Impact Factor
ISSN: 1449-1907
Int J Med Sci 2021; 18(3):778-784. doi:10.7150/ijms.55361 This issue Cite
Research Paper
Oral treatment for diabetes using α-glucosidase inhibitors was a risk factor for chronic obstructive pulmonary disease: a cohort study
1. Division of Nephrology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
2. The School of Medicine, Chung Shan Medical University, Taichung, Taiwan
3. School of Medical Applied Chemistry, Chung Shan Medical University, Taichung, Taiwan.
4. Department of Pharmacology, School of Medicine, Chung Shan Medical University, Taichung, Taiwan
5. Department of Pharmacy, Chung Shan Medical University Hospital, Taichung, Taiwan
6. School of Pharmacy, China Medical University, Taichung, Taiwan.
7. Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
8. Department of Internal Medicine, School of Medicine, Chung Shan Medical University, Taichung, Taiwan
9. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
# The authors contributed equally to this work
Received 2020-11-2; Accepted 2020-11-17; Published 2021-1-1
Abstract
Objectives: Currently, diabetes mellitus (DM) and chronic obstructive pulmonary disease (COPD) have proven to be risk factors for each other. This study aimed to determine the risk relationship between COPD and five common oral medications for DM among patients with DM.
Methods: This population-based cohort study was conducted from 2008 to 2013. Patient data were retrieved from the Longitudinal Health Insurance Database (LHID) of the National Health Insurance Research Database (NHIRD). After pairing by gender, age, and index date, time-to-event analysis and multiple regression analysis were performed to determine the factors associated with COPD in patients taking oral medication for DM, including age, gender, income, and comorbidities. We identified 1,028 patients who took oral medication for DM and 1,028 controls who did not take oral medication for DM.
Results: We observed that the use of α-glucosidase inhibitors was associated with a higher risk of COPD (hazard ratio [HR]: 1.964, 95% confidence interval [CI]: 1.207-2.380). Furthermore, compared with the control group, α-glucosidase inhibitor users had a higher risk of COPD (HR: 2.295, 95% CI: 1.304-4.038), and no significant difference was observed in other oral medications for DM.
Conclusions: Based on present results, we could suggest that patients with DM who used α-glucosidase inhibitors are probably a higher risk of COPD. We recommend that in the future, treatment with α-glucosidase inhibitors upregulate the occurrence of COPD might through gastrointestinal side effects and malnutrition.
Keywords: diabetes mellitus, chronic obstructive pulmonary disease, α-glucosidase inhibitor, cohort study, cox regression
Introduction
The relationship between diabetes mellitus (DM) and chronic obstructive pulmonary disease (COPD) has currently been confirmed.1,2 The literature has also reported that the risk of death in patients with COPD and DM is higher than that in patients with general COPD.3 Several medicines have yielded satisfactory results in the treatment of COPD. Potassium channel modulators can effectively dilate the bronchi, reduce cough and mucus production, and inhibit tracheal inflammation4. A corresponding study was also conducted on oral medicines for DM. The use of metformin was not related to the deterioration of COPD, but the concentration of plasma lactic acid slightly increased in patients in a statistically significant manner.5 Studies have also demonstrated that metformin can reduce mortality in patients with DM and in those with COPD and DM.3
Most reports investigated the effects of oral medicines for DM on patients with COPD. We studied the effect of oral medicines for DM on the subsequent development of COPD in patients. In the future, patients with DM should have more options and receive additional recommendations in the use of medicines. This study used the Taiwan National Health Insurance Research Database (NHIRD) to determine the relationship between oral medicines for DM and the development of COPD in patients after a series of adjustments.
Methods
Data source
The NHIRD is a claims database covering 98% of the population in Taiwan. The data are derived from the National Health Insurance programme implemented by the National Health Research Institutes. The Taiwan Longitudinal Health Insurance Database 2010 (LHID 2010) consists of a sample of 1 million claims randomly drawn from the NHIRD. No significant difference was observed in the statistical age, gender, annual births, and average insured amount. The strength of this database for research purposes hinges on its large sample size and vertical nature.6 It provides information on patient characteristics, medical service, hospital drug compensation, general practice, community pharmacy, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes. All NHIRD applicants must be researchers or clinicians in universities, research institutions, or hospitals. The data form NHIRD must be used for research purposes only. All applications should be reviewed by peer experts to ensure the rationality of the research. Ethical approval for this research was obtained from the institutional review board (IRB) of Chung Shan Medical University (CS2-15106).
Participants
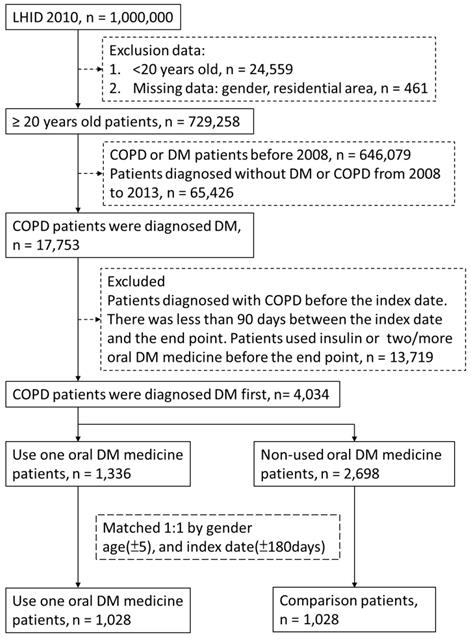
Patients with DM were identified according to the ICD-9-CM code 250, A181. The identification of patients with COPD was conducted using ICD-9-CM codes 490.xx-496.xx. A patient with DM or COPD with at least one hospital admission or three or more outpatient claims was considered to be newly diagnosed as having DM or COPD. The index date was the date of DM diagnosis between 2008 and 2013, and the end point was the date of COPD diagnosis. Exclusion criteria were as follows: (1) missing data including gender and residential area, (2) patients younger than 20, (3) end point before the index date, (4) patients diagnosed as having DM or COPD before 2008, (5) and less than 90 days between the index date and the end point. The patients who used oral medication were defined as cases. We randomly selected individuals not using oral medicine, and we gender-matched, age-matched, and index date-matched them with the patients from the case group to form the control group. There were 1,028 and 1,028 controls.
Oral medicines for DM
Based on the Anatomical Therapeutic Chemical classification system, we divided patients between biguanides, α-glucosidase inhibitors, sulphonylureas, meglitinides, thiazolidinediones (TZDs), and no oral medicine use for DM. We excluded patients who used more than one medication or were treated with insulin during the follow-up period. Patients having taken medicine for at least a month were included in the study.
Comorbidity
COPD-related comorbidity7 included hypertension (ICD-9-CM code: 401.xx, 402.xx, 403.xx, 404.xx, 405.xx), hyperlipidemia (ICD-9-CM code: 272.xx), cerebrovascular disease (CVD; ICD-9-CM code: 430.xx, 431.xx, 432.xx, 433.xx, 434.xx, 435.xx, 436.xx, 437.xx, 438.xx), anxiety (ICD-9-CM code: 300.0), substance abuse (ICD-9-CM code: 304.xx, 305.xx), congestive heart failure (ICD-9-CM code: 428.xx), peripheral vascular disease (ICD-9-CM code: 443.9), depression (ICD-9-CM code: 311.xx), gastro-esophageal refluxdisease (GERD; ICD-9-CM code: 530.81).
Statistical analysis
The chi-square test was used to analyse the category variables between the case and control groups. A two-tailed test was used to compare the continuous variables. Univariate and multivariate stratified Cox regression models were subsequently used to calculate the hazard ratio (HR) and 95% CI. Multivariable models were adjusted for COPD-related comorbidities, gender, age, low income, and urbanisation level.8 A further analysis of the risk relationship between users of oral medicine for DM and their controls was conducted. Statistical analyses were performed using the SAS 9.3 software package, and P < 0.05 was considered statistically significant.
Results
From 1 January 2008 to 31 December 2013, a total of 1,028 oral medicine users were compared with 1,028 controls. The descriptive demographic data namely age, gender, income, urbanization level, and comorbidities are presented in Table 1. Patients taking oral medicine were compared with their controls. No difference existed in age and gender. Patients were mostly male (55.88%) and 57 years old on average. There were more patients with a low income in the case group (50.58%) and more patients with a non-low income in the control group (50.88%). No difference was observed between the case and control groups. The patients generally lived in moderately urbanized areas (cases: 29.67%, controls: 30.64%).
Table 2 presents the Cox regression analysis of risk factors associated with COPD development. The HR of α-glucosidase inhibitor users was 1.697 (95% CI: 1.208-2.383) and was statistically significant (P = 0.0023). Low income was also a risk factor for COPD (HR: 1.143, 95% CI: 1.044-1.25, P = 0.0037). Compared with patients living in moderately urbanized areas, those living in agricultural areas had a higher risk of developing COPD (HR: 1.482, 95% CI: 1.197-1.835, P = 0.0003). Patients with hyperlipidaemia (HR: 0.808, 95% CI: 0.738-0.884, P < 0.0001) and cardiovascular disease (HR: 0.874, 95% CI: 0.771-0.991, P = 0.0352) had a lower risk of COPD development.
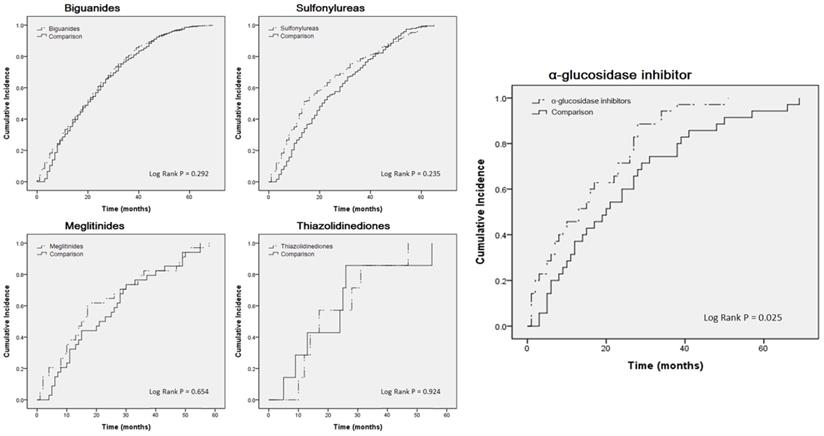
For further analysis, we compared the case group to the control group. Table 3 indicates that, apart from the α-glucosidase inhibitor users (crude HR: 1.718, 95% CI: 1.052-2.807; adjusted HR: 2.295, 95% CI: 1.304-4.038), no statistically significant differences were observed among the users of other medicines. A Kaplan-Meier curve was also used for analysis (Fig. 2). Only α-glucosidase inhibitor users had a significantly higher incidence of COPD than did the controls (log-rank test, P = 0.025).
Flow-chart of participant selection.
Basic characteristics of the study participants from 2008 to 2013.
| Oral medicine | Comparison | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 1,028) | (n = 1,028) | P | ||||||
| Gender | ||||||||
| female | 505 | (49.12%) | 505 | (49.12%) | 1.0000 | |||
| male | 523 | (50.88%) | 523 | (50.88%) | ||||
| Age in 2008 (Mean ± SD) | 57.14±13.64 | 57.23±13.79 | 0.8773 | |||||
| Low-income | ||||||||
| Yes | 505 | (49.12%) | 520 | (50.58%) | 0.5082 | |||
| No | 523 | (50.88%) | 508 | (49.42%) | ||||
| Urbanization level | ||||||||
| Highly urbanization | 303 | (29.47%) | 252 | (24.51%) | 0.0741 | |||
| Moderate urbanization | 315 | (30.64%) | 305 | (29.67%) | ||||
| Emerging town | 156 | (15.18%) | 167 | (16.25%) | ||||
| General town | 146 | (14.2%) | 159 | (15.47%) | ||||
| Aged Township | 20 | (1.95%) | 27 | (2.63%) | ||||
| Agricultural town | 42 | (4.09%) | 61 | (5.93%) | ||||
| Remote township | 46 | (4.47%) | 57 | (5.54%) | ||||
| Comorbidity | ||||||||
| Hypertension | 569 | (55.35%) | 693 | (67.41%) | <.0001 | |||
| Hyperlipidemia | 514 | (50%) | 594 | (57.78%) | 0.0004 | |||
| Osteoarthritis | 410 | (39.88%) | 351 | (34.14%) | 0.0070 | |||
| Cardiovascular disease | 160 | (15.56%) | 195 | (18.97%) | 0.0411 | |||
| Anxiety | 340 | (33.07%) | 294 | (28.6%) | 0.0280 | |||
| Substance abuse | 32 | (3.11%) | 30 | (2.92%) | 0.7965 | |||
| Congestive heart failure | 54 | (5.25%) | 64 | (6.23%) | 0.3430 | |||
| Peripheral vascular disease | 30 | (2.92%) | 40 | (3.89%) | 0.2239 | |||
| Depression | 31 | (3.02%) | 27 | (2.63%) | 0.5942 | |||
| GERD | 28 | (2.72%) | 17 | (1.65%) | 0.0973 | |||
Abbreviation: SD, standard deviation.
Hazards ratios of COPD with diabetes and compare with oral treatment medicine.
| Adjusted | ||||
|---|---|---|---|---|
| HR 95%CI | P-value | |||
| Diabetes medicine (reference: no used) | ||||
| Biguanides (n = 761) | 1.089 (0.990-1.198) | 0.0812 | ||
| α-glucosidase inhibitor (n = 35) | 1.694 (1.207-2.380) | 0.0023 | ||
| Sulfonylureas (n = 191) | 1.080 (0.922-1.264) | 0.3409 | ||
| Meglitinides (n = 34) | 1.054 (0.746-1.488) | 0.7656 | ||
| Thiazolidinediones (n = 7) | 1.233 (0.584-2.604) | 0.5828 | ||
| Gender (reference: female) | ||||
| Male | 1.015 (0.926-1.113) | 0.7444 | ||
| Age (reference: general population) | ||||
| Age | 0.999 (0.995-1.003) | 0.6085 | ||
| Low income (reference: no) | ||||
| Yes | 1.143 (1.044-1.250) | 0.0036 | ||
| Urbanization level (reference: moderate urbanization) | ||||
| Highly urbanization | 1.085 (0.967-1.218) | 0.1650 | ||
| Emerging town | 1.078 (0.941-1.236) | 0.2764 | ||
| General town | 1.080 (0.940-1.241) | 0.2755 | ||
| Aged Township | 0.972 (0.719-1.314) | 0.8529 | ||
| Agricultural town | 1.482 (1.197-1.835) | 0.0003 | ||
| Remote township | 1.041 (0.841-1.288) | 0.7138 | ||
| Comorbidity (reference: without) | ||||
| Hypertension | 1.027 (0.929-1.135) | 0.6052 | ||
| Hyperlipidemia | 0.808 (0.738-0.884) | <.0001 | ||
| Osteoarthritis | 0.926 (0.839-1.023) | 0.1309 | ||
| Cardiovascular disease | 0.874 (0.771-0.991) | 0.0352 | ||
| Anxiety | 0.954 (0.864-1.053) | 0.3501 | ||
| Substance abuse | 0.930 (0.716-1.209) | 0.5881 | ||
| Congestive heart failure | 0.977 (0.806-1.184) | 0.8122 | ||
| Peripheral vascular disease | 0.900 (0.706-1.146) | 0.3910 | ||
| Depression | 0.964 (0.737-1.261) | 0.7916 | ||
| GERD | 1.097 (0.812-1.482) | 0.5465 | ||
Abbreviation: CI, confidence interval.
Adjusted with gender, age, low income, urbanization level, comorbidity.
Kaplan-Meier curves estimating cumulative incidence of COPD patients between oral treatment medicine users and control cohorts.
Oral treatment medicine subgroups of hazards ratios of COPD with diabetes.
| Event | Observed | Incidence Density | Crude | Adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| Person-months | (Per 1000 person-month) | HR 95%CI | p-value | HR 95%CI | p-value | |||
| Biguanides medicine | ||||||||
| Biguanides | 761 | 16589 | 45.87 | 1.054 (0.953-1.166) | 0.3056 | 1.063 (0.960-1.719) | 0.2411 | |
| Comparison | 761 | 17512 | 43.46 | Reference | Reference | |||
| α-glucosidase inhibitor medicine | ||||||||
| α-glucosidase inhibitors | 35 | 546 | 64.10 | 1.718 (1.052-2.807) | 0.0307 | 2.295 (1.304-4.038) | 0.0040 | |
| Comparison | 35 | 837 | 41.82 | Reference | Reference | |||
| Sulfonylureas medicine | ||||||||
| Sulfonylureas | 191 | 4796 | 39.82 | 1.127 (0.921-1.379) | 0.2471 | 1.172 (0.946-1.451) | 0.1466 | |
| Comparison | 191 | 4074 | 46.88 | Reference | Reference | |||
| Meglitinides medicine | ||||||||
| Meglitinides | 34 | 710 | 47.89 | 1.112 (0.688-1.798) | 0.6641 | 0.959 (0.538-1.711) | 0.9590 | |
| Comparison | 34 | 808 | 42.08 | Reference | Reference | |||
| Thiazolidinediones medicine | ||||||||
| Thiazolidinediones | 7 | 159 | 44.03 | 0.948 (0.314-2.862) | 0.9242 | - | 0.9993 | |
| Comparison | 7 | 157 | 44.59 | Reference | Reference | |||
Abbreviation: CI, confidence interval.
Adjusted with gender, age, low income, urbanization level, comorbidity.
Discussion
In this study, patients with DM using α-glucosidase inhibitors had a higher risk of developing COPD than those using other oral medicines. Sulphonylureas bind to and shut down the ATP-sensitive potassium channel on the cell membrane of the pancreatic beta cell, and they prevent the potassium from depolarising by blocking it.9 In turn, the fusion of insulin particles with the cell membrane increases, and so does the secretion of mature insulin. The potassium channel has been proven to effectively alleviate the symptoms of COPD, such as decreased airway hyper responsiveness, bronchiectasis, decreased cough, and decreased mucus production as well as inhibition of airway inflammation and remodeling.10 Therefore, closing the potassium channel may cause COPD to worsen. One of the sulphonylureas, glyburide, which binds to (+)-[3H] isradipine, causes pathological changes in the cardiopulmonary structure and function of rats with monocrotaline-induced pulmonary hypertension. This evidence suggests that sulphonylureas had a tendency to aggravate lung injury and related diseases such as COPD. Our results reveal that sulphonylureas were trending towards the development of COPD, but the statistics were nonsignificant. The mechanism of action of meglitinide was the same as that of sulphonylureas, that is, shutting down the ATP-dependent potassium channel.11 In contrast to sulphonylureas, meglitinide has a fast onset and a short duration of action. Compared with those caused by sulphonylureas, the side effects of hypoglycaemia and weight gain caused by meglitinide are relatively mild.12 This may also affect patients with COPD because of the inhibition of the potassium channels. Our results indicated that patients who used meglitinide did not face a risk of COPD; instead they exhibited a decreasing trend compared with their matched group. Studies have demonstrated that for patients with DM, repaglinide can replace meglitinide and treat early cystic fibrosis-related diseases.13 Therefore, it may also be useful in the treatment of COPD. However, the detailed pathological relationship requires clarification.
One of the biguanides, metformin appears to be irrelevant for the treatment of COPD, regardless of whether the patient has diabetes.14,15 However, studies have demonstrated that metformin can effectively inhibit the mortality of patients with COPD and the development of COPD.16, 17 Therefore, whether biguanides inhibit the development of COPD remains to be discussed. In our results, there was no significant difference in the risk of COPD between biguanides cases and controls. Patients with COPD and DM who were exposed to TZDs had a small but significant risk of acute exacerbations of COPD.18 TZDs exert antidiabetic effects by activating the mechanism of the γ isoform of the peroxisome proliferator-activated receptor (PPARγ) (nuclear receptor) and expression of PPARγ in alveolar macrophages, an in vitro alveolar macrophage model and in vivo associated with COPD Animal model studies have displayed the potential to fight inflammation.19 However, studies have also indicated that the long-term use of TZDs in patients with type 2 diabetes causes pneumonia or lower respiratory tract infections as well as severe pneumonia or lower respiratory tract infections. The risk is increased.20 TZDs also increases the risk of heart failure (HF)21 and HF is often highly correlated with COPD.22 In our results, TZDs were not associated with COPD in patients with DM.
The results indicated that patients with DM who used α-glucosidase inhibitors are probably a higher risk of COPD. α-Glucosidase inhibitors, the pseudo-carbohydrates, competitively inhibit activity of α-glucosidases which hydrolyze non-absorbable oligosaccharides and polysaccharides into absorbable monosaccharides in the brush border of enterocytes.23 α-Glucosidase inhibitors delay carbohydrate digestion and prolong the carbohydrate digestion duration, thus reducing monosaccharides absorption rates.24,25 Therefore, patients taken with α-glucosidase inhibitors may have the potential to develop into malnutrition. Addition, α-glucosidase inhibitors cause the gastrointestinal side effects, such as bloating, nausea, diarrhea, and flatulence.24 Malnutrition in COPD is described by variable prevalence rates ranging between 30-60%.26 Malnutrition and poor nutrition play as the risk factor for patients with COPD. Compared with healthy individuals, patients with COPD had significantly higher rates of 0-3 hours of urinary lactulose to rhamnose and sucralose to erythritol and 5-24 hours of urinary galactooligosaccharide to erythritol.25 These findings indicated that intestinal permeability would be significant reduction in carbohydrate metabolism in the patients with COPD25 The patients with COPD also suffered by lower gastrointestinal symptoms, including constipation and bloating. The study suggests improving the management of gastrointestinal symptoms and maintaining a clear bowel to improve the condition of patients with COPD.24 Based on these findings, the gastrointestinal side effects and malnutrition caused by α-glucosidase inhibitors may be one of the main reasons for the development of COPD, and further experiments need to be clarified. We recommend that patients with DM use α-glucosidase inhibitors in combination with other medicines to alleviate the gastrointestinal side effects and malnutrition.
In the analysis of multiple population studies, poor education systems, low-income families, and low composite socioeconomic status (SES) indices were associated with individuals with COPD whose annual income is below the minimum wage in the United States.27 It have a much greater impact on smoking-related diseases, which is the same as ours.28 In Poland, 8.5% of men and 4.9% of women have symptoms of chronic airflow obstruction. Livestock farmers have an increased risk of chronic bronchitis, COPD, and reduced forced expiratory volume in 1 second (FEV1).29 Exposure to mineral dust by working in the soil has also been suggested to result in COPD.30 This was also confirmed in our results. Interestingly, among the patients in the case group, those with DM with high blood lipids or cardiovascular disease had a lower risk of COPD. Studies have demonstrated that patients with hyperlipidaemia and COPD experience lung hyperinflation and airway obstruction less often than patients without hyperlipidaemia, but the effects of the drug need to be clarified.29 In fact, α-glucosidase inhibitors can effectively reduce triglycerides and increase HDL; they can also inhibit the risk of cardiovascular disease32 Moreover, biguanides and TZDs effectively reduce the risk of cardiovascular disease.33,34 But, the risk of heart failure is increased by TZDs.21 However, the relationship between the risk of cardiovascular disease and anti-diabetic agents including sulphonylureas and meglitinides are not clear.35 At present, we also found that there are more patients using biguanides due to the lower risk of cardiovascular disease. Therefore, we further compared various drug users with their controls, but the results were similar.
There are several limitations to this study. We did not have a clear understanding of the lives or exercise habits of patients, for example, their cigarette smoking habit. We also could not accurately obtain the actual values of blood sugar levels, FVC, and FEV1 in patients with diabetes. Although we did not directly track the severity of diabetes and COPD from NHIRD could not directly track patients, adjusted these comorbidities of COPD for indirectly explain the severity. These comorbidities present high-risk factors for COPD. Despite the large sample size, the number of participants for whom a comparison between several medicines could be made remained limited after strict screening and matching. Especially for patients with DM who used TZDs, the result was unexpected. For other users of oral medicine for DM, we believe that there is credible by adjustment and matching.
Conclusion
In summary, we suggest that patients with DM who administration of the α-glucosidase inhibitors are probably a higher risk of COPD. Although α-glucosidase inhibitors have a satisfactory inhibitory effect on blood lipids and cardiovascular diseases. However, the gastrointestinal side effects and malnutrition of the α-glucosidase inhibitors probably results in higher incidence of COPD occur in the patients with DM. In future medications, the side effects of α-glucosidase inhibitors should be alleviated and the occurrence of COPD reduced.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Rogliani P, Calzetta L, Segreti A, Barrile A, Cazzola M. Diabetes mellitus among outpatients with COPD attending a university hospital. Acta Diabetol. 2014;51:933-940
2. Ho TW, Huang CT, Ruan SY, Tsai YJ, Lai F, Yu CJ. Diabetes mellitus in patients with chronic obstructive pulmonary disease-The impact on mortality. PLoS One. 2017;12:e0175794
3. Yen FS, Chen W, Wei JC, Hsu CC, Hwu CM. Effects of metformin use on total mortality in patients with type 2 diabetes and chronic obstructive pulmonary disease: A matched-subject design. PLoS One. 2018;13:e0204859
4. Malerba M, Radaeli A, Mancuso S, Polosa R. The potential therapeutic role of potassium channel modulators in asthma and chronic obstructive pulmonary disease. J Biol Regul Homeost Agents. 2010;24:123-130
5. Hitchings AW, Archer JRH, Srivastava SA, Baker EH. Safety of Metformin in Patients with Chronic Obstructive Pulmonary Disease and Type 2 Diabetes Mellitus. COPD. 2015;12:126-131
6. Hsing AW, John P, loannidins A. National population science lessons from Taiwan national health insurance research database. JAMA Internal Med. 2015;175:1527-1529
7. Divo MJ, Casanova C, Marin JM, Pinto-Plata VM, de-Torres JP, Zulueta JJ. et al. COPD comorbidities network. Eur Respir J. 2015;46:640-50
8. Liu CY, Hung YT, Chuang YL, Chen YJ, Weng WS, Liu JS. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manage. 2006;4:1-22
9. Pelaia G, Gallelli L, Vatrella A, Grembiale RD, Maselli R, De Sarro GB. et al. Potential role of potassium channel openers in the treatment of asthma and chronic obstructive pulmonary disease. Life Sci. 2002;70:977-990
10. Malerba M, Radaeli A, Mancuso S, Polosa R. The potential therapeutic role of potassium channel modulators in Asthma and chronic obstructive pulmonary disease. J Biol Regul Homeost Agents. 2010;24:123-130
11. Blicklé JF. Meglitinide analogues: a review of clinical data focused on recent trials. Diabetes Metab. 2006;32:113-120
12. Guardado-Mendoza R, Prioletta A, Jiménez-Ceja LM, Sosale A, Folli F. The role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch Med Sci. 2013;9:936-943
13. Ballmann M, Hubert D, Assael BM, Staab D, Hebestreit A, Naehrlich L. et al. Repaglinide versus insulin for newly diagnosed diabetes in patients with cystic fibrosis: a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:114-121
14. Hitchings AW, Archer JR, Srivastava SA, Baker EH. Safety of Metformin in Patients with Chronic Obstructive Pulmonary Disease and Type 2 Diabetes Mellitus. COPD. 2015;12:126-131
15. Hitchings AW, Lai D, Jones PW, Baker EH, Metformin in COPD Trial Team. Metformin in COPD Trial Team. Metformin in severe exacerbations Of chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2016;71:587-593
16. Yen FS, Chen W, Wei JC, Hsu CC, Hwu CM. Effects of metformin use on total mortality in patients with type 2 diabetes and chronic obstructive pulmonary disease: A matched-subject design. PLoS One. 2018;13:e0204859
17. Tseng CH. Metformin and risk of chronic obstructive pulmonary disease in diabetes patients. Diabetes Metab. 2019;45:184-190
18. Rinne ST, Liu CF, Feemster LC, Collins BF, Bryson CL, O'Riordan TG. et al. Thiazolidinediones are associated with a reduced risk of COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2015;10:1591-1597
19. Lea S, Plumb J, Metcalfe H, Spicer D, Woodman P, Fox JC. et al. The effect of peroxisome proliferator-activated receptor-γ ligands on in vitro and in vivo models of COPD. Eur Respir J. 2014;43:409-420
20. Singh S, Loke YK, Furberg CD. Long-term use of thiazolidinediones and the associated risk of pneumonia or lower respiratory tract infection: systematic review and meta-analysis. Thorax. 2011;66:383-388
21. Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am J Cardiovasc Drugs. 2011;11:115-128
22. de Miguel Díez J, Chancafe Morgan J, Jiménez García R. The association between COPD and heart failure risk: a review. Int J Chron Obstruct Pulmon Dis. 2013;8:305-312
23. Derosacor G, Maffioli P. α-Glucosidase Inhibitors and Their Use in Clinical Practice. Arch Med Sci. 2012;8:899-906
24. Sun Y, Zheng F, Li Y, Wu R, Liu Y, Liu M. et al. Correlation between lower gastrointestinal tract symptoms and quality of life in patients with stable chronic obstructive pulmonary disease. J Tradit Chin Med. 2013;33:608-614
25. Rutten EPA, Lenaerts K, Buurman WA, Wouters EFM. Disturbed Intestinal Integrity in Patients With COPD: Effects of Activities of Daily Living. Chest. 2014;145:245-252
26. Hancu A. Nutritional Status as a Risk Factor in COPD. Maedica (Buchar). 2019Jun;14:140-143
27. Grigsby M, Siddharthan T, Chowdhury MA, Siddiquee A, Rubinstein A, Sobrino E. et al. Socioeconomic status and COPD among low- and middle-income countries. Int J Chron Obstruct Pulmon Dis. 2016;11:2497-2507
28. Lowe KE, Make BJ, Crapo JD, Kinney GL, Hokanson JE, Kim V. et al. Association of low income with pulmonary disease progression in smokers with and without chronic obstructive pulmonary disease. ERJ Open Res. 2018 4
29. Szczyrek M, Krawczyk P, Milanowski J, Jastrzębska I, Zwolak A, Daniluk J. et al. Chronic obstructive pulmonary disease in farmers and agricultural workers - an overview. Ann Agric Environ Med. 2011;18:310-313
30. Weinhold B. Occupational Health: Agriculture-COPD Link Bolstered. Environ Health Perspect. 2007;115:A444
31. Kahnert K, Lucke T, Huber RM, Behr J, Biertz F, Vogt A. et al. Relationship of hyperlipidemia to comorbidities and lung function in COPD: Results of the COSYCONET cohort. PLoS One. 2017;12:e0177501
32. Standl E, Theodorakis MJ, Erbach M, Schnell O, Tuomilehto J. On the potential of acarbose to reduce cardiovascular disease. Cardiovasc Diabetol. 2014;13:81
33. Lamanna C, Monami M, Marchionni N, Mannucci E. Effect of metformin on cardiovascular events and mortality: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2011;13:221-228
34. Wilcox R, Bousser MG, Betteridge DJ, Schernthaner G, Pirags V, Kupfer S. et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from proactive (prospective pioglitazone clinical trial In macrovascular events 04) Stroke. 2007; 38: 865-873.
35. James Xu, Rohan Rajaratnam. Cardiovascular safety of non-insulin pharmacotherapy for type 2 diabetes. Cardiovasc Diabetol. 2017;16:18
Author contact
![]() Corresponding author: Yu-Hsiang Kuan. Address: Department of Pharmacology, School of Medicine, Chung Shan Medical University and Chung Shan Medical University Hospital, No. 110, Sec. 1, Jianguo N. Rd., Taichung 402, Taiwan, ROC. Tel.: +886-4-24730022 Ext. 11662. Fax: +886-4-24739030. E-mail address: kuanyhedu.tw (Yu-Hsiang Kuan)
Corresponding author: Yu-Hsiang Kuan. Address: Department of Pharmacology, School of Medicine, Chung Shan Medical University and Chung Shan Medical University Hospital, No. 110, Sec. 1, Jianguo N. Rd., Taichung 402, Taiwan, ROC. Tel.: +886-4-24730022 Ext. 11662. Fax: +886-4-24739030. E-mail address: kuanyhedu.tw (Yu-Hsiang Kuan)

 Global reach, higher impact
Global reach, higher impact