could be associated with lowered IOP and dysfunction of the optic nerve and retinal ganglion cells through antioxidant mechanisms. In addition, male and female SD rats that were administered mycelial C. cicadae for 90 days in a sub-chronic toxicity study revealed no observable adverse effects at a dose of > 2000 mg/kg, which suggests that C. cicadae is safe for use in functional food development [26].
Conclusions
In conclusion, this study has indicated that C. cicadae mycelia could be used as a food supplement with substantial benefits for relieving glaucoma symptoms through significant IOP-lowering and antioxidant activities. Subsequent studies could focus on the investigation of molecular mechanisms underlying the therapeutic effects of C. cicadae mycelia associated with glaucoma. C. cicadae mycelia could provide an alternative natural plant resource for glaucoma treatment.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-7
2. Lee SH, Kim T-W, Lee EJ, Girard MJA, Mari JM, Ritch R. Ocular and Clinical Characteristics Associated with the Extent of Posterior Lamina Cribrosa Curve in Normal Tension Glaucoma. Scientific Reports. 2018;8:961
3. Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology. 2014;121:2081-90
4. Smith RS, Zabaleta A, Savinova OV, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001;1:3
5. Chan HC, Chang RC, Koon-Ching Ip A, Chiu K, Yuen WH, Zee SY. et al. Neuroprotective effects of Lycium barbarum Lynn on protecting retinal ganglion cells in an ocular hypertension model of glaucoma. Exp Neurol. 2007;203:269-73
6. Morrison JC, Johnson EC, Cepurna WO. Hypertonic Saline Injection Model of Experimental Glaucoma in Rats. Methods Mol Biol. 2018;1695:11-21
7. Lee DA, Higginbotham EJ. Glaucoma and its treatment: a review. Am J Health Syst Pharm. 2005;62:691-9
8. Rocha-Sousa A, Rodrigues-Araújo J, Gouveia P, Barbosa-Breda J, Azevedo-Pinto S, Pereira-Silva P. et al. New therapeutic targets for intraocular pressure lowering. ISRN Ophthalmol. 2013;2013:261386 -
9. Chiu C-P, Hwang T-L, Chan Y, El-Shazly M, Wu T-Y, Lo IW. et al. Research and development of Cordyceps in Taiwan. Food Science and Human Wellness. 2016;5:177-85
10. Liu T, Liu Z, Yao X, Huang Y, Qu Q, Shi X. et al. Identification of cordycepin biosynthesis-related genes through de novo transcriptome assembly and analysis in Cordyceps cicadae. R Soc Open Sci. 2018;5:181247
11. Hsu J-H, Jhou B-Y, Yeh S-H, Chen Y-L, Chen C-C. Healthcare functions of Cordyceps cicadae. J Nutr Food Sci. 2015;5:1-7
12. Weng SC, Chou CJ, Lin LC, Tsai WJ, Kuo YC. Immunomodulatory functions of extracts from the Chinese medicinal fungus Cordyceps cicadae. J Ethnopharmacol. 2002;83:79-85
13. Ng TB, Wang HX. Pharmacological actions of Cordyceps, a prized folk medicine. J Pharm Pharmacol. 2005;57:1509-19
14. Guo P, Kai Q, Gao J, Lian ZQ, Wu CM, Wu CA. et al. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J Pharmacol Sci. 2010;113:395-403
15. Zhu R, Chen YP, Deng YY, Zheng R, Zhong YF, Wang L. et al. Cordyceps cicadae extracts ameliorate renal malfunction in a remnant kidney model. J Zhejiang Univ Sci B. 2011;12:1024-33
16. Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013;65:474-93
17. Lu MY, Chen CC, Lee LY, Lin TW, Kuo CF. N(6)-(2-Hydroxyethyl)adenosine in the Medicinal Mushroom Cordyceps cicadae Attenuates Lipopolysaccharide-Stimulated Pro-inflammatory Responses by Suppressing TLR4-Mediated NF-κB Signaling Pathways. J Nat Prod. 2015;78:2452-60
18. Yang S, Zhang H. Optimization of the fermentation process of Cordyceps sobolifera Se-CEPS and its anti-tumor activity in vivo. J Biol Eng. 2016;10:8
19. Li IC, Lin S, Tsai YT, Hsu JH, Chen YL, Lin WH. et al. Cordyceps cicadae mycelia and its active compound HEA exert beneficial effects on blood glucose in type 2 diabetic db/db mice. J Sci Food Agric. 2019;99:606-12
20. Negi PS, Jayaprakasha GK, Jena BS. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chemistry. 2003;80:393-7
21. Lee CF, Fan CW, Chiang NN, Chang HC, Chen C, Huang YS. et al. Protective effect of Corchorus capsularis L. leaves on ethanol-induced acute gastric mucosal lesion in rats. J Vet Med Sci. 2019;81:1636-42
22. Horng CT, Huang JK, Wang HY, Huang CC, Chen FA. Antioxidant and antifatigue activities of Polygonatum Alte-lobatum Hayata rhizomes in rats. Nutrients. 2014;6:5327-37
23. DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric Method for Determination of Sugars and Related Substances. Analytical Chemistry. 1956;28:350-6
24. Park SH, Kim YK, Kim MS, Lee SH. Antioxidant and Antibacterial Properties of Hovenia (Hovenia dulcis) Monofloral Honey Produced in South Korea. Food Sci Anim Resour. 2020;40:221-30
25. Chatatikun M, Chiabchalard A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement Altern Med. 2017;17:487
26. Chen YL, Yeh SH, Lin TW, Chen CC, Chen CS, Kuo CF. A 90-Day Subchronic Toxicity Study of Submerged Mycelial Culture of Cordyceps cicadae (Ascomycetes) in Rats. Int J Med Mushrooms. 2015;17:771-81
27. Lin T-Y, Chang H-H, Tang Y-J, Chen C-C, Lee L-Y, Lin D. Fermented Cordyceps cicadae Mycelia Extracts Ameliorate Dry Eye Symptoms through Reduction of Cornea Epithelial Cell Apoptosis and Maintenance of Conjunctival Goblet Cells in a Mouse Dry Eye Model. Journal of Food and Nutrition Research. 2017;5:320-30
28. Pang JJ, Frankfort BJ, Gross RL, Wu SM. Elevated intraocular pressure decreases response sensitivity of inner retinal neurons in experimental glaucoma mice. Proc Natl Acad Sci U S A. 2015;112:2593-8
29. Horng CT, Tsai ML, Chien ST, Kao WT, Tsai MK, Chang TH. et al. The activity of lowering intraocular pressure of cassiae seed extract in a DBA/2J mouse glaucoma model. J Ocul Pharmacol Ther. 2013;29:48-54
30. Lorenzetti OJ. Effects of corticosteroids on ocular dynamics in rabbits. J Pharmacol Exp Ther. 1970;175:763-72
31. Hopkins RL, Leinung MC. Exogenous Cushing's syndrome and glucocorticoid withdrawal. Endocrinology and metabolism clinics of North America. 2005;34:371-84 ix
32. Harasymowycz P, Birt C, Gooi P, Heckler L, Hutnik C, Jinapriya D. et al. Medical management of glaucoma in the 21st century from a Canadian perspective. Journal of ophthalmology. 2016. 2016
33. Moshirfar M, Parker L, Birdsong OC, Ronquillo YC, Hofstedt D, Shah TJ. et al. Use of Rho kinase Inhibitors in Ophthalmology: A Review of the Literature. Med Hypothesis Discov Innov Ophthalmol. 2018;7:101-11
34. Lee AJ, Goldberg I. Emerging drugs for ocular hypertension. Expert opinion on emerging drugs. 2011;16:137-61
35. Tian B, Gabelt BAT, Crosson CE, Kaufman PL. Effects of Adenosine Agonists on Intraocular Pressure and Aqueous Humor Dynamics in Cynomolgus Monkeys. Experimental Eye Research. 1997;64:979-89
36. Crosson CE. Adenosine receptor activation modulates intraocular pressure in rabbits. J Pharmacol Exp Ther. 1995;273:320-6
37. Avila MY, Stone RA, Civan MM. A(1)-, A(2A)- and A(3)-subtype adenosine receptors modulate intraocular pressure in the mouse. Br J Pharmacol. 2001;134:241-5
38. WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42:2849-55
39. Chen A, Li C, Fan M. Scavenging and anti-fungal activities of the metabolite of Paecilomyces cicadae. Mycosystema. 2008;27:405-12
40. Sharma SK, Gautam N, Atri NS. Optimized extraction, composition, antioxidant and antimicrobial activities of exo and intracellular polysaccharides from submerged culture of Cordyceps cicadae. BMC Complement Altern Med. 2015;15:446
41. Wang X, Qin A, Xiao F, Olatunji OJ, Zhang S, Pan D. et al. N(6) -(2-hydroxyethyl)-adenosine from Cordyceps cicadae protects against diabetic kidney disease via alleviation of oxidative stress and inflammation. J Food Biochem. 2019;43:e12727
42. Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105-14
Author contact
![]() Corresponding author: Fu-An Chen, Tel.: +886-87624002#2617, E-mail: fachenedu.tw
Corresponding author: Fu-An Chen, Tel.: +886-87624002#2617, E-mail: fachenedu.tw

 Global reach, higher impact
Global reach, higher impact