3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(4):944-952. doi:10.7150/ijms.50275 This issue Cite
Review
Endovascular treatment of blunt injury of the extracranial internal carotid artery: the prospect and dilemma
1. Department of Neurosurgery, The First Hospital of Jilin University, Changchun, 130021, China
2. Department of Neurology, The First Hospital of Jilin University, Changchun, 130021, China
*Guangming Wang and Chao Li contributed equally to this work.
Received 2020-7-3; Accepted 2020-12-18; Published 2021-1-1
Abstract
The extracranial internal carotid artery (ICA) refers to the anatomic location that reaches from the common carotid artery proximally to the skull base distally. The extracranial ICA belongs to the C1 segment of the Bouthillier classification and is at considerable risk for injury. Currently, the understanding of endovascular treatment (EVT) for blunt injury of the extracranial ICA is limited, and a comprehensive review is therefore important. In this review, we found that extracranial ICA blunt injury should be identified in patients presenting after blunt trauma, including classical dissection, pseudoaneurysm, and stenosis/occlusion. Computed tomography angiography (CTA) is the first-line method for screening for extracranial ICA blunt injury, although digital subtraction angiography (DSA) remains the “gold standard” in imaging. Antithrombotic treatment is effective for stroke prevention. However, routine EVT in the form of stenting should be reserved for patients with prolonged neurological symptoms from arterial stenosis or considerably enlarged pseudoaneurysm. Endovascular repair is now emerging as a favored therapeutic option given its demonstrated safety and positive clinical and radiographic outcomes.
Keywords: endovascular treatment, blunt injury, extracranial internal carotid artery
Introduction
The extracranial internal carotid artery (ICA) refers to the anatomic location from the common carotid artery proximally to the skull base distally, and the segment ICA of the skull base is not included (Figure 1). In 1996, Bouthiller classified the ICA into seven segments: C1, cervical; C2, petrous; C3, lacerum; C4 cavernous; C5, clinoid; C6, ophthalmic; and C7, communicating [1]. This classification is practical and clarifies all segments of the ICA, and the extracranial ICA therefore refers to the C1 segment.
Extracranial ICA is at high risk for injury because it is more mobile and vulnerable to stretching [2]. The most common cause is blunt trauma from motor vehicle crashes, which results in a disruption in one or more layers of the extracranial ICA [3-5]. Extracranial traumatic vascular injury is present in approximately 1% to 2% of patients after blunt trauma, and the incidence of extracranial ICA injury ranges from 0.08 to 0.33%, among which 52-79% of these patients are asymptomatic. For most of these patients, conservative medical treatment is sufficient [2, 6-10]. However, for a small number of patients with disastrous implications, surgical intervention is needed [11].
Currently, endovascular treatment (EVT) has been used with encouraging outcomes [12, 13]. However, the understanding of EVT for blunt injury of the extracranial ICA is limited, and a comprehensive review is therefore important. In this paper, a literature search was performed using the PubMed database and relevant search terms. After a review of the obtained literature, the current status of EVT for blunt injury of extracranial ICA was summarized in terms of pathogenesis, classification and grading, imaging examination, EVT prospects and controversies, EVT options, prognosis and complications.
Pathogenesis
The blunt injury of the extracranial ICA begins with intimal damage resulting in dissection, which is the final common pathway for vascular injury. The dissection can be progressive and extend to the distal site, resulting in ICA stenosis/occlusion [14, 15].
When dissection disrupted almost all layers of the ICA wall with preservation of only the adventitia, pseudoaneurysm potentially occurred in 10% of cases with extracranial ICA blunt injury [6, 16]. Pseudoaneurysms were defined as more than 50% focal dilatation of the ICA diameter and tended to occur in the upper cervical segment; moreover, they could grow [16, 17].
These injuries could be solitary or multiple, with one or more patterns of injury, and bilateral extracranial ICA could even be involved [9, 18-20].
Any kind of blunt injury of the extracranial ICA could result in platelet activation and thrombosis to cause distal embolization [21]. In addition, when stenosis/occlusion occurs, the subsequent hemodynamics are compromised if collateral circulation is not satisfactory [6, 22]. The majority of ischemic strokes occur relatively early; however, 17-36% of cases develop symptoms >24 h post injury, and 8% develop symptoms after a week [2, 9, 23].
Classification and grading
For extracranial ICA blunt injury, there are several classifications, such as the Biffl scale, Borgess classification, and Seth supplemental classification to Biffl [24-27].
The Borgess classification includes four types. Type I dissections have an intact intima, with type IA displaying persistent antegrade flow and type IB demonstrating complete occlusion. Type II dissections have an intimal tear, with a small disruption of the intima and a small sidewall aneurysm in type IIA and a clear intimal flap and pseudoaneurysm in type IIB [24]. However, for blunt carotid arterial injuries, the Borgess classification is limited.
The anatomy of the extracranial ICA region. A-B: Lateral view (A) and posterior anterior view (B) of CTA showing carotid veins around the extracranial ICA. C-D: Lateral view (C) and posterior anterior view (D) of CTA showing that the extracranial ICA extends from the bifurcation to the skull base, and the extracranial ICA belongs to the C1 segment of the Bouthiller classification (asterisk). Abbreviations: CTA: computed tomography angiography; ICA: internal carotid artery
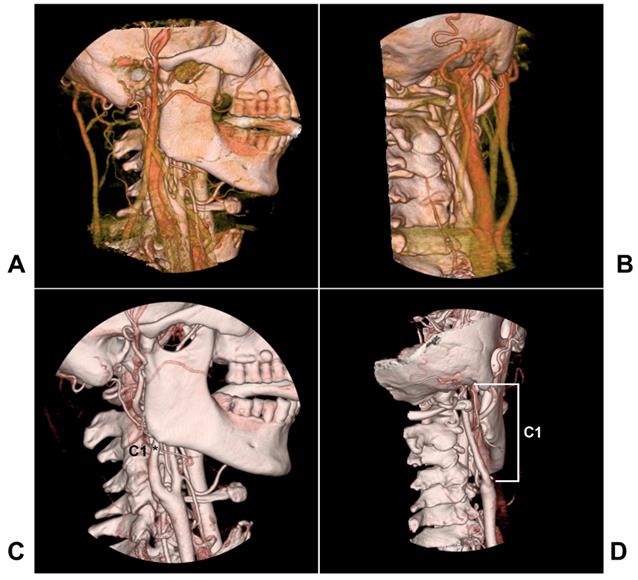
Currently, the Biffl et al. scale is commonly utilized to assign vascular injuries and has become the standard for extracranial ICA blunt injury, from grade I to V as follows: grade I, irregularity or dissection with <25% stenosis; grade II, dissection with >25% luminal narrowing or a raised intimal flap; grade III, pseudoaneurysm; grade IV, complete occlusion; and grade V, ICA transection, active contrast extravasation [25, 26].
In this classification, grade V is very rare in extracranial ICA blunt injury because it is often caused by skull base fracture, which is not attributed to the extracranial ICA. Therefore, in our review, traumatic extracranial ICA transection was excluded, and only classical dissection, pseudoaneurysm, and traumatic stenosis/occlusion are discussed.
Furthermore, Seth et al. (2013) modified the Biffl et al. scale, further subdividing grade 2 and 3 injuries were further subdivided into 2a or 3a (non-flow-limiting when<70% luminal narrowing was present) and 2b or 3b (flow-limiting when >70% luminal narrowing was present) [27].
High grades had a greater risk of stroke; for instance, in the Biffl et al. (1999) study, the stroke rates with type I, type II, type III, and type IV ICA injuries were 3, 11, 33, and 44%, respectively [26]. In addition, it must be emphasized here that follow-up imaging in extracranial ICA blunt injury is very important, as these injuries are variable; for instance, approximately 5% of low-grade injuries (88% pseudoaneurysms) can change to a higher grade [3, 28].
Imaging examination
Available imaging
Extracranial ICA blunt injury may be diagnosed using digital subtraction angiography (DSA), computed tomography angiography (CTA), magnetic resonance imaging/angiography (MRI/MRA), and ultrasonography; of these, ultrasonography alone is not an adequate test and is not recommended for screening, and DSA remains the “gold standard” [3, 29-33].
DSA can detect extracranial ICA blunt injury accurately with a sensitivity greater than 99% and a specificity of 100%; it also affords the opportunity to administer EVT [6, 33]. However, this does not mean that DSA is preferred as the initial screening test [34].
Currently, the Eastern Association for the Surgery of Trauma's Blunt Cerebrovascular Injury Practice Management Guidelines recommend that CTA is the first-line method for screening for extracranial ICA blunt injury due to its increasing use and relative reliability [35].
MRI/MRA is most useful for evaluating patients with clinical evidence of stroke rather than as a screening tool [36]. In addition, they are useful in the detection of dissections and intramural hematomas [37]. Sometimes, routine computed tomography (CT) is also useful, as only CT can obtain secondary images of brain infarction and find high-density middle cerebral artery signs [38].
In addition, intraarterial optical coherence tomography is a new technique that provides in vivo evidence of arterial injury and thrombi not visible on CTA or DSA [39].
Imaging characteristics
The imaging of extracranial ICA blunt injury varies as a result of various pathogeneses [40].
Classical dissection
Classical dissections include intramural hematomas, intimal tears and raised intimal flaps [8]. Intramural hematomas present with eccentric/circumferential mural thickening extending along the long artery [18]. Intimal tears present with subtle mural thickening and contour irregularity, and raised intimal flaps present with a linear pattern of filling defect/false lumen opacification known as the ''double lumen'' sign and even pseudofenestration [41].
Pseudoaneurysm
Pseudoaneurysms present with eccentric luminal dilatation and adjacent outpouchings [42, 43], and they are further classified into saccular and fusiform types [44]. Saccular laminae have a distinct neck and a roundish dome and arise from a disruption of the elastic laminae [45]. Fusiform laminae have a smooth, tapering shape and arise from a less extensive disruption of the elastic laminae [33]. Saccular aneurysms have a greater tendency to enlarge than fusiform aneurysms [44].
Stenosis/occlusion
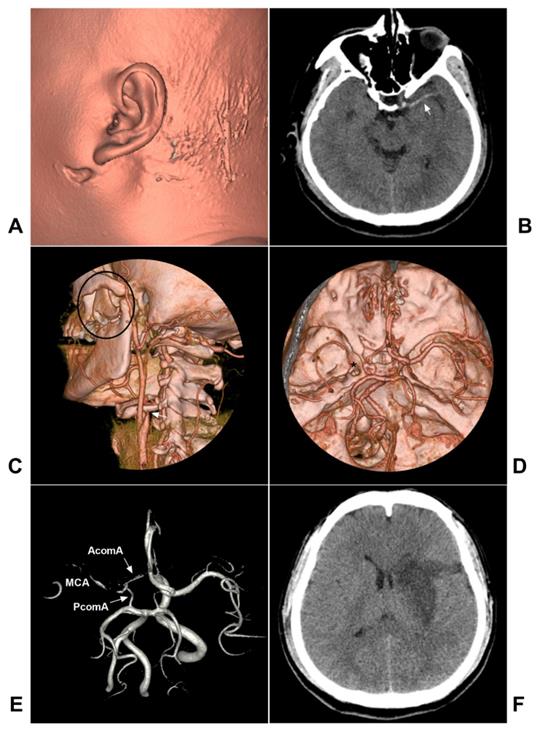
Dissection-related stenosis usually appears as a smooth and tapered narrowing that varies in severity and length depending on the extent of the dissection, and the occlusion demonstrates a tapered, flame-like appearance [15]. On DSA, antegrade and retrograde collateral obstruction channels, including the anterior and posterior communicating arteries and ophthalmic artery, can be seen in the acute phase of arterial occlusion, and rapid assessment of collateral circulation on DSA is thus necessary [46, 47]. A typical case is shown in Figure 2.
Traumatic extracranial ICA occlusion. A: CTA soft tissue reconstruction shows the left face injury in front of the ear. B: CT showed the left MCA high-density sign (arrow). C: CTA reconstruction showed maxillary bone fracture (black ellipse) and extracranial ICA occlusion (arrow). D: CTA reconstruction showed a thin intracranial MCA (asterisk). E: MRA showed the connections from AcomA and PcomA to MCA. F: Follow-up CT showed infarction of the left basal ganglia region. Abbreviations: AcomA: anterior communicating artery; CT: computed tomography; CTA: CT angiography; ICA: internal carotid artery; MCA: middle cerebral artery; MRA: magnetic resonance angiography; PcomA: posterior communicating artery
EVT prospects and controversy
EVT prospect
Low-grade extracranial ICA blunt injury should be treated with early systemic anticoagulation or antiplatelet therapy [6, 48-51]. The Eastern Association for the Surgery of Trauma recommends against the routine use of endovascular stents as an adjunct to antithrombotic therapy in patients with asymptomatic grade II or stable III extracranial ICA blunt injuries [52, 53].
For flow-limiting dissections of grade II, balloon angioplasty or stenting is used [54]. For expanding or symptomatic pseudoaneurysms (grade III) (depth>15 mm), stenting with/without coiling is necessary [55, 56]. For vessel occlusion (grade IV) with acute flow-related infarcts, mechanical thrombectomy or recanalization EVT may be considered to restore flow [57].
EVT dilemma
For severe and complex extracranial ICA blunt injuries, EVT reconstruction is sometimes difficult and fails, thus causing several pitfalls, including microcatheterization of the true carotid lumen [27]. In addition, microcatheterization may worsen the dissection or release thrombi, or the microcatheter may accidentally remain in the false lumen [58, 59].
For some extracranial ICA blunt injuries, arterial occlusion is sometimes the last resort [11, 60]. However, when performing arterial occlusion, the balloon occlusion test (BOT) is not absolutely safe, as it is not reliable for semicomatose patients [61, 62]. In addition, patients who passed the BOT can exhibit ischemic events [63-65]. Moreover, arterial occlusion carries a risk of migration of the intraluminal thrombus into the intracranial circulation [66].
In addition, for extracranial ICA blunt injury, EVT using stents requires antiplatelet therapy, which can be problematic in trauma patients. If the antiplatelet agents are not used routinely, acute thrombosis occurs in 45% of patients; thus, it seems prudent to limit EVT [11].
Endovascular option
Outline
EVT for extracranial ICA blunt injury can be divided into reconstruction and deconstruction techniques [2].
Reconstruction
Reconstructive EVT includes mechanical thrombectomy, balloon angioplasty, traditional carotid artery stenting (CAS) and flow-diverting stent (FDS) deployment, and intracranial stent deployment [67, 68].
Mechanical thrombectomy
If thrombosis forms in the ICA true lumen after blunt injury, endovascular clot removal is an attractive option for the treatment of acute stroke in the early stage; however, the clot is often dissected, and it is difficult or dangerous to remove the intramural hematoma [2, 69]. In addition, mechanical thrombectomy should be used in the stenting combination [70].
Carotid artery stent deployment
For symptomatic dissection, CAS allows better containment of the mural thrombus between the stent and vessel wall, with a >99% technique success rate [2, 71]. During CAS, the stent should cross the entire length of the dissection; when using overlapping stents, the initial stent should be placed at the proximal margin of the dissection [27, 72]. Currently, CAS is the mainstay for most proximal and middle extracranial ICA dissections [55].
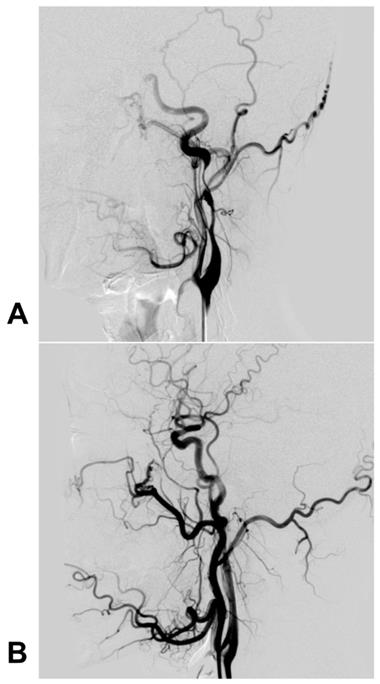
For pseudoaneurysms, CAS with/without coil embolization of the aneurysm cavity is a feasible technique [73]. For instance, Kadkhodayan et al. (2005) reported that 9 traumatic carotid dissections with/without associated pseudoaneurysms were effectively treated with CAS; in this report, some pseudoaneurysms were given coiling simultaneously [71]. Currently, there is no consensus on whether coiling is needed during CAS for pseudoaneurysms, but it is reasonable that large and wide-necked coiling should be recommended [74-76]. A typical case is shown in Figure 3.
Carotid stent deployment for traumatic extracranial ICA dissection with severe stenosis. A: The preoperative DSA revealed a long dissection with severe stenosis of the extracranial ICA, and the intracranial blood supply was poorly visible. B: DSA after carotid stenting showed that the extracranial ICA recovered to a normal diameter, and the intracranial blood supply was normal. Abbreviations: DSA: digital subtraction angiography; ICA: internal carotid artery; MRA: magnetic resonance angiography; MCA: middle cerebral artery; PcomA: posterior communicating artery
Covered stent deployment
By setting up a direct physical barrier, the covered stent allows the immediate closure of the leakage while maintaining the patency of the parent artery; for extracranial ICA pseudoaneurysm, the covered stent graft has appeal [43, 67, 77]. In Wang et al. (2020), 5 patients with traumatic carotid pseudoaneurysms underwent covered stenting, and complete exclusion of the pseudoaneurysm and patency of the parent artery were maintained during follow-up [78].
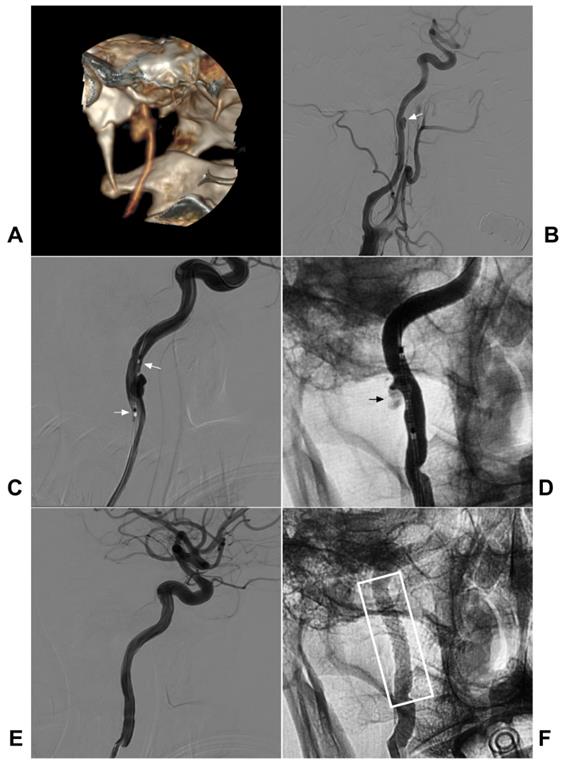
During covered stent deployment, to ensure good adherence and avoid endoleak, the stent diameter is selected mainly according to the larger diameter of the proximal and distal ICA, and the length of the stent must be long enough [42, 79]. A typical case is shown in Figure 4.
Flow-diverting stent deployment
The FDS is a self-expanding tubular mesh made of cobalt-chromium with more than 30% metal coverage and 3 times the vessel wall coverage compared with traditional intracranial stents [80]. The woven tubes can provide a significantly increased potential for thrombosis by diverting blood flow away from the aneurysm and reconstructing the diseased parent artery as well as providing a scaffold for endothelial healing [81].
It is difficult to access extracranial ICA blunt injuries with the inflexible carotid stent delivery system when they are located in the high extracranial ICA near the skull base because of ICA tortuosity [82]. In contrast, the FDS is flexible, enabling the device to more efficiently conform to vessel curves [83].
Covered stent deployment for the traumatic extracranial ICA pseudoaneurysm. A-B: CTA (A) and DSA (B) showed a pseudoaneurysm in the high segment extracranial CIA (arrow in B). C: A covered stent (MicroPort Medical Co., Shanghai, China) crossed the pseudoaneurysm (arrows show the distal and proximal markers). D: After covered stent deployment, endoleak was observable (arrow). E: After the balloon dilation was repeated, the endoleak disappeared. F: X-ray showing the covered stent (white frame). Abbreviations: CTA: CT angiography; DSA: digital subtraction angiography; ICA: internal carotid artery
Therefore, the FDS should be suitable for treating distal extracranial ICA dissections and pseudoaneurysms [55]. For instance, Brzezicki et al. (2016) reported 13 extracranial ICA dissections, of which 9 were caused by trauma. After FDS deployment, complete revascularization was achieved in 91% of vessels, and 50% of pseudoaneurysms were completely or near completely obliterated immediately [84].
With FDS deployment, a higher risk of FDS migration may occur in the extracranial ICA than in intracranial vessels because of the high luminal pressure and frequent positional changes with neck movement; thus, proximal migration of the FDS is oversized relative to that of the extracranial ICA [81]. For some extracranial ICA injuries, CAS can be performed after FDS deployment because the FDA can easily pass the complex and tortuous ICA segment [85].
In addition, multiple overlaying FDSs can be used when necessary [81].
Intracranial stent deployment
Although intracranial stents can be used for this indication, they may not possess enough radial force to exclude false lumen or provide enough flow diversion to promote thrombosis of the pseudoaneurysm; because of their large cell design, they can rarely be used alone [74]. Therefore, the intracranial stent cannot be used routinely [86].
However, if coiling is combined, it may be a good choice [87]. For instance, Joo et al. (2016) successfully treated an extracranial ICA pseudoaneurysm with stenting and coiling, and the EVT was satisfactory after a one-year follow-up [88].
Deconstruction
For extracranial ICA blunt injuries that cannot be treated by stenting reconstruction, such as some severe pseudoaneurysms, therapeutic arterial occlusion must be performed [16]. Certainly, the BOT must be performed before considering this option [89]. For arterial occlusion, the embolic materials include detachable balloons, coils, and liquid embolic agents [90, 91].
Prognosis and complication
For extracranial ICA blunt injury, the prognosis can be judged by the modified Rankin Scale (mRS), and poor outcome is defined as recurrent stroke or a mRS score of 2 or more [48].
In general, for extracranial ICA blunt injury, the survivors who accept EVT have a good prognosis due to the high success rate [92, 93]. The systematic review of Pavlos et al. (2020) included twenty-four studies comprising 191 patients (204 lesions) and showed that EVT for extracranial ICA blunt injury was associated with excellent outcomes and efficiently prevented recurrent stroke and TIA and demonstrated excellent recanalization and pseudoaneurysm occlusion rates with very low retreatment rates [94].
Complications of EVT in extracranial ICA blunt injury are low [95]. In a retrospective study, Kadkhodayan et al. (2005) demonstrated a complication rate of 6.9% [71]. The complications of EVT in extracranial ICA blunt injury were associated with injury grading or the EVT procedure. For instance, for extracranial ICA blowouts, Choi et al. (2018) reported a procedure-related complication rate of 37.5% and a cerebral infarction rate of 25% [96].
Conclusion
Extracranial ICA blunt injury should be identified in patients presenting after blunt trauma, including classical dissection, pseudoaneurysm, and stenosis/occlusion. CTA is the first-line method for screening for extracranial ICA blunt injury. Antithrombotic treatment is effective for stroke prevention. However, routine EVT in the form of stenting should be reserved for patients with prolonged neurological symptoms from arterial stenosis or considerably enlarging pseudoaneurysm. Endovascular repair is now emerging as a favored therapeutic option given its demonstrated safety and positive clinical and radiographic outcomes.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38:425-32 discussion 32-3
2. Foreman PM, Harrigan MR. Blunt Traumatic Extracranial Cerebrovascular Injury and Ischemic Stroke. Cerebrovasc Dis Extra. 2017;7:72-83
3. Elbanna KY, Mohammed MF, Choi JI, Dawe JP, Joos E, Baawain S. et al. What Are the Expected Findings on Follow-up Computed Tomography Angiogram in Post-traumatic Patients With Blunt Cerebrovascular Injury? Can Assoc Radiol J. 2018;69:266-76
4. Esnault P, Cardinale M, Boret H, D'Aranda E, Montcriol A, Bordes J. et al. Blunt cerebrovascular injuries in severe traumatic brain injury: incidence, risk factors, and evolution. J Neurosurg. 2017;127:16-22
5. Anto VP, Brown JB, Peitzman AB, Zuckerbraun BS, Neal MD, Watson G. et al. Blunt cerebrovascular injury in elderly fall patients: are we screening enough? World J Emerg Surg. 2018;13:30
6. Harrigan MR. Ischemic Stroke due to Blunt Traumatic Cerebrovascular Injury. Stroke. 2020;51:353-60
7. Davis JW, Holbrook TL, Hoyt DB, Mackersie RC, Field TO Jr, Shackford SR. Blunt carotid artery dissection: incidence, associated injuries, screening, and treatment. J Trauma. 1990;30:1514-7
8. Abu Mughli R, Wu T, Li J, Moghimi S, Alem Z, Nasir MU. et al. An Update in Imaging of Blunt Vascular Neck Injury. Can Assoc Radiol J. 2020: 846537120909468.
9. George E, Khandelwal A, Potter C, Sodickson A, Mukundan S, Nunez D. et al. Blunt traumatic vascular injuries of the head and neck in the ED. Emerg Radiol. 2019;26:75-85
10. Hundersmarck D, Slooff WM, Homans JF, van der Vliet QMJ, Moayeri N, Hietbrink F. et al. Blunt cerebrovascular injury: incidence and long-term follow-up. Eur J Trauma Emerg Surg. 2019
11. DuBose J, Recinos G, Teixeira PG, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: expanding experience. J Trauma. 2008;65:1561-6
12. Alimi YS, Di Mauro P, Fiacre E, Magnan J, Juhan C. Blunt injury to the internal carotid artery at the base of the skull: six cases of venous graft restoration. J Vasc Surg. 1996;24:249-57
13. Garg K, Rockman CB, Lee V, Maldonado TS, Jacobowitz GR, Adelman MA. et al. Presentation and management of carotid artery aneurysms and pseudoaneurysms. J Vasc Surg. 2012;55:1618-22
14. Rattan A, Kataria R, Kumar A, Azam Q. Blunt carotid injury with thrombotic occlusion: Is an intervention always required for best outcome? Trauma Case Rep. 2019;24:100263
15. Morton RP, Hanak BW, Levitt MR, Fink KR, Peterson EC, Vilela MD. et al. Blunt traumatic occlusion of the internal carotid and vertebral arteries. J Neurosurg. 2014;120:1446-50
16. Foreman PM, Griessenauer CJ, Falola M, Harrigan MR. Extracranial traumatic aneurysms due to blunt cerebrovascular injury. J Neurosurg. 2014;120:1437-45
17. Biasi L, Azzarone M, De Troia A, Salcuni P, Tecchio T. Extracranial Internal Carotid Artery Aneurysms: case report of a saccular wide-necked aneurysm and review of the literature. Acta Biomed. 2008;79:217-22
18. Ariyada K, Shibahashi K, Hoda H, Watanabe S, Nishida M, Hanakawa K. et al. Bilateral Internal Carotid and Left Vertebral Artery Dissection after Blunt Trauma: A Case Report and Literature Review. Neurol Med Chir (Tokyo). 2019;59:154-61
19. Jenkins JM, Norton J, Hampton T, Weeks R. Rare case of bilateral traumatic internal carotid artery dissection. BMJ Case Rep. 2016. 2016
20. Chomel A, Vernet M, Lile A, Messant I, Combes JC, Freysz M. Traumatic bilateral dissections of the internal carotid artery: an infrequent diagnosis not to be missed. J Neurosurg Anesthesiol. 2002;14:309-12
21. Bajko Z, Maier S, Motataianu A, Balasa R, Vasiu S, Stoian A. et al. Stroke Secondary to Traumatic Carotid Artery Injury - A Case Report. J Crit Care Med (Targu Mures). 2018;4:23-8
22. Wang GM, Xue H, Guo ZJ, Yu JL. Cerebral infarct secondary to traumatic internal carotid artery dissection. World J Clin Cases. 2020;8:4773-84
23. Burlew CC, Sumislawski JJ, Behnfield CD, McNutt MK, McCarthy J, Sharpe JP. et al. Time to stroke: A Western Trauma Association multicenter study of blunt cerebrovascular injuries. J Trauma Acute Care Surg. 2018;85:858-66
24. Perry BC, Al-Ali F. Spontaneous cervical artery dissection: the borgess classification. Front Neurol. 2013;4:133
25. Nunnink L. Blunt carotid artery injury. Emerg Med (Fremantle). 2002;14:412-21
26. Biffl WL, Moore EE, Offner PJ, Brega KE, Franciose RJ, Burch JM. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845-53
27. Seth R, Obuchowski AM, Zoarski GH. Endovascular repair of traumatic cervical internal carotid artery injuries: a safe and effective treatment option. AJNR Am J Neuroradiol. 2013;34:1219-26
28. Wagenaar AE, Burlew CC, Biffl WL, Beauchamp KM, Pieracci FM, Stovall RT. et al. Early repeat imaging is not warranted for high-grade blunt cerebrovascular injuries. J Trauma Acute Care Surg. 2014;77:540-5 quiz 650
29. Grandhi R, Weiner GM, Agarwal N, Panczykowski DM, Ares WJ, Rodriguez JS. et al. Limitations of multidetector computed tomography angiography for the diagnosis of blunt cerebrovascular injury. J Neurosurg. 2018;128:1642-7
30. Lumsden S, Rosta G, Bismuth J, Lumsden AB, Garami Z. Spontaneous Recanalization After Carotid Artery Dissection: The Case for an Ultrasound-Only Monitoring Strategy. Methodist Debakey Cardiovasc J. 2017;13:243-7
31. Bonow RH, Witt CE, Mosher BP, Mossa-Basha M, Vavilala MS, Rivara FP. et al. Transcranial Doppler Microemboli Monitoring for Stroke Risk Stratification in Blunt Cerebrovascular Injury. Crit Care Med. 2017;45:e1011-e7
32. Rutman AM, Vranic JE, Mossa-Basha M. Imaging and Management of Blunt Cerebrovascular Injury. Radiographics. 2018;38:542-63
33. Spanos K, Karathanos C, Stamoulis K, Giannoukas AD. Endovascular treatment of traumatic internal carotid artery pseudoaneurysm. Injury. 2016;47:307-12
34. Sporns PB, Niederstadt T, Heindel W, Raschke MJ, Hartensuer R, Dittrich R. et al. Imaging of Spontaneous and Traumatic Cervical Artery Dissection: Comparison of Typical CT Angiographic Features. Clin Neuroradiol. 2019;29:269-75
35. Bromberg WJ, Collier BC, Diebel LN, Dwyer KM, Holevar MR, Jacobs DG. et al. Blunt cerebrovascular injury practice management guidelines: the Eastern Association for the Surgery of Trauma. J Trauma. 2010;68:471-7
36. Vranic JE, Huynh TJ, Fata P, Barber J, Bonow RH, Levitt MR. et al. The ability of magnetic resonance black blood vessel wall imaging to evaluate blunt cerebrovascular injury following acute trauma. J Neuroradiol. 2020;47:210-5
37. Young CC, Bonow RH, Barros G, Mossa-Basha M, Kim LJ, Levitt MR. Magnetic resonance vessel wall imaging in cerebrovascular diseases. Neurosurg Focus. 2019;47:E4
38. Harrigan MR, Griffin RL, Deveikis JP, Prattipati V, Chimowitz MI, Jansen JO. New ischemic lesions on brain magnetic resonance imaging in patients with blunt traumatic cerebrovascular injury. J Trauma Acute Care Surg. 2020;88:796-802
39. Griessenauer CJ, Foreman PM, Deveikis JP, Harrigan MR. Optical coherence tomography of traumatic aneurysms of the internal carotid artery: report of 2 cases. J Neurosurg. 2016;124:305-9
40. Shakir HJ, Davies JM, Shallwani H, Siddiqui AH, Levy EI. Carotid and Vertebral Dissection Imaging. Curr Pain Headache Rep. 2016;20:68
41. Gesheva S, Bayona MT, Taussky P, Grandhi R. Unusual case of traumatic cervical internal carotid artery dissection presenting as pseudofenestration. BMJ Case Rep. 2019 12
42. Spanos K, Giannoukas AD. Endovascular treatment of a traumatic distal internal carotid pseudoaneurysm. Eur J Vasc Endovasc Surg. 2015;50:174
43. Zaghlool D, Franz R. Treatment of a high large extracranial carotid artery pseudoaneurysm from trauma using a Viabahn graft. Ann Vasc Surg. 2015;29:837 e1-7
44. Griessenauer CJ, Foreman P, Shoja MM, Kicielinski KP, Deveikis JP, Walters BC. et al. Carotid and vertebral injury study (CAVIS) technique for characterization of blunt traumatic aneurysms with reliability assessment. Interv Neuroradiol. 2015;21:255-62
45. Scavee V, De Wispelaere JF, Mormont E, Coulier B, Trigaux JP, Schoevaerdts JC. Pseudoaneurysm of the internal carotid artery: treatment with a covered stent. Cardiovasc Intervent Radiol. 2001;24:283-5
46. Shahan CP, Gray RI, Croce MA, Fabian TC. Impact of circle of Willis anatomy in traumatic blunt cerebrovascular injury-related stroke. Trauma Surg Acute Care Open. 2017;2:e000086
47. Watanabe Y, Mezaki T, Yamamoto Y, Kuzuhara S, Ueyama M. [Internal carotid artery occlusion related to seat belt shoulder strap: report of two cases]. Rinsho Shinkeigaku. 1996;36:670-4
48. Yaghi S, Maalouf N, Keyrouz SG. Cervical artery dissection: risk factors, treatment, and outcome; a 5-year experience from a tertiary care center. Int J Neurosci. 2012;122:40-4
49. Barrera D, Sercy E, Orlando A, Mains CW, Madayag R, Carrick MM. et al. Associations of Antithrombotic Timing and Regimen with Ischemic Stroke and Bleeding Complications in Blunt Cerebrovascular Injury. J Stroke Cerebrovasc Dis. 2020;29:104804
50. Hanna K, Douglas M, Asmar S, Khurrum M, Bible L, Castanon L. et al. Treatment of Blunt Cerebrovascular Injuries: Anticoagulants or Antiplatelet Agents? J Trauma Acute Care Surg. 2020
51. Burlew CC, Biffl WL, Moore EE, Pieracci FM, Beauchamp KM, Stovall R. et al. Endovascular stenting is rarely necessary for the management of blunt cerebrovascular injuries. J Am Coll Surg. 2014;218:1012-7
52. Daou B, Hammer C, Mouchtouris N, Starke RM, Koduri S, Yang S. et al. Anticoagulation vs Antiplatelet Treatment in Patients with Carotid and Vertebral Artery Dissection: A Study of 370 Patients and Literature Review. Neurosurgery. 2017;80:368-79
53. Kim DY, Biffl W, Bokhari F, Brakenridge S, Chao E, Claridge JA. et al. Evaluation and management of blunt cerebrovascular injury: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2020;88:875-87
54. Shahan CP, Sharpe JP, Stickley SM, Manley NR, Filiberto DM, Fabian TC. et al. The changing role of endovascular stenting for blunt cerebrovascular injuries. J Trauma Acute Care Surg. 2018;84:308-11
55. Amuluru K, Al-Mufti F, Roth W, Prestigiacomo CJ, Gandhi CD. Anchoring Pipeline Flow Diverter Construct in the Treatment of Traumatic Distal Cervical Carotid Artery Injury. Interv Neurol. 2017;6:153-62
56. Cohen JE, Ben-Hur T, Rajz G, Umansky F, Gomori JM. Endovascular stent-assisted angioplasty in the management of traumatic internal carotid artery dissections. Stroke. 2005;36:e45-7
57. Muller H, Bradac GB. Bilateral occlusion of the extracranial internal carotid artery secondary to closed neck injury. Case report and review of the literature. Neurochirurgia (Stuttg). 1984;27:53-5
58. Lo YL, Yang TC, Liao CC, Yang ST. Diagnosis of traumatic internal carotid artery injury: the role of craniofacial fracture. J Craniofac Surg. 2007;18:361-8
59. Maras D, Lioupis C, Magoufis G, Tsamopoulos N, Moulakakis K, Andrikopoulos V. Covered stent-graft treatment of traumatic internal carotid artery pseudoaneurysms: a review. Cardiovasc Intervent Radiol. 2006;29:958-68
60. Patsalides A, Fraser JF, Smith MJ, Kraus D, Gobin YP, Riina HA. Endovascular treatment of carotid blowout syndrome: who and how to treat. J Neurointerv Surg. 2010;2:87-93
61. Wan WS, Lai V, Lau HY, Wong YC, Poon WL, Tan CB. Endovascular treatment paradigm of carotid blowout syndrome: review of 8-years experience. Eur J Radiol. 2013;82:95-9
62. Lee SJ, Hwang SC, Park JM, Kim BT. Perfusion Study for Internal Carotid Artery Trapping of a Traumatic Pseudoaneurysm in an Unconscious Patient. Korean J Neurotrauma. 2015;11:170-4
63. Fujimoto K, Hashimoto H, Yonezawa T, Masui K, Nishi N, Ikeuchi H. et al. [Massive epistaxis due to traumatic internal carotid artery pseudoaneurysm: a case report]. No Shinkei Geka. 2008;36:339-43
64. Wang AY, Chen CC, Lai HY, Lee ST. Balloon test occlusion of the internal carotid artery with stump pressure ratio and venous phase delay technique. J Stroke Cerebrovasc Dis. 2013;22:e533-40
65. Abud DG, Spelle L, Piotin M, Mounayer C, Vanzin JR, Moret J. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol. 2005;26:2602-9
66. Singh AK, Okudera H, Kobayashi S. Traumatic carotid artery occlusion following blunt cervical injury. J Clin Neurosci. 1999;6:265-8
67. Assadian A, Senekowitsch C, Rotter R, Zolss C, Strassegger J, Hagmuller GW. Long-term results of covered stent repair of internal carotid artery dissections. J Vasc Surg. 2004;40:484-7
68. Fanelli F, Salvatori FM, Ferrari R, Pacella S, Rossi P, Passariello R. Stent repair of bilateral post-traumatic dissections of the internal carotid artery. J Endovasc Ther. 2004;11:517-21
69. Hoving JW, Marquering HA, Majoie C. Endovascular treatment in patients with carotid artery dissection and intracranial occlusion: a systematic review. Neuroradiology. 2017;59:641-7
70. Fields JD, Lutsep HL, Rymer MR, Budzik RF, Devlin TG, Baxter BW. et al. Endovascular mechanical thrombectomy for the treatment of acute ischemic stroke due to arterial dissection. Interv Neuroradiol. 2012;18:74-9
71. Kadkhodayan Y, Jeck DT, Moran CJ, Derdeyn CP, Cross DT 3rd. Angioplasty and stenting in carotid dissection with or without associated pseudoaneurysm. AJNR Am J Neuroradiol. 2005;26:2328-35
72. Malek AM, Higashida RT, Phatouros CC, Lempert TE, Meyers PM, Smith WS. et al. Endovascular management of extracranial carotid artery dissection achieved using stent angioplasty. AJNR Am J Neuroradiol. 2000;21:1280-92
73. Assali AR, Sdringola S, Moustapha A, Rihner M, Denktas AE, Lefkowitz MA. et al. Endovascular repair of traumatic pseudoaneurysm by uncovered self-expandable stenting with or without transstent coiling of the aneurysm cavity. Catheter Cardiovasc Interv. 2001;53:253-8
74. Kasprzak D, Bojakowski K, Nowicki M, Zawadzki M, Andziak P. False Aneurysms Complicating Internal Carotid Artery Dissection. Vasc Endovascular Surg. 2019;53:259-63
75. Chaer RA, Derubertis B, Kent KC, McKinsey JF. Endovascular treatment of traumatic carotid pseudoaneurysm with stenting and coil embolization. Ann Vasc Surg. 2008;22:564-7
76. Coldwell DM, Novak Z, Ryu RK, Brega KE, Biffl WL, Offner PJ. et al. Treatment of posttraumatic internal carotid arterial pseudoaneurysms with endovascular stents. J Trauma. 2000;48:470-2
77. Choudhri O, Heit J, Do HM. Endovascular reconstruction of enlarging traumatic internal carotid artery pseudoaneurysm. Neurosurg Focus. 2014;37:1
78. Wang K, Peng XX, Liu AF, Zhang YY, Lv J, Xiang L. et al. Covered Stenting Is an Effective Option for Traumatic Carotid Pseudoaneurysm with Promising Long-Term Outcome. J Korean Neurosurg Soc. 2020
79. Fusonie GE, Edwards JD, Reed AB. Covered stent exclusion of blunt traumatic carotid artery pseudoaneurysm: case report and review of the literature. Ann Vasc Surg. 2004;18:376-9
80. Ghorbani M, Shojaei H, Bavand K, Azar M. Surpass Streamline Flow-Diverter Embolization Device for Treatment of Iatrogenic and Traumatic Internal Carotid Artery Injuries. AJNR Am J Neuroradiol. 2018;39:1107-11
81. Shields LBE, Shields CB, Ghiassi M, Dashti SR, Yao TL, Zhang YP. et al. Pipeline Embolization Device for Treatment of Craniocervical Internal Carotid Artery Dissections: Report of 3 Cases. World Neurosurg. 2019;132:106-12
82. Sami MT, Gattozzi DA, Soliman HM, Reeves AR, Moran CJ, Camarata PJ. et al. Use of Pipeline embolization device for the treatment of traumatic intracranial pseudoaneurysms: Case series and review of cases from literature. Clin Neurol Neurosurg. 2018;169:154-60
83. Nariai Y, Kawamura Y, Takigawa T, Hyodo A, Suzuki K. Pipeline embolization for an iatrogenic intracranial internal carotid artery pseudoaneurysm after transsphenoidal pituitary tumor surgery: Case report and review of the literature. Interv Neuroradiol. 2020;26:74-82
84. Brzezicki G, Rivet DJ, Reavey-Cantwell J. Pipeline Embolization Device for treatment of high cervical and skull base carotid artery dissections: clinical case series. J Neurointerv Surg. 2016;8:722-8
85. Prasad V, Gandhi D, Jindal G. Pipeline endovascular reconstruction of traumatic dissecting aneurysms of the intracranial internal carotid artery. J Neurointerv Surg. 2014;6:e48
86. Pham MH, Rahme RJ, Arnaout O, Hurley MC, Bernstein RA, Batjer HH. et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68:856-66 discussion 66
87. Matsuura JH, Rosenthal D, Jerius H, Clark MD, Owens DS. Traumatic carotid artery dissection and pseudoaneurysm treated with endovascular coils and stent. J Endovasc Surg. 1997;4:339-43
88. Ong JL, Jalaludin S. Traumatic Pseudoaneurysm of Right Extracranial Internal Carotid Artery: A Rare Entity and Recent Advancement of Treatment with Minimally Invasive Technique. Malays J Med Sci. 2016;23:78-81
89. Wilseck Z, Savastano L, Chaudhary N, Pandey AS, Griauzde J, Sankaran S. et al. Delayed extrusion of embolic coils into the airway after embolization of an external carotid artery pseudoaneurysm. J Neurointerv Surg. 2018;10:e18
90. Gandhi CD, El-Gengahy A, Cornett-Thompson OE, Kraus J, Prestigiacomo CJ. The novel use of Onyx for the rapid treatment of a traumatic carotid injury. J Neurointerv Surg. 2012;4:e18
91. Zussman B, Gonzalez LF, Dumont A, Tjoumakaris S, Rosenwasser R, Hasan D. et al. Endovascular management of carotid blowout. World Neurosurg. 2012;78:109-14
92. Giannopoulos S, Trinidad E, Aronow H, Soukas P, Armstrong EJ. Epsilonndovascular Repair of Extracranial Carotid Artery Aneurysms: A Systematic Review. Vasc Endovascular Surg. 2020;54:254-63
93. Asif KS, Lazzaro MA, Teleb MS, Fitzsimmons BF, Lynch J, Zaidat O. Endovascular reconstruction for progressively worsening carotid artery dissection. J Neurointerv Surg. 2015;7:32-9
94. Texakalidis P, Karasavvidis T, Giannopoulos S, Tzoumas A, Charisis N, Jabbour P. et al. Endovascular reconstruction of extracranial traumatic internal carotid artery dissections: a systematic review. Neurosurg Rev. 2020;43:931-40
95. Cohen JE, Gomori JM, Itshayek E, Spektor S, Shoshan Y, Rosenthal G. et al. Single-center experience on endovascular reconstruction of traumatic internal carotid artery dissections. J Trauma Acute Care Surg. 2012;72:216-21
96. Choi HC, Park SE, Choi DS, Shin HS, Kim JE, Choi HY. et al. Ruptured extracranial carotid artery: Endovascular treatment with covered stent graft. J Neuroradiol. 2018;45:217-23
Author contact
![]() Corresponding author: Jinlu Yu, E-mail: jlyuedu.cn, jinluyucom. Department of Neurosurgery, The First Hospital of Jilin University, 1 Xinmin Avenue, Changchun 130021, China.
Corresponding author: Jinlu Yu, E-mail: jlyuedu.cn, jinluyucom. Department of Neurosurgery, The First Hospital of Jilin University, 1 Xinmin Avenue, Changchun 130021, China.

 Global reach, higher impact
Global reach, higher impact