3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2020; 17(14):2163-2170. doi:10.7150/ijms.47357 This issue Cite
Review
Therapeutic effects and mechanisms of actions of Descurainia sophia
1. Department of Chinese Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation.
2. School of Post-Baccalaureate Chinese Medicine, Tzu Chi University, Hualien, Taiwan.
3. Department of Research, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan.
4. Department of pulmonary medicine, Buddhist Taichung Tzu Chi General Hospital, Taichung, Taiwan.
5. Division of Pulmonary Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan.
6. School of Medicine, Tzu-Chi University, Hualien, Taiwan.
#These authors contributed equally to this work.
Received 2020-4-23; Accepted 2020-7-28; Published 2020-8-1
Abstract
Descurainia sophia Webb ex Prantl has been used in traditional medicine globally. It has been shown that Descurainia sophia, together with many other bioactive compounds, can modulate the biological functions of various genes. We have viewed the clinical benefits and mechanisms of action of Descurainia sophia associated with its current uses and outlined potential further applications. There are many studies documenting its numerous clinical effects in cancer, respiratory, gastrointestinal, and cardiac systems. Further, Descurainia sophia has been shown to exhibit anti-inflammatory, anti-oxidative, and anthelmintic activities. The clinical studies did not indicate any significant adverse effects of Descurainia sophia, demonstrating that it is a safe and effective herbal medicine. However, more clinical studies demonstrating the therapeutic effects of Descurainia sophia are still warranted.
Keywords: Descurainia sophia, phytochemical effects, traditional Chinese medicine
Introduction
Complementary and alternative medicine is popular around the world [1] and has been combined with conventional medicine to treat diverse diseases. About 38% of US and some 75% of the Republic of Korea population have been reported to use traditional medicine in their healthcare [1]. Further, 76% Japanese and 93% Chinese patients used traditional medicine as complementary treatment for their diseases [1]. Herbal medicine is thus an important part of complementary and alternative medicine.
Descurainia sophia (DS) L. Webb ex Prantl belongs to the Cruciferae (Brassiaceae) family [2]. The DS plant can be found growing in temperate climate including the United States, Southern Africa, East Asia, Australia and New Zealand [2]. DS has been used in herbal medicine in China, India and many other countries around the world. It has been demonstrated that DS can regulate a variety of genes closely related to numerous biological functions [3]. About sixty-seven compounds have been isolated from DS and identified by gas chromatography-mass spectrometry [4]. However, fifty-one compounds accounted for 98% of the total amount [4]. The aerial part and seeds of DS have also found to contain several such bioactive compounds [2]. Many phytochemicals have been identified from DS (aerial parts and seeds), including unique compounds of DS (descurainin, descurainin A, descurainoside, descurainoside A, and descurainoside B) that have biological activity [4-6] (Table 1).
Phytochemical constituents of Descurainia sophia
| Reference | Category | Phytochemical constituents |
|---|---|---|
| [4-6] | unique compounds | descurainin, descurainin A, descurainoside, descurainoside A, descurainoside B |
| [4-6] | cardiac glycosides | erysimoside, evobioside, helveticoside, strophanthidin |
| [4-6] | coumarins | bergapten, isoscopoletin, psoralene, scopoletin, xanthotoxin, xanthotoxol |
| [5, 6] | fatty acids | arachic acid, capric acid, eicosenoic acid, erucic acid, lauric acid, linoleic acid, linolenic acid, myristic acid, oleic acid, palmitic acid, stearic acid |
| [4-6] | flavonoids | drabanemoroside, isoquercitrin, isorhamnetin, isorhamnetin-3-O-β-D-glucopyranoside, kaempferol, quercetin, quercetin 3-O-α-L-rhamnopyranosyl-(1 → 2)-α-L-arabinopyranose, quercetin-3-O-β-D-glucopyranoside |
| [5] | flavonol glycoside | artabotryside A |
| [4, 5] | glucosinolates | gluconapin, sinigrin |
| [4-6] | lactones | descurainolide A and B |
| [5] | lignan | syringaresinol |
| [5] | lipids | epoxyacylglyceride, triacylglyceride |
| [5, 6] | nor-lignan | descuraic acid |
| [5] | phenolic compounds | 3,4,5-tritrimethoxy cinnamic acid, isovanillic acid, p-benzoic acid, p-hydroxybenzaldehyde, sinapic acid, syringic acid |
| [5, 6] | phytosterol | daucosterol |
| [5] | sinapoyl glycosides | 1,2-di-O-sinapoyl-β-D-glucopyranose, 1,2-disinapoylgentiobiose, 1,3-di-O-sinapoyl-β-D-glucopyranose |
The known therapeutic effects and possible active constituents of Descurainia sophia
| Reference | Therapeutic effects and possible active constituents of DS |
|---|---|
| [3, 10] | Cytotoxic effects; EEDS showed cytotoxic effects on cancer cell lines: A549 (Lung), HepG2 (Liver), PC-3 (Prostate), HCT116 (Colon), SNU-638 (Stomach), SK-OV-3 (Ovary), and SK-MEL-28 (Skin); Helveticoside is considered the primary active cytotoxic constituent. |
| [3, 10, 18] | Anti-inflammatory effects; Coumarins, flavonoids, lignan, quercetin, syringaresinol. |
| [6, 19] | Antioxidant effects; EEDS, cardiac glycoside |
| [18] | Antipyretic activity; EEDS, neoisomenthyl acetate, alloaromadendrene. |
| [18] | Analgesic activity; Benzyl, allyl, propenyl-isothiocyanate and allyl disulfide constituents. |
| [6, 9, 21] | Gastrointestinal tract regulation effects; Bowel smooth muscle relaxation; Enhance bowel movement; Stool softener (allyl disulfide); Treatment of constipation and hemorrhoids. |
| [9, 12, 25-27] | Cardioprotective effects; Inotropic effects (helveticoside as Na+/K+ ATPase inhibitor); Diuretic effect (inhibition of renal tubular to reabsorb water, sodium, and chloride); Improve cardiac remodeling and function (activation of PI3k/Akt/mTOR dependent signaling, to attenuate cardiomyocyte apoptosis). |
| [6, 28] | Anthelmintic activities; Therapeutic effects on mice infected with Himeonolepis nana. |
Many studies addressed the effect of DS in cancer, respiratory system, gastrointestinal system, and inflammatory diseases [4-6]. As evidence of pharmacological activity of DS increases, clinical uses for DS are likely to increase. Therefore, we review the use of DS herbal medicine and its mechanism of action. The known compounds of DS and their therapeutic effects are summarized in Table 2.
Descurainia sophia activity in cancer
Despite advances in clinical medicine, the prevalence of cancer continues to rise and the disease remains the leading cause of death globally [7]. Mutations in regulatory genes involved in maintaining the balance between cell proliferation and cell death lead to rapid cell growth, cell division and invasion [8]. Conventional treatments of cancer such as chemotherapies or radiotherapies lack specificity. These therapies cannot distinguish normal cells from cancer cells, thus causing a great damage and death to both normal and cancer cells, resulting in significant adverse effects such as bone-marrow suppression, fatigue, gastrointestinal complications, loss of appetite, body-weight loss, etc., and often in significant negative effects on the health-related quality of life. In addition, such side effects may limit treatment programs and hence may affect the prognosis for patients. Ideally, anticancer-treatment strategy should specifically target cancer cells without affecting normal cells.
Cytotoxic activity of Descurainia sophia
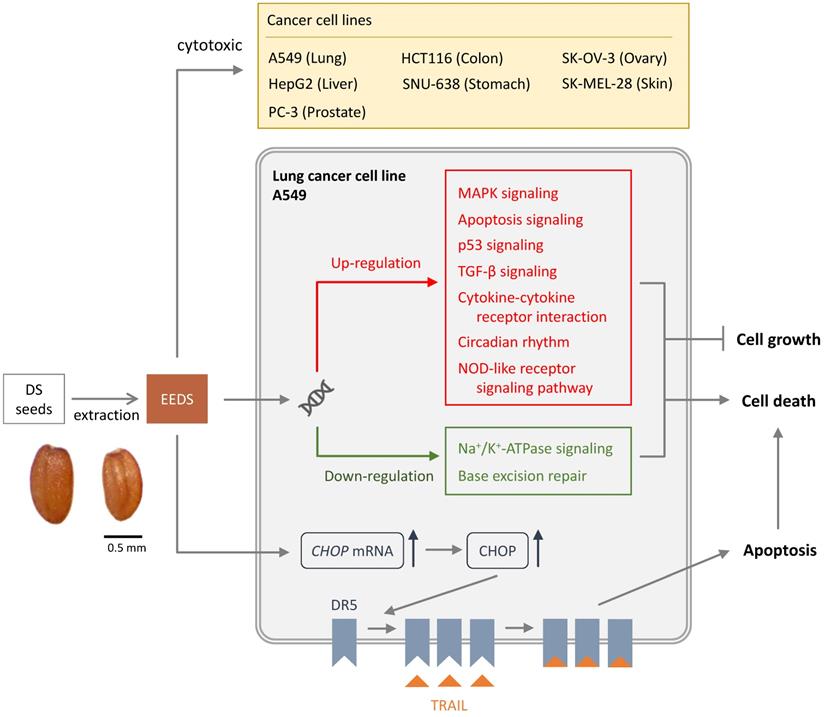
Many studies showed that DS possess prominent cytotoxic activity against a number of human cancer-cell lines such as lung, liver, colon, prostate, ovary, skin, and stomach [9]. DS is therefore offers an option for use in cancer therapy [9]. There are several constituents isolated from DS showed cytotoxic effects on cancer cell lines [3]. Helveticoside has been considered as the main active cytotoxic constituent of DS [10]. Quercetin also showed a potent cytotoxicity against cancer cell lines [10]. Therefore, DS herb appears to show potential for the development of an anticancer supplement.
Mechanisms of cytotoxic activity of Descurainia sophia
Studies are available on the DS anti-cancer mechanism of action. It has been shown that ethanolic extract of DS seeds (EEDS) has anti-cancer activity via both suppression in cancer-cell growth and cytotoxic effect [3]. It is suggested that EEDS significantly inhibited cell growth by regulation of the genes involved in cell growth signaling [3]. EEDS can also induce apoptotic cell death in lung cancer cells [3]. The pathway of mitogen-activated protein kinase (MAPK) has been suggested to be important in the cancer invasion and growth of lung cancer [11]. EEDS has been shown to up-regulate the MAPK pathway and therefore has anti-lung cancer effects [3]. The other possible mechanism of anti-cancer effect of DC is that helveticoside act as anti-cancer agent by inhibiting Na+/K+ ATPase activity [12]. The potential of Na+/K+ ATPase inhibitors to have anti-cancer effects has been demonstrated in many cancers such as prostate, breast, lung and leukemia [13].
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily, can selectively induce cancer-cell apoptosis through the death receptors [14]. As a cytotoxic cytokine, TRAIL selectively induces apoptosis in tumor cells through homotrimeric binding to the membrane bound death receptor 5 (DR5) [14]. This pathway recruits Fas-related death-domain proteins and activates caspase pathway. Some cancer cells are highly malignant due to their resistance to TRAIL-induced programmed cell death. The mechanisms of TRAIL resistance include reduced expression of death receptors, overexpression of inhibitor of apoptosis, and decreased release of caspases into the cytosol [14]. TRAIL resistance in cancer cells can be overcome by reversing the mechanisms of resistance, such as by upregulating death receptors [14]. TRAIL is an attractive anticancer agent because it has the ability to induce apoptosis in cancer cells while sparing most of normal cells. EEDS is an appropriate herbal addition to anticancer drugs for its beneficial effect elicited by acting on the TRAIL pathway [5]. It has been shown that in TRAIL-resistant cancer cells, DR5 are significantly up-regulated by EEDS [5]. The mechanism by which EEDS (Figure 1) up-regulates DR5 is mediated by endoplasmic reticulum stress-induced transcription factors CCAAT/enhancer-binding protein homologous protein (CHOP) [5]. By effectively upregulating death receptors, EEDS can sensitize TRAIL-refractory cancer cells [5].
Descurainia sophia activity in respiratory diseases
DS is commonly used in traditional medicine to treat asthma and cough [4]. Consequently, DS would be expected to have therapeutic effects on the respiratory system.
Anti-Asthmatic Effects
Asthma is a multi-factorial disease determined by multiple factors such as genetic, epigenetic, and environmental [15]. Although there are multiple causes of asthma, airway inflammation is an important trigger factor. In recent years, epigenetic processes regulating immune cells were recognized as playing a role in mechanisms of asthma. Allergic inflammation is the main pathogenesis of allergic asthma. The type-2 cytokines such as IL-4, IL-5, and IL-13 are important in the initiation and progression of asthma [16]. The regulation of the methylation of genes related to these cytokines plays an essential role in asthma [2].
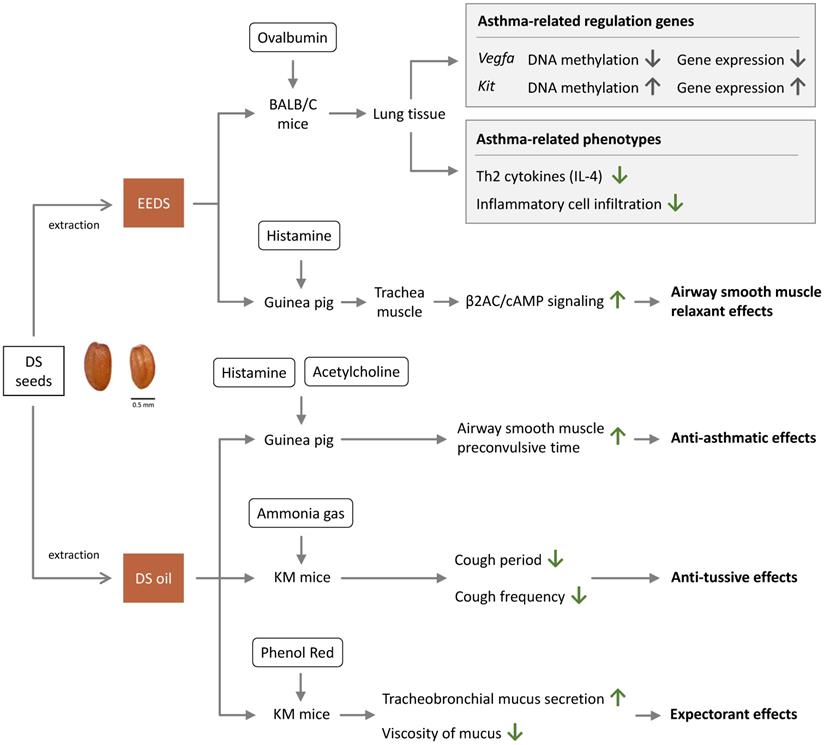
The mechanisms of therapeutic effects of DS are summarized in Figure 2. A study that examined the effects of EEDS in mouse model asthma showed that EEDS decreased infiltration of immune cells into the airways [2]. This study revealed that EEDS decrease infiltration of immune cells around the airway [2]. According to this study, EEDS decreased expression of Th2 cytokines and infiltration of inflammatory cell into the lungs [2]. The study provided a valuable insight into the anti-asthmatic effects of DS by explaining the mechanism of traditional use of DS in treating asthma [2]. The study also demonstrated that in ovalbumin (OVA)-induced asthmatic mouse model, EEDS could regulate DNA methylation and gene expression, including two functionally significant hub genes: down-regulated vascular endothelial growth factor A (Vegfa) and up-regulated proto-oncogene receptor tyrosine kinase (Kit) [2].
Another study reported that DS increased the pre-spasm time of smooth muscle induced by acetylcholine and histamine in guinea pigs [4]. Descurainoside A and descurainolide B contained in EEDS were shown to have smooth-muscle relaxation effects via the β2-adrenergic receptor/cyclic adenosine monophosphate (β2AR/cAMP) signal pathway [17]. The studies indicated that DS is beneficial in controlling asthma through regulating inflammatory processes and smooth-muscle relaxation.
Anti-tussive effects
A study demonstrated that DS could prolong the latent period of cough (increasing it by 23.1%) in ammonia-liquor spray induced Kunming mice (KM mice) [4]. DS could also decrease cough frequency (decreasing frequency by 15.7%) in phenol red intraperitoneal injected KM mice, thus proving the anti-tussive activities of DS in vivo [4].
Expectorant effects
Gong et al. suggested that DS is able to increase tracheobronchial mucus secretion and reduce mucus viscosity, showing that DS exhibited about 40% expectorant effect as compared with control [4].
Anti-inflammatory and anti-oxidative effects of Descurainia sophia
The anti-inflammatory, antipyretic, analgesic and antioxidant effects of DS were assessed in many experimental studies [6, 18, 19]. Mirzaei et al. showed EEDS antioxidant effects in vitro [19]. Nimrouzi et al. suggested that DS antioxidant effect is due to free radical scavenging [6]. The antipyretic activity of DS was almost the same as that of diclofenac sodium at a dose of 400 mg/kg body weight in hyperthermic rats [18]. Decoction of DS seeds was used as an antipyretic when treating smallpox and measles [20]. The DS extract was also reported to have analgesic activity compared with paracetamol [18].
Cytotoxic effects on cancer cells of Descurainia Sophia.
Anti-asthma effects of Descurainia sophia.
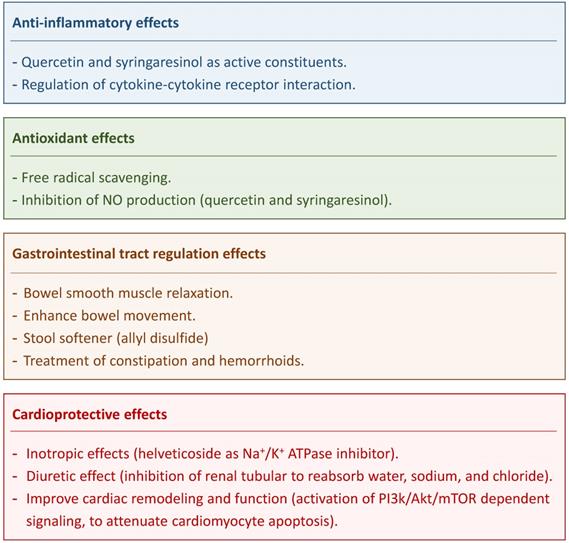
Quercetin and syringaresinol are active constituents of DS that impart it with anti-inflammatory effects [10]. The anti-inflammatory activity of DS that were prominent especially after 2 and 3 hours of intake was ascribed to coumarins [18]. Flavonoids and lignan are also DS components that inhibited nitric oxide production in lipopolysaccharides (LPS)-stimulated macrophages [10]; two compounds (quercetin and syringaresinol) were isolated from DS that exerted dose-dependent inhibitory effect on NO production in LPS-stimulated RAW264.7 cells. [10] Kim et al. identified the functional involvement of DS components with responsive genes involved in the regulation of cytokine-cytokine receptor interaction and thus displaying anti-inflammatory effects [3].
Descurainia sophia activity in gastrointestinal tract
Chronic constipation is a common symptom of gastrointestinal (GI) disorders. It is estimated that 12-30% of population suffers from constipation worldwide [21]. DS is used as the initial treatment for constipation in traditional medicine in Iran [21]. DS can act via the bowel smooth-muscle relaxation and as a stool softener [6, 9, 21]. Impregnated formulations of DS seeds stimulate mucus production and absorb water from the GI lumen thus softening the stool. Allyl disulfide compound present in DS seeds are most likely acting as a smooth-muscle relaxants in the GI tract and thus promoting defecation [6, 9]. Pasalar et al. found that DS could enhance bowel movement, ease defecation, and decrease abdominal distension [22]. Therefore, DS is considered to offer an effective treatment for constipation and hemorrhoids [6]. A clinical trial showed that a 4-weeks treatment with DS is safe (no significant adverse effects being observed) and effective in treating chronic functional constipation [21].
Descurainia sophia activity in cardiac diseases
Na+/K+ ATPase is a target for the treatment of arrhythmia and congestive heart failure (CHF) [23]. Cardiac glycosides have been used to treat heart failure and arrhythmia for many years [12]. Cardiac glycosides exert the positive inotropic effect through inhibiting Na+/K+ ATPase activity to increase intracellular sodium, followed by inhibition of the Na+/Ca2+ exchanger to increase intracellular calcium levels for strengthening the force of the heartbeat [24]. Helveticoside of DS is a cardiac glycoside and thus might have therapeutic effect in cardiac diseases [9, 12]. One previous study suggested that DS could significantly increase the urinary output in the chronic heart failure of rats based on the manifestation the diuretic effect resulted from the inhibition of renal tubular to reabsorb water, sodium, and chloride [25]. Zhou et al. showed that DS could decrease pleural effusion and pulmonary edema in critically ill patients [26]. Another study reported that DS could improve remodeling and cardiac function in chronic heart failure rats via attenuating cardiomyocyte apoptosis by regulating the balance between Bax and Bcl-2, blocking caspase cascades with the activation of PI3k/Akt/mTOR dependent signaling [27].
Anthelmintic activities of Descurainia sophia
The anthelmintic activity of DS was confirmed experimentally [6]. Maraghi et al. also showed that the EEDS is effective in treating mice infected with Hymenolepis nana, demonstrating that administration of EEDS daily for 7 days cured mice infected with Hymenolepis nana [28].
Drug-metabolizing enzyme activities of Descurainia sophia
Since DS is used extensively in herbal medicine, it is important to address its drug-metabolizing-enzyme (DME) activities. A study assaying enzyme activity showed that EEDS is an inhibitor with a moderate effect on CYP1A2, CYP2C9, and CYP2C19 [29]. Since CYP1A2, CYP2C9, and CYP2C19 are major enzymes to many vital processes, such as metabolism of endogenous compounds and elimination of environmental toxins, it is essential to consider the dosage, duration, and interactions when using DS. Many nonsteroidal anti-inflammatory drugs (NSAID) are a substrate for CYP2C9, including celecoxib, diclofenac, ibuprofen, or naproxen. As DS seeds are clinically used in the herbal formulation for anti-inflammation or treating respiratory diseases, DS seeds and NSAID will compete to bind with CYP2C9 [29]. The substrates for CYP1A2 include amitriptyline and erlotinib. The substrates for CYP2C9 include ibuprofen, warfarin, and tamoxifen. The substrates for CYP2C19 include diazepam, mephenytoin, methadone and bortezomib [29, 30]. Clinically, the potential adverse effects of DS-drug interactions should be considered when using DS in combination with drugs that are metabolized by CYP1A2, CYP2C9, or CYP2C19 [29].
Limitations
Despite DS having a wide range of pharmacological effects and being used widely in the complementary and alternative medicine, only few clinical trials evaluated its clinical benefits. Well-designed clinical trials should be conducted to determine the DS clinical benefits and safety.
Conclusions
DS is considered to be a safe and effective herb and is commonly used in complementary and alternative medicine. In this review, we provided information suggesting that DS might be effective in cancer, respiratory diseases, gastrointestinal diseases, cardiac diseases, and other conditions (Figure 3). DS is rich in pharmacological constituents that could be used or developed further as therapeutic agents. While there have been many studies addressing DS' mechanisms of action, well-designed clinical trials need to be conducted to prove its clinical benefits.
Abbreviations
β2AR: β2-adrenergic receptor; cAMP: cyclic adenosine monophosphate; CHF: congestive heart failure; CHOP: CCAAT/enhancer-binding protein homologous protein; DME: drug-metabolizing-enzyme; DS: Descurainia sophia; EEDS: ethanolic extract of DS seeds; GI: gastrointestinal; LPS: lipopolysaccharides; MAPK: mitogen-activated protein kinase; TNF: tumor necrosis factor; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand.
Major therapeutic effects of Descurainia sophia.
Acknowledgements
This work was supported by Buddhist Tzu Chi Medical Foundation, Hualien, Taiwan (TCMF-CP-109-02) and Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan (TCRD-TPE-109-64).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Liu MZ, Zhang YL, Zeng MZ, He FZ, Luo ZY, Luo JQ. et al. Pharmacogenomics and herb-drug interactions: merge of future and tradition. Evid Based Complement Alternat Med. 2015;2015:321091
2. Baek SJ, Chun JM, Kang TW, Seo YS, Kim SB, Seong B. et al. Identification of epigenetic mechanisms involved in the anti-asthmatic effects of Descurainia sophia seed extract based on a multi-Omics Approach. Molecules. 2018;23:pii E2879
3. Kim BY, Lee J, Park SJ, Bang OS, Kim NS. Gene expression profile of the A549 human non-small cell lung carcinoma cell line following treatment with the seeds of Descurainia sophia, a potential anticancer drug. Evid Based Complement Alternat Med. 2013;2013:584604
4. Gong JH, Zhang YL, He JL, Zheng XK, Feng W, Wang XL. et al. Extractions of oil from Descurainia sophia seed using supercritical CO2, chemical compositions by GC-MS and evaluation of the anti-tussive, expectorant and anti-asthmatic activities. Molecules. 2015;20:13296-312
5. Park JS, Lim CJ, Bang OS, Kim NS. Ethanolic extract of Descurainia sophia seeds sensitizes A549 human lung cancer cells to TRAIL cytotoxicity by upregulating death receptors. BMC Complement Altern Med. 2016;16:115
6. Nimrouzi M, Zarshenas MM. Phytochemical and pharmacological aspects of Descurainia sophia Webb ex Prantl: modern and traditional applications. Avicenna J Phytomed. 2016;6:266-72
7. Nagai H, Kim YH. Cancer prevention from the perspective of global cancer burden patterns. J Thorac Dis. 2017;9:448-51
8. Yamaguchi R, Perkins G. An Emerging Model for Cancer Development from a Tumor Microenvironment Perspective in Mice and Humans. Adv Exp Med Biol. 2020;1225:19-29
9. Sun K, Li X, Liu JM, Wang JH, Li W, Sha Y. A novel sulphur glycoside from the seeds of Descurainia sophia (L.). J Asian Nat Prod Res. 2005;7:853-6
10. Lee YJ, Kim NS, Kim H, Yi JM, Oh SM, Bang OS. et al. Cytotoxic and anti-inflammatory constituents from the seeds of Descurainia sophia. Arch Pharm Res. 2013;36:536-41
11. Wang B, Zhu XX, Pan LY, Chen HF, Shen XY. PP4C facilitates lung cancer proliferation and inhibits apoptosis via activating MAPK/ERK pathway. Pathol Res Pract. 2020 p: 152910
12. Kim BY, Lee J, Kim NS. Helveticoside is a biologically active component of the seed extract of Descurainia sophia and induces reciprocal gene regulation in A549 human lung cancer cells. BMC Genomics. 2015;16:713
13. Alevizopoulos K, Calogeropoulou T, Lang F, Stournaras C. Na+/K+ ATPase inhibitors in cancer. Curr Drug Targets. 2014;15:988-1000
14. von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer. 2017;17:352-66
15. Enilari O, Sinha S. The Global Impact of Asthma in Adult Populations. Ann Glob Health. 2019;85:Pii 2
16. Beckert H, Meyer-Martin H, Buhl 2, Taube C, Reuter S. Single and Synergistic Effects of Type 2 Cytokines on Eosinophils and Asthma Hallmarks. J Immunol. 2020;204:550-8
17. Hou Y, Cheng B, Zhou M, Fang R, Jiang M, Hou W. et al. Searching for synergistic bronchodilators and novel therapeutic regimens for chronic lung diseases from a traditional Chinese medicine, Qingfei Xiaoyan Wan. PLoS One. 2014;9:e113104
18. Mohamed NH, Mahrous AE. Chemical constituents of Descurainia sophia l. and its biological activity. Rec Nat Prod. 2009;3:58-67
19. Mirzaei A, Mohammadi J, Mirzaei N, Mirzaei M. The antioxidant capacities and total phenolic contents of some medicinal plants in Iran. J Fasa Univ Med Sci. 2011;1:160-7
20. Bekker NP, Ul'chenko NT, Glushenkova AI. Lipids from Descurainia sophia seeds. Chem Nat Compd. 2005;41:346-7
21. Choopani R, Ghourchian A, Hajimehdipoor H, Kamalinejad M, Ghourchian F. Effect of Descurainia sophia (L.) Webb ex Prantl on Adult Functional Constipation: A Prospective Pilot Study. J Evid Based Complementary Altern Med. 2017;22:646-51
22. Pasalar M, Bagheri Lankarani K, Mehrabani D, Tolidei HR, Naseri M. The effect of Descureania Sophia L. and Prunus Domestica L. in prevention of constipation among Iranian Hajj Pilgrims, Saudi Arabia. Res J Pharm Biol Chem Sci. 2013;4:1195-204
23. Shattock MJ, Ottolia M, Bers DM, Blaustein MP, Boguslavskyi A, Bossuyt J. et al. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J Physiol. 2015;593:1361-82
24. Demiryürek AT, Demiryürek S. Cardiotoxicity of digitalis glycosides: roles of autonomic pathways, autacoids and ion channels. Auton Autacoid Pharmacol. 2005;25:35-52
25. Zhang X, Fan C, Yingmei Yu, Wang Liu, Yanzhuo Zhang. Diuretic effect of aqueous extract of Tinglizi on CHF rats. Chin JMAP. 2010;27:210-4
26. Zhou G, Zhang CH. Application Tinglizi Jujube Soup for Treating Critically Ill Patients in ICU. Journal of Liaoning University of TCM. 2011;13:180
27. Luo Y, Sun Z, Hu P, Wu Y, Yu W, Huang S. Effect of Aqueous Extract from Descurainia sophia (L.) Webb ex Prantl on Ventricular Remodeling in Chronic Heart Failure Rats. Evid Based Complement Alternat Med. 2018;2018:1904081
28. Maraghi S TJN. Study of in vitro and in vivo effects of Descurainia sophia extract on Hymenolepis nana in comparison with niclosamide. Hakim. 2002;5:57-62
29. Yi JM, Kim YA, Lee YJ, Bang OS, Kim NS. Effect of an ethanol extract of Descurainia sophia seeds on Phase I and II drug metabolizing enzymes and P-glycoprotein activity in vitro. BMC Complement Altern Med. 2015;15:441
30. Pan Y, Tiong KH, Abd-Rashid BA, Ismail Z, Ismail R, Mak JW. et al. Inhibitory effects of cytochrome P450 enzymes CYP2C8, CYP2C9, CYP2C19 and CYP3A4 by Labisia pumila extracts. J Ethnopharmacol. 2012;143:586-91
Author contact
![]() Corresponding author: Dr. Chou-Chin Lan, Division of Pulmonary Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan. No.289, Jianguo Rd., Xindian Dist., New Taipei City 23142, Taiwan; Telephone +886-2-6628-9779 ext. 2259; Fax: +886-2-6628-9009; E-mail: bluescopycom.tw.
Corresponding author: Dr. Chou-Chin Lan, Division of Pulmonary Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, Taiwan. No.289, Jianguo Rd., Xindian Dist., New Taipei City 23142, Taiwan; Telephone +886-2-6628-9779 ext. 2259; Fax: +886-2-6628-9009; E-mail: bluescopycom.tw.

 Global reach, higher impact
Global reach, higher impact