3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2020; 17(12):1819-1832. doi:10.7150/ijms.42612 This issue Cite
Research Paper
Resistance training promotes reduction in Visceral Adiposity without improvements in Cardiomyocyte Contractility and Calcium handling in Obese Rats
1. Centre for Physical Education and Sports, Department of Sports, Federal University of Espírito Santo, Vitória, Espírito Santo, Brazil.
2. Centre for Health Sciences, Department of Nutrition, Federal University of Espírito Santo, Vitória, Espírito Santo, Brazil.
3. Center of Health Sciences, Department of Morphology, Federal University of Espírito Santo, Vitória, Espírito Santo, Brazil.
4. Center of Health Sciences, Department of Physiological Sciences, Federal University of Espírito Santo, Vitória, Espírito Santo, Brazil.
#These authors contributed equally to this work.
Received 2019-11-30; Accepted 2020-3-23; Published 2020-7-11
Abstract
Resistance training (RT) improves the cardiomyocyte calcium (Ca2+) cycling during excitation-contraction coupling. However, the role of RT in cardiomyocyte contractile function associated with Ca2+ handling in obesity is unclear. Wistar rats were distributed into four groups: control, sedentary obese, control plus RT, and obesity plus RT. The 10-wk RT protocol was used (4-5 vertical ladder climbs, 60-second interval, 3× a week, 50-100% of maximum load). Metabolic, hormonal, cardiovascular and biochemical parameters were determined. Reduced leptin levels, epididymal, retroperitoneal and visceral fat pads, lower body fat, and adiposity index were observed in RT. Obesity promoted elevation of collagen, but RT did not promote modifications of LV collagen in ObRT. RT induced elevation in maximum rates of contraction and relaxation, and reduction of time to 50% relaxation. ObRT group did not present improvement in the cardiomyocyte contractile function in comparison to Ob group. Reduced cardiac PLB serine16 phosphorylation (pPLB Ser16) and pPLB Ser16/PLB ratio with no alterations in sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) and phospholamban (PLB) expression were observed in Ob groups. Resistance training improved body composition reduced fat pads and plasma leptin levels but did not promote positive alterations in cardiomyocyte contractile function, Ca2+ handling and phospholamban phosphorylation.
Keywords: high-fat diet, obesity, resistance training, contractile function, calcium handling
Introduction
Obesity is a chronic metabolic disease that is characterized by excessive accumulation of adipose tissue [1,2]. Currently, it is considered a global epidemic and an important public health issue, besides being an independent risk factor for cardiovascular diseases [3]. In this context, a close relationship has been observed between accumulation of adipose tissue and impairment of cardiac function in both human and experimental models [4-9].
Experimental studies have been developed to elucidate the pathophysiological mechanisms related to cardiac functional impairment induced by obesity, including abnormalities in myocardial intracellular calcium (Ca2+) handling, one of the major regulatory mechanisms of contractility and relaxation [7,10-12]. Thus, impairment of myocardial function assigned to obesity may be related to mechanisms involving the Ca2+ influx and release into the cytosol, and/or damage in the recapture and/or extrusion of this ion by the sarcoplasmic reticulum [7,10-13]. Generally, relaxation impairment observed in obese rats may be related to phospholamban (PLB) phosphorylation of Ser16 and Thr17 by protein kinase A (PKA) or CaMKII impairment, provided that both are physiologically relevant to control SERCA2a activity [14,15].
Non-pharmacological approaches, such as physical exercise, have been used as an efficient and viable tool to improve adiposity parameters and minimize and/or reverse the cardiac damage promoted by obesity [16-18]. Physical exercise has been able to alter the activity and expression of regulatory proteins, improving contraction and relaxation in several physiological and pathological models [19-22]. The literature has consistently shown that endurance training improves myocardial contractility associated with the benefits of intracellular Ca2+ handling, as well as promoting increased sensitivity of the myofilaments to this ion in isolated cardiomyocytes [23,24].
Resistance Training (RT) is effective in improving body composition [16,29-31]. Other studies also showed that RT was able to reduce the adipocyte area and body fat percentage [16,31]. Regarding the role of RT in cardiovascular system, limited data exist about the effects on healthy or unhealthy heart [16,25-28]. Nevertheless, it has been demonstrated that RT performed for 1 and 3 months can generate benefits to heart, such as increased LV velocity of contraction and relaxation [25]. In addition, RT may have positive effects on cardiac function in cardiopathy patients and experimental models of chronic heart failure and diabetes mellitus [16,26-28]. However, these functional benefits observed in the heart depend on the training variables, such as intensity, frequency, duration, and type of exercise used [26].
Nevertheless, the effects of RT on Ca2+ handling are poor studied. Melo et al. [19] have shown improvement in contraction and relaxation of cardiomyocytes in healthy rats, which are related to increased protein expression of SERCA2a. However, to the best of our knowledge, no studies evaluated the myocardial Ca2+ handling adaptations to chronic RT in obesity. Thus, the aim of this study was to investigate the effects of RT on the process of cardiac remodeling, contractile function and Ca2+ handling in obese rats. The hypothesis was that RT promotes improvement in contractile function in obese cardiomyocytes, which may be related to adjustments in Ca2+ handling.
Material and Methods
Animals and Care
Thirty-day-old male Wistar rats (≈ 150 g) obtained from the Animal Quarters of the Federal University of Espírito Santo (Vitória, Espírito Santo, Brazil) were housed in individual cages. The environment was controlled in terms of light (12h light/dark cycle starting at 6 am), clean-air room temperature (23 ± 3°C), and relative humidity (60 ± 5%). All experiments and procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Animals” published by “U.S. National Institutes of Health” and current Brazilian laws. The University of Espírito Santo Ethics Committee approved the experimental protocol (CEUA-UFES 16/2016).
Experimental Design
After 7 days of acclimatization, the rats were distributed into two groups: control (C, n=29), and obese (Ob, n=29). The C group was fed a standard diet (Nuvilab CR1-Nuvital, Colombo, Paraná, Brazil) containing 13.9% of its kcal from fat, 55.9% from carbohydrates, and 30.2% from protein. Ob animals were alternately submitted to four palatable high-fat diets (RC Focus 2413, 2414, 2415, and 2416) containing 49.2% of its kcal from fat, 28.9% from carbohydrates, and 21.9% from protein [7]. Each diet was changed daily, and the rats were maintained on their respective diets for 26 consecutive weeks. The high-fat diet was calorically rich (high-fat diet = 3.65 kcal/g versus low-fat diet = 2.92 kcal/g) due to its higher fat energy (consisting of saturated and unsaturated fatty acids, which provided 20% and 80% of the fat-derived calories, respectively). These experimental diets provided sufficient amounts of protein, vitamins, and minerals according to the Nutrient Requirements of Laboratory Animals. High-fat diets components have been previously described [10]. All animals had free access to water and chow (40 g/day). After starting the experimental protocol, body weight (BW) was recorded weekly.
The experimental protocol was divided into three periods: induction (3 weeks), exposure to obesity (13 weeks), and resistance training protocol (10 weeks). At the end of the protocol of exposure to obesity (13 weeks of obesity initial moment), rats were submitted to composition and redistribution of groups. For constitution of two homogeneous groups, ensuring that C group was composed only of animals with characteristics of control animals and Ob group was composed only of animals with characteristics of obese animals, a confidence interval of 95% (CI) was created based on the BW means of control and obese rats. A separation point (SP) was applied between groups; a BW medium point between C upper limit and Ob lower limit. Based on this point, animals with BW above the SP were excluded from C group, and animals with BW below the SP were excluded from Ob group, as previously described [5]. Thus, 25 animals from C group (C; n=25) and 21 animals from Ob group (Ob; n=21) remained in the study.
After the composition of groups, animals were redistributed into two more groups as absence or presence of resistance training (RT). Therefore, in the second stage of experimental protocol, this study was composed of four groups: sedentary control (C; n=10), control submitted to resistance training (RT; n=11), sedentary obese (Ob; n=9), and obese submitted to resistance training (ObRT; n=11).
It's important to highlight that 5 animals died from undetermined causes throughout the experimental protocol.
Resistance Training (RT) Protocol
Familiarization
The RT protocol was adapted from Hornberger & Farrar [33], which consisted of climbing a vertical ladder (110 cm high, 18 cm wide, with 2 cm-grid steps, 80◦ incline) with a load apparatus affixed to the base of the rat's tail by means of plastic insulation tape. Resting chamber (20 x 20 x 20 cm) was placed on the top of the ladder, which served as a shelter during rest between climbing sets [33]. The weight attached to the base of the tail was gradually increased with exercise progression. The sets were characterized from voluntarily ladder climbs of rats to the top of the ladder. Familiarization of RT and ObRT groups with the load apparatus was gradual by climbing for 3 non-consecutive days without any weight prior the RT protocol. In the familiarization period, the animals were stimulated to perform four complete climbs on the ladder, with a 60-second-rest interval between climbs [33].
Maximum load carrying test (MLCT)
Three MLCTs were performed during the experimental protocol (after the familiarization period, before starting the experimental protocol, and after 10 weeks of RT protocol). The initial load was 50% of BW (first climb), and for each series completed, 30g was added to the load. The test was conducted until the animal was unable to climb the ladder. The interval between each series was 120 seconds for all tests. The highest loaded load (HLL) successfully throughout the ladder was considered the maximum load (ML), which was used for the prescription of the RT protocol intensities. In the second and third MLCTs, the load of the last training session for the beginning of the test (50% ML) was used. The following parameters were analyzed: absolute (g) and relative loads (%), and for comparison of the results obtained between MLCTs, the delta (Δ) of force was calculated using the formula: final MLCT- initial MLCT multiplied by 100/initial MLCT and expressed as a percentage (%) [33].
Resistance training period
Training sessions consisted of one set of climbing, using progressive loads, interspaced with 60-second intervals. The animals had to perform 4-5 climbs to the top of the ladder. The load was progressively increased from 50% of the maximum load in the first series, through 75%, 90%, until 100% of the maximum load in the fourth series. After that, if the animal completed the fourth series, it was submitted to a fifth series with 100% of the maximum load plus 30g to failure. Failure was determined when the rat could not progress up the ladder after three successive gentle stimuli to the tail. Resistance training was performed in the afternoon, for 3 times a week, from Monday to Friday for 10 weeks.
Comorbidities Associated with Obesity
Blood pressure measurements
After the end of the experimental protocol, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were assessed indirectly by the non-invasive tail-cuff plethysmography (Insight Equipment, Ribeirão Preto, SP, Brazil). The animals were housed in a heated chamber, with average temperature of 37ºC for 15 minutes [34]. After this period, a rubber cuff with pressure transducer from 0 to 300 mmHg was connected to the proximal tail. The values of SBP and DBP were obtained through the transducer signals coupled to the computer and analyzed on software (Software Flow Pressure Meter, Insight Equipment, Ribeirão Preto, SP, Brazil). The average of three pressure readings was recorded for each animal. In addition, the mean arterial pressure (MAP) was obtained by the following formula: MAP = (SBP + 2 × DBP)/3 [35].
Glucose tolerance test (GTT)
After the end of the experimental protocol, all rats were fasted for 4-6 h prior to the glucose tolerance test. After fasting, a blood sample was collected from the tip of the tail. The basal blood glucose level of each animal was immediately determined using a handheld glucometer (Accuchek Advantage; Roche Diagnostics, Indianapolis, IN, USA). Subsequently, an injection of glucose solution (2 g/kg body wt) dissolved in water was administered intraperitoneally, and blood glucose levels were measured after 15, 30, 60, 90, and 120 min [36]. Glucose intolerance was evaluated by the area under the curve (AUC) for glucose.
Euthanasia and Collection of Biological Material
At the end of the experimental protocol, the animals were fasted for 12 to 15 hours and received an injection with sodium heparin (1000U/kg/i.p; Heparamax-s, Blau Pharmaceutic S.A., São Paulo, Brazil) for blood anticoagulation. After 30 minutes, rats were anesthetized with ketamine (50 mg/kg/ip, Dopalen, Sespo Indústria e Comércio Ltda - Vetbrands Division, Jacareí, São Paulo, Brazil) and xylazine (10 mg/kg / ip; Anasedan, Sespo Indústria e Comércio Ltda - Vetbrands Division, Jacareí, São Paulo, Brazil), and euthanized. After median thoracotomy, the heart, ventricles, fat pads of adipose tissue and tibia were separated, dissected, weighed, and measured.
Determination of Obesity
After 26 weeks, a criterion based on the adiposity index was used to determine obesity [37,38]. Body weight was measured weekly and body fat (BF) amount was determined from the dissection of the epididymal, retroperitoneal, and visceral fat deposits. The adiposity index (AI) was calculated using the following formula: AI = (total BF/final body weight) × 100 [39].
Lipid and hormonal profile
Blood samples were collected from the abdominal artery and centrifuged at 5000 rpm for 10 minutes (Eppendorf Centrifuge 5804-R, Hamburg, Germany) in dry tubes, and the plasma was collected and stored in Eppendorf tubes in the freezer at -80°C (Thermo Fisher Scientific LLC, Asheville, NC, USA). The tissues used for further analysis were collected and frozen immediately with liquid nitrogen and then packaged and stored in the freezer at -80ºC (ColdLab Ultra Freezer CL374-86V, Piracicaba, São Paulo, Brazil). Plasma was evaluated by levels of total cholesterol (T-Chol) and high-density cholesterol (HDL), and hormones (insulin and leptin). T-Chol and HDL were measured with an automatic enzymatic analyzer system (Biochemical analyzer BS-200, Mindray, China). Leptin levels were determined with the enzyme-linked immunosorbent assay (ELISA) method using commercial kits (Linco Research Inc., St. Louis, MO, USA).
Muscle morphometric
At the end of the experimental period, animals previously anesthetized had their soleus and tibialis muscles from the left hindlimb were removed, and then weighed.
Cardiac remodeling
Cardiac remodeling was measured by post-mortem morphological analysis, histological study, isolated cardiomyocyte contractile function, as well as by intracellular Ca2+-cycling protein analysis by Western blotting.
Postmortem Morphological Analysis
Cardiac remodeling at the macroscopic level, which identifies presence or absence of cardiac hypertrophy, was determined by analyzing the following parameters: heart and left ventricle (LV) weights, heart and LV normalized by tibia length.
Histological Study
LV fragments were placed in 4% paraformaldehyde solution pH 7.4, transferred to 70% ethanol solution and embedded in paraffin. Thick sections of 6 mm thickness were cut from tissue block and stained with hematoxylin-eosin solution. After HE staining was developed, slides were mounted and visualized under microscopy (40×; AX70, Olympus Optical CO, Hamburg, Germany) to determine the myocyte cross-sectional area (CSA), which was determined for at least 50 myocytes per slide with rounded shape and nucleus visible at the center of the cell [40]. CSA (μm2) was used as an indicator of cell size, characterizing presence or absence of cardiac hypertrophy.
LV interstitial collagen fraction (%) was determined for the entire picrosirius red stained cardiac section. Further analysis of the quantification of the interstitial collagen fraction was performed using 30 to 40 fields per fragment. The histological sections were enlarged 40 times with the aid of a biological microscope (BEL Photonics Research Bio 3, Porto Alegre, Rio Grande do Sul, Brazil). The components of the cardiac tissue were identified according to color level as follows: red for collagen fibers, yellow for myocytes, and white for interstitial space. Perivascular collagen was excluded from this analysis. The analyses were performed through software (Image Pro-plus, Media Cybernetics, Silver Spring, Maryland, USA).
Cardiomyocyte preparation
Under anesthesia, rats from each group were euthanized and the hearts were quickly removed by median thoracotomy and enzymatically isolated as previously described [41]. Briefly, the hearts were cannulated and retrograde perfusion of the aorta was performed in Langendorff system (37oC) with a modified isolation digestion buffer solution (DB), a calcium-free solution containing 0.1 mM ethylene glycol-bis (ß-aminoethyl ether)-N, N, N', N'-tetraacetic acid (EGTA) and N-[2-hydro-ethyl]-piperazine-N'-[2-ethanesulfonic acid] (HEPES) equilibrated with 5% CO2-95% O2 for ~3 to 5 min. The composition of DB solution was (mM): 130 NaCl, 1.4 MgCl2, 5.4 KCl, 25 HEPES, 22 glucose, 0.33 NAH2PO4, and pH 7.39. Afterwards, the hearts were perfused for 15-20 minutes with a DB solution containing 1 mg/ml collagenase type II (Worthington Biochemical Corporation, UK) and Ca2+ (1 mM). The digested hearts were then removed from the cannula, cut down and placed into small conical flasks with DB solution containing collagenase supplemented with 0.1% bovine serum albumin and Ca2+ (1 mM). After that, this process was performed 2 more times without collagenase, with addition of 1.6 and 3.12 µL (1.0 mM CaCl2; stock solution). Each stage containing cells and solutions was held for approximately 10 minutes. Then, the supernatant was removed, and the myocytes were resuspended in Tyrode's buffer containing (in mM): 140 NaCl, 10 HEPES, 0.33 NaH2PO4; 1 MgCl2, 5 KCl, 1.8 CaCl2, 10 glucose. Only calcium-tolerant, quiescent, rod-shaped cardiomyocytes showing clear cross-striations were studied. The isolated cardiomyocytes were used within 2-3 h of isolation.
Cardiomyocyte contractility
Briefly, isolated cells were placed in an experimental chamber with a glass coverslip base mounted on the stage of an inverted microscope (IonOptix, Milton, MA, USA) edge detection system with a 40× objective lens (Nikon Eclipse - TS100, USA). Cells were immersed in Tyrode's solution containing 1.8 mM CaCl2 and field stimulated at 1 Hz (20 V, 5ms duration square pulses). Cell shortening in response to electrical stimulation was measured with a video-edge detection system at a 240-Hz frame rate (Ionwizard, Ion Optix, Milton, MA, USA), and the contractile parameters were evaluated. Fractional shortening (expressed as a percentage of resting cell length), maximal rate of contraction and relaxation, times to 50% contraction, and relaxation were measured. The total numbers of cells analyzed are described in the legend of each figure.
Western Blot
Protein expression of sarcoplasmic reticulum Ca2+-ATPase (SERCA2a), phospholamban (PLB), PLB serine16 phosphorylation (pPLB ser16) and β-actin were determined by Western blot analysis. SERCA2a/PLB and pPLBser16/PLB ratios were also determined. Briefly, LV samples from C (n= 5), Ob (n= 4), RT (n= 4), and ObRT (n= 5) rats were frozen in liquid nitrogen and homogenized in a buffer containing 50 mM potassium phosphate buffer (pH 7.0), 0.3 M sucrose, 0.5 mM dithiothreitol (DTT), 1 mM EDTA (pH 8.0), 0.3 mM phenylmethylsulfonyl fluoride (PMSF), 10 mM sodium fluoride (NaF), and phosphatase inhibitor cocktail (1:100; Sigma-Aldrich, St. Louis, MO, USA). The samples were subjected to SDS-PAGE in 12-15% polyacrylamide gels depending on the molecular weight of the protein. After electrophoresis, the separated proteins were transferred to nitrocellulose membranes (Bio-Rad Biosciences, USA), using the Mini Trans-Blot system (Bio-Rad, Hercules, CA, USA), and containing transfer buffer (25 mM Tris base; 190 mM glycine; 20% methanol and 10% SDS). Equal loading of the samples (50 mg) and transfer efficiencies were monitored with 0.5% Ponceau S staining of the membrane. The blotted membrane was blocked (5% non-fat dry milk, 10 mM Tris-HCl (pH 7.5R), 100mM, NaCl, and 100% Tween 20) for 2h at room temperature and then incubated overnight at 4ºC with specific primary antibodies PLB Monoclonal Antibody, mouse IgG (ThermoFisher Scientific, USA), PLBser16 Antibody, rabbit IgG (Badrilla, UK), SERCA2a, Polyclonal Antibody, goat (Santa Cruz Biotechonology, USA). Binding of the primary antibody was detected with peroxidase-conjugated secondary antibodies (rabbit, mouse or goat IgG-HRP (Santa Cruz Biotechonology, USA) for 1.5 hours at room temperature. Protein bands were visualized via chemiluminescent detection (ECL Prime Western Blotting Detection, GE Healthcare Life Sciences, USA) in a western blot detection imaging system (FX PRO, Bruker BioSpin Corporation, USA), and quantified by densitometry using Image J Analysis software. Targeted bands were normalized to the expression of cardiac β-actin (Cell Signaling Technology, USA).
Statistical analysis
The results are reported as means ± standard deviation (SD) and submitted to the Kolmogorov-Smirnov test. As all data presented adherence to normality, two-way analysis of variance (ANOVA) followed by Tukey's or Bonferroni post hoc tests were performed using Prism 8.0 software (GraphPad Prism version 8.01 for Windows; GraphPad Software, San Diego, CA, USA). The level of significance was 5%.
Results
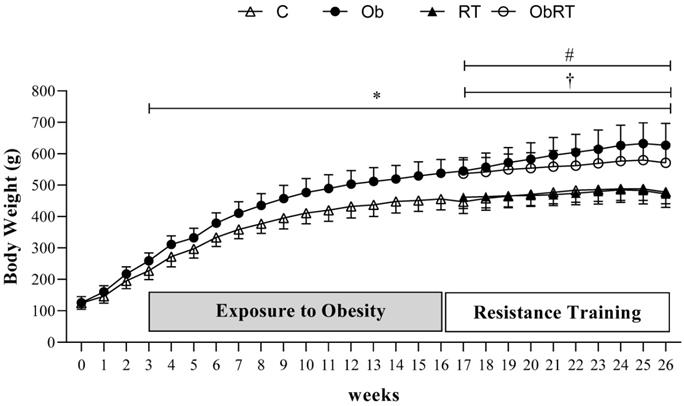
Figure 1 shows the evolution of body weight during 26 weeks of experimental protocol. The BW was similar in the first three weeks of treatment between groups; however, Ob group presented higher BW than C in the 3rd week, considered the initial moment of obesity. From 3rd to 16th week, there was statistical difference in BW between groups (C vs. Ob), characterizing the period of exposure to obesity. After that, C and Ob rats were redistributed into two more groups as absence or presence of resistance training (RT). Thus, Ob and ObRT groups presented an elevation of BW when compared with the respective control groups (C and RT), which remained significantly greater during the experimental period (17th to 26th week); however, the resistance training was not be able to reduce the body weight in ObRT group when compared to Ob rats. In addition, RT also promoted reduced BW in relation to Ob group (Ob>RT) from the 17th to 26th week.
After 26 weeks, Ob rats demonstrated a final body weight 31% and 33% greater than C and RT, respectively (Table 1). In addition, obesity promoted a substantial elevation of epididymal, retroperitoneal and visceral fat pads compared to C and RT groups, respectively. Specifically, these rats showed elevation of 125% and 128% in body fat, respectively, when compared with C and RT rats. Adiposity index was also significantly greater in this group (69%) than in C and RT rats. Nevertheless, considering the Ob and ObRT groups, the resistance training led to a significant reduction of epididymal fat (28.8%) and visceral fat pads (26.9%) when compared to Ob rats, but it was not able to reduce FBW, retroperitoneal fat pad, body fat (BF), and adiposity index (AI). In addition, ObRT group showed differences in FBW, epididymal, retroperitoneal and visceral fat pads in relation to RT (ObRT > RT), as well as for BF and AI, demonstrating obesity effect (Table 1).
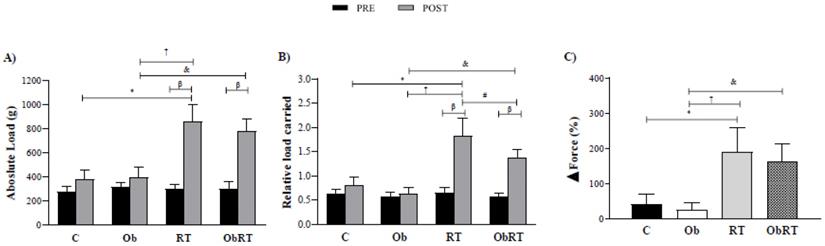
There was no statistically significant difference in absolute and relative training loads among groups in the initial MLCT (pre training), but the maximum workload capacity of the resistance training groups (RT vs. C and ObRT vs. Ob) was increased throughout the training period (Figure 2). Experimental groups, RT and ObRT increased significantly their maximum carrying load capacity (125% and 98%) compared with C and Ob, respectively (Figure 2A). RT also promoted elevation of absolute load when compared with Ob. In relation to relative load carried, RT and ObRT groups presented higher values than sedentary groups (C and Ob). In addition, RT group had increased relative load when compared with ObRT and Ob rats (Figure 2B). Furthermore, the ∆Force was elevated in resistance training groups (RT and ObRT) when compared with the respective control groups (Figure 2C). There was no difference between RT and ObRT.
General characteristics
| Variables | Experimental Groups | |||
|---|---|---|---|---|
| C | Ob | RT | ObRT | |
| FBW (g) | 478 ± 37 | 627 ± 69* | 472 ± 43† | 571 ± 60# |
| Epididymal fat pad (g) | 7.4 ± 2.0 | 15.6 ± 4.6* | 6.9 ± 1.0† | 11.1 ± 3.2#& |
| Retroperitoneal fat pad (g) | 12.1 ± 2.6 | 28.2 ± 7.1* | 12.7 ± 1.1† | 24.6 ± 7.3# |
| Visceral fat pad (g) | 8.2 ± 1.5 | 18.6 ± 5.9* | 7.7 ± 0.9† | 13.6 ± 4.6#& |
| BF (g) | 27.7 ± 5.3 | 62.4 ± 17.1* | 27.3 ± 2.1† | 49.3 ± 14.4# |
| AI (%) | 5.8 ± 0.9 | 9.8 ± 1.8* | 5.8 ± 0.7† | 8.5 ± 1.8# |
Values presented as mean ± SD. n = number of animals. Sedentary control group (C, n = 10), sedentary obese (Ob, n = 9), control submitted to resistance training (RT, n = 11) and obese submitted to RT (ObRT, n = 11). FBW = final body weight; BF: body fat; IA = adiposity index. Two-way ANOVA followed by Tukey post hoc test. p<0.05. *C vs. Ob; †RT vs. Ob; #RT vs. ObRT; &Ob vs. ObRT.
Evolution of body weight during 26 weeks of experimental protocol. Sedentary control group (C, n = 10), sedentary obese (Ob, n = 9), control submitted to resistance training (RT, n = 11) and obese submitted to RT (ObRT, n = 11). Data presented as the mean ± SD. Two-way ANOVA for repeated measurements followed by Bonferroni post hoc test. p<0.05 *C vs. Ob; †RT vs. Ob; #RT vs. ObRT.
Maximum load carrying test (MLCT) data before (PRE) and after (POST) 26 weeks of experimental protocol. A) Absolute load (g); B) relative load carried (Absolute load/FBW) and C) Delta ∆ force training (%). Sedentary control group (C, n = 10), sedentary obese (Ob, n = 9), control submitted to resistance training (RT, n = 11) and obese submitted to RT (ObRT, n = 11). Data presented as the mean ± SD. Two-way ANOVA complemented with the post hoc Tukey test. p<0.05. *C vs. Ob; †RT vs. Ob; #RT vs. ObRT; &Ob vs. ObRT; βpre vs. post.
The comorbidities associated with obesity are summarized in Table 2. Obesity did not promote significant metabolic and hemodynamic alterations, but this condition only caused elevation in leptin levels (Ob > C). Nevertheless, the resistance training was able to promote a reduction in the leptin levels in ObRT in relation to Ob group (42.1%). In addition, AUC was elevated in Ob group, but there was no significant difference when compared with C (p = 0.07). However, RT was effective in the reduction of glucose and leptin levels (RT < ObRT and Ob). The other parameters, including AUC, SBP, DBP, T-Chol, and HDL, were similar among groups.
Comorbidities associated with Obesity
| Variables | Experimental Groups | |||
|---|---|---|---|---|
| C | Ob | RT | ObRT | |
| Glucose (mg/dL) | 109 ± 6 | 113 ± 8 | 101 ± 7† | 106 ± 11 |
| AUC (mg/dL/min) | 220 ± 15 | 270 ± 55 | 233 ± 33 | 270 ± 54 |
| SBP (mmHg) | 124 ± 14 | 129 ± 17 | 129 ± 17 | 124 ± 18 |
| DBP (mmHg) | 95 ± 13 | 112 ± 21 | 104 ± 16 | 98 ± 21 |
| T-Chol (mg/dL) | 68 ± 9 | 62 ± 7 | 64 ± 10 | 54 ± 7 |
| HDL (mg/dL) | 24 ± 3 | 25 ± 2 | 25 ± 3 | 25 ± 5 |
| Leptin (ng/mL) | 6 ± 3 | 19 ± 5* | 5 ± 2† | 11 ± 7#& |
Values presented as mean ± SD. 9 animals per group. Sedentary control group (C), sedentary obese (Ob), control submitted to resistance training (RT) and obese submitted to RT (ObRT). AUC: area under the curve for glucose; SBP: systolic blood pressure; DBP: diastolic blood pressure; T-Chol: total cholesterol; HDL: high-density lipoprotein. ANOVA followed by Tukey post hoc test. p<0.05. *C vs. Ob; †RT vs. Ob; #RT vs. ObRT; &Ob vs. ObRT.
Soleus and tibialis muscles weights of animals from ObRT group were similar when compared to the RT and Ob groups, respectively. In addition, no differences were found between the ObRT and RT groups for the tibia length, as well as the soleus and tibialis normalized muscle weight (Table 3). These results were also observed in the comparison between the Ob and ObRT groups.
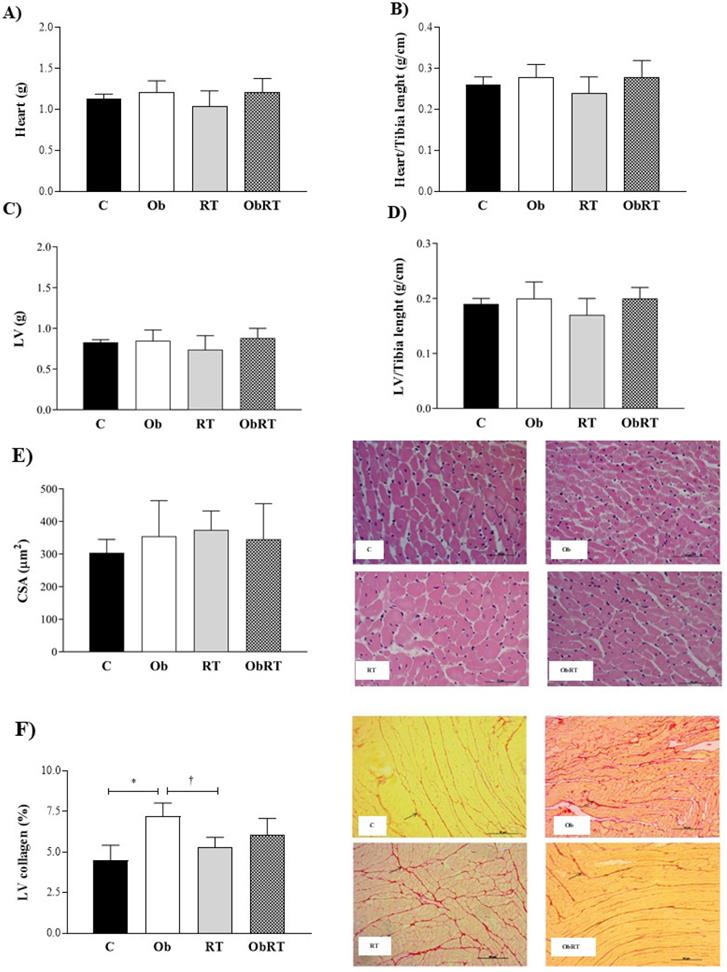
There were no differences in heart, LV, heart/Tibia, LV/Tibia and CSA among groups (Figure 3A-E). Histological analysis from LV samples revealed that interstitial collagen fraction (%) was elevated in Ob group when compared with C and RT rats (Figure 3F). However, RT was not able to reduce or prevent increase of LV collagen in ObRT, since there was no statistical difference (p = 0.25) between Ob and ObRT groups (Figure 3F).
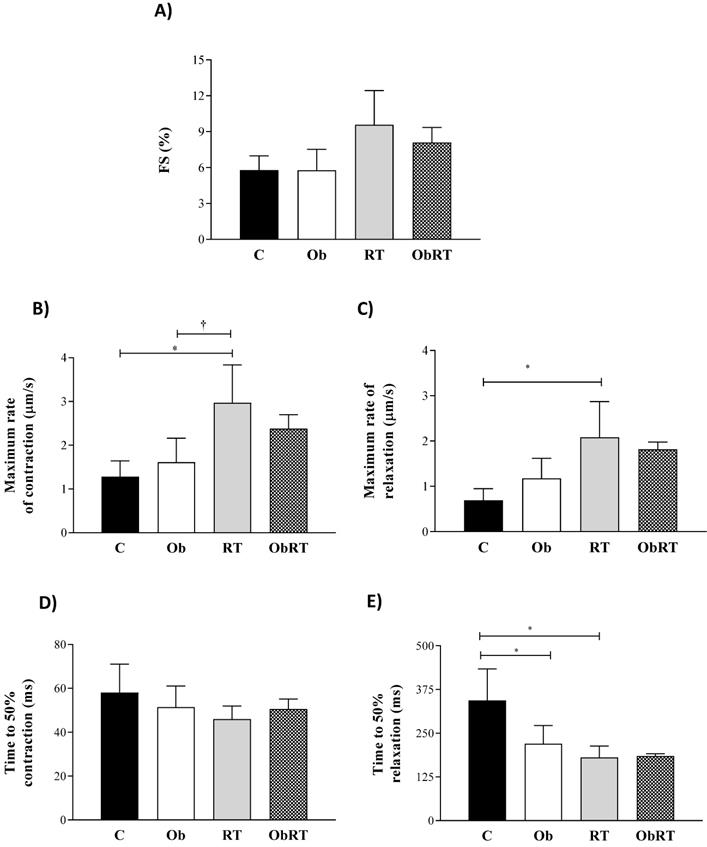
Figure 4 summarizes the mechanical properties of isolated cardiomyocytes from all groups. RT induced a marked adaptive response in cardiomyocyte contractile function visualized by elevation in maximum rates of contraction and reduction of time to 50% relaxation in relation to C and Ob groups (Figures 4B and E). In addition, the RT promoted an elevation in maximum rates of relaxation when compared to C rats (Figure 4C). However, there was no difference in the fractional shortening among the groups, respectively (Figure 4A); fractional shortening was elevated by 65% in RT group in relation to C and Ob groups, but no differences were observed (p = 0.06). The time to 50% relaxation was reduced in Ob and RT when compared with C rats, respectively (Figure 4D), resulting in improvement of cardiomyocyte relaxation. Moreover, no differences were observed for the time to 50% contraction among groups (Figure 4D). In addition, RT was not able to improve cardiomyocyte contractile function in ObRT, since there was no statistical difference between Ob and ObRT groups (Figure 4A-E).
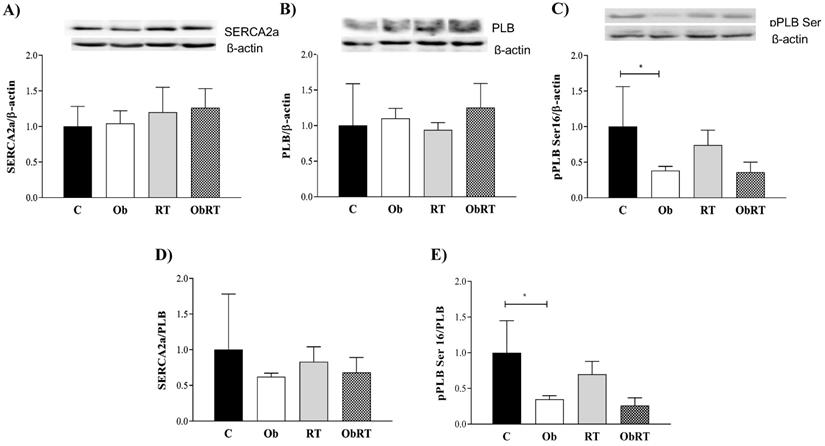
The levels of intracellular Ca2+ cycling proteins SERCA2a, PLB and pPLB Ser16 were assessed to determine the mechanism for obesity-induced changes in cardiac function and the role of RT in this process (Figure 5). There was no difference in SERCA2a, PLB and SERCA2a/PLB ratio among groups (Figure 5A, B and D). Figure 5C shows that obesity only promoted change in pPLB Ser16 (C: 1.00 ± 0.56 vs. Ob: 0.38 ± 0.06; p <0.05) when compared with C rats. Additionally, pPLB Ser16/PLB ratio (C: 1.00 ± 0.45 vs. Ob: 0.35 ± 0.05; p<0.05) was significantly diminished in Ob rats. Nevertheless, the resistance training was not able to reverse these alterations associated with obesity (Ob vs. ObRT).
Morphological post mortem and histological studies performed after 26 weeks of experimental protocol. 5 animals per group. A) Heart weight; B) Heart normalized by tibia length; C) left ventricle (LV); D) LV normalized by tibia length; E) cross sectional area (CSA) obtained by HE staining for reticulum, and F) interstitial collagen of myocardium (40x magnification); representative picrosirius red-stained left ventricle (LV) section. Arrows: interstitial collagen. Sedentary control group (C), sedentary obese (Ob), control submitted to resistance training (RT) and obese submitted to RT (ObRT). Data presented as the mean ± SD. Two-way ANOVA complemented with the post hoc Tukey's test. p<0.05. *C vs. Ob; †RT vs. Ob.
Muscle morphometric data
| Variables | Experimental Groups | |||
|---|---|---|---|---|
| C | Ob | RT | ObRT | |
| Soleus Weight (g) | 0.176 ± 0.028 | 0.192 ± 0.035 | 0.167 ± 0.028 | 0.199 ± 0.038 |
| Tibialis Weight (g) | 0.858 ± 0.108 | 0.956 ± 0.148 | 0.865 ± 0.130 | 1.002 ± 0.137 |
| Tibia Length (cm) | 4.34 ± 0.13 | 4.41 ± 0.10 | 4.34 ± 0.11 | 4.45 ± 0.04 |
| Soleus /tibia (mg/cm) | 40.52 ± 5.93 | 43.68 ± 8.06 | 38.42 ± 5.84 | 44.89 ± 8.81 |
| Tibialis/tibia (mg/cm) | 197.61 ± 22.07 | 216.67 ± 33.12 | 199.29 ± 26.98 | 225.25 ± 30.81 |
Values presented as mean ± SD. Sedentary control group (C; n = 10), sedentary obese (Ob; n=7), control submitted to resistance training (RT; n = 10) and obese submitted to RT (ObRT; n = 9). ANOVA followed by Tukey post hoc test.
Data are presented as the mean ± SD. Four (4) animals per group. Contractile cardiomyocytes from sedentary control rats (C; cells = 11), sedentary obese (Ob; cells = 34), control submitted to resistance training (RT; cells = 29) and obese submitted to RT (ObRT; cells = 41). A) Fractional shortening (FS) expressed as % of resting cell length. B) Maximum rate of contraction. C) Maximum rate of relaxation. D) Time to 50% contraction. E) Time to 50% relaxation. Two-way ANOVA followed by post hoc Tukey's test. p<0.05. * vs. C; †RT vs. Ob.
Cardiac protein expression of intracellular Ca2+ handling after 26 weeks of experimental protocol. A) Sarcoplasmic reticulum (SR) Ca2+-ATPase (SERCA2a); B) phospholamban (PLB); C) PLB serine-16 phosphorylation (pPLB Ser16); D) SERCA2a/PLB; E) pPLB Ser16/PLB. Sedentary control group (C, n = 5), sedentary obese (Ob, n = 4), control submitted to resistance training (RT, n = 4) and obese submitted to RT (ObRT, n = 5). Data presented as the mean ± SD. Two-way ANOVA complemented with the post hoc Tukey's test. p<0.05. * vs. C.
Discussion
The main findings of this study indicate that obesity promotes elevation of adiposity and hyperleptinemia, and RT was able to promote strength increase, reducing leptin levels and epididymal and visceral fat pads without changes in total adiposity and other comorbidities. Obesity caused increased deposit of myocardial collagen and reduced expression of pPLBser16, as well as of pPLBser16/PLB ratio, without contractile cardiomyocyte impairment. Surprisingly, RT was not able to reverse this damage.
The development of obesity was confirmed by higher body weight in Ob rats when compared with group C during the 3rd week of experimental protocol, reiterating the efficiency of high-fat diets to induce obesity in animal models [42-44]. In the current study, RT was not able to modulate the body weight in ObRT in comparison with Ob group from 17th to 26th weeks. These findings are in disagreement with the studies found in the literature, indicating that RT was a viable non-pharmacological strategy to reduce body weight [26,31,44]. On the other hand, with regard to the effect of RT associated with the high-fat diet, it was possible to verify the effectiveness of this type of training to reduce epididymal (40.5%) and visceral (37%) fat pads, but without changes in total adiposity. Our findings are in agreement with Speretta et al. [45], who observed that resistance training was able to decrease the area of visceral and epididymal adipocytes. In addition, Leite et al. [16] showed that RT, performed 3 times a week for 12 weeks, was able to reduce fat percentage and increase fat-free mass, determining factors of body composition.
In contrast, the retroperitoneal fat deposit did not change with RT. The literature reports that body fat depots present distinct metabolic behavior, evidenced by lower expression of β3 adrenergic receptors in retroperitoneal fat [46]. The authors emphasize that different body fat deposits may be more or less susceptible to lipolysis conditions according to the expression of β3 adrenergic receptors. Moreover, as explained by Tchernof et al. [47], visceral fat is more lipolytic than subcutaneous fat, considering the higher number of β-adrenergic receptors in this region. Another explanation for adiposity reduction induced by exercise may be related to its capacity to modulate mechanisms such as daily energy expenditure, resting metabolic rate and post-exercise oxygen consumption, contributing to body weight loss [48].
The literature has demonstrated that resistance training induces higher protein synthesis and muscle hypertrophy in several models [49,50]. In the current study, RT and ObRT increased the absolute and relative load and strength (RT> C; ObRT> Ob), but they did not be able to promote elevation in soleus and tibialis muscles (skeletal muscle mass). However, the relative load in the MCLT remained lower in ObRT compared with RT rats (ObRT < RT). Interestingly, this finding point out that the condition of obesity triggers reduced functionality, since despite the greater body weight in ObRT group, the capacity to produce strength was not higher than the RT group, possibly due to the large adiposity in ObRT. Miller et al. [51] highlighted that changes in body composition influence the physical functionality, which can result in reduced functionality and physical disability. Obesity, evidenced by the excessive accumulation of adipose tissue, is a condition that favors the development of less functional capacity [52-54], corroborating the findings with the current study. Bollinger ratified that absolute strength seems to be greater in obesity, however when the strength is normalized by body weight, the relative muscle strength is reduced [55]. In addition, other studies have demonstrated that in the condition of obesity, the normalization of the absolute load by the body weight in dynamic conditions, such as stair training, represents a relevant indicator of functional performance [56,57]. Nevertheless, the high-intensity RT sessions enable the strength development and gain in both groups (RT and ObRT). The high-intensity RT sessions enable the strength development and gain. Recent studies have shown that this type of training increases the rat's muscular strength [45,58-61]. Schoenfeld suggests that regardless of the magnitude of the load (low or high), muscle hypertrophy is achieved if training is done until voluntary muscle failure [62], but in experimental models, it is difficult to determine whether rats climb the ladder until failure. Adaptations to training are related to the amount of work performed during the exercise sessions [60]. In this sense, the physical exercise induces momentary adjustments in physiology, biology, and cellular biochemistry [56], and muscle strength gain may occur through neural and/or structural adaptations [64].
Obesity has been associated with several comorbidities, such as glucose intolerance, hyperinsulinemia, insulin resistance, dyslipidemia, and hypertension [65,66]. Obesity model presented only hyperleptinemia but without elevations in fasting glucose, total cholesterol and cardiovascular parameters, as well as reduction in HDL levels. Moreover, RT in obese animals did not cause changes in blood pressure, but it was able to significantly decrease plasma leptin levels compared with their respective control group. According to literature, the physical exercise is widely accepted to prevent or treat metabolic diseases, promoting different beneficial effects in obese patients and rats [16,45,67,68], but these alterations were not observed by RT used in this study. While the aerobic exercise has a clinically significant effect on cardiorespiratory fitness and metabolic control, resistance training improves insulin sensitivity and glucose tolerance, whereas improving lean body mass [69]. Sertié et al. [70] identified that 10 weeks of aerobic training performed 5 days a week was ineffective in altering plasma triglyceride and cholesterol levels in obese Wistar rats. Specifically, regarding the resistance training, Speretta et al. [71] showed that RT was be able to prevent the increase in total cholesterol in HFD rats, but it did not promote improvements in TG and HDL levels. These findings together with previous studies suggest that resistance training induces metabolic alterations in a time-dependent manner, accomplishing favorable modulation of metabolic serum levels, especially lipid profile, and thus promoting antiatherogenic effects [72]. Nevertheless, it has been well documented regarding this type of exercise that increased volume rather than increased intensity has greater impact on lipid profile [73]. Another explanation to absence of metabolic beneficial alterations could be related to the way they are mediated, that is, more strongly through exercise itself or exercise-induced weight loss and subsequent improvements in body composition [74]. Regarding glucose intolerance, Caponi et al. [75] showed a positive correlation between exercise and GLUT 4 expression in skeletal muscle and heart, showing that physical training improves insulin sensitivity and it has beneficial effects on metabolic syndrome. Therefore, we believe that the training volume (3 times a week, 4-5 climbs) in the current study was low to promote greater metabolic changes.
The literature reports that physiological cardiac hypertrophy may occur through stimulation generated by physical exercise resulting in normal or supranormal ventricular function [3]. On the other hand, the maladaptive remodeling can be associated with increase in left ventricle (LV) end-diastolic-pressure, pulmonary congestion, and LV hypertrophy [3,76]. In addition to myocyte hypertrophy, another element involved in the cardiac remodeling process is the interstitial connective tissue. Increases in collagen content may cause myocardial dysfunction due to impairment of the ventricular compliance, as well as changes in cardiac geometry. In this study, elevation of myocardial collagen was observed in obesity condition but without LV hypertrophy. Nevertheless, resistance training was not able to promote LV hypertrophy or to reverse or prevent the collagen accumulation in Ob group.
Some researchers point out that the overload generated on the heart depends on the type of training, aerobic or anaerobic [20,77,78]. In this context, in RT, there is increase in the pressure overload on the heart, which may generate physiological cardiac hypertrophy [20, 77,78]. An explanation for our results may be related to absence of pressure overloads, which are evidenced by normal systolic and diastolic arterial pressures among the experimental groups. Another mechanism may be related to leptin, which partially mediates the process of cardiac hypertrophy by the mitogen-activated protein kinase (MaPK p38), which regulates various cellular processes; however, our results showed that RT promotes reduction in leptin levels in obesity condition. Thus, absence of cardiac hypertrophy in this study can be explained by the total RT volume. According to Cassilhas et al. [79], some adaptive effects are not seen with different training volumes.
As regards the cardiomyocyte contractile function, obesity did not cause impairment of cardiac function demonstrated by similar fractional shortening percentage and lower time to 50% relaxation compared with group C, but RT alone promoted improvement in maximum rate of contraction and relaxation. After exercise stimulus, heart rate and cardiac contractility increase to meet the metabolic demands of the body, which is facilitated by elevation of cardiomyocyte contraction; it is partially achieved by the increased release of stored Ca2+ within the sarcoplasmic reticulum (SR) binding to components of the contractile apparatus and sarcomere remodeling [80,81]. However, in disagreement with our initial hypothesis, RT was not able to promote favorable adaptations to cardiovascular system in obesity condition as improvement in contractile function during excitation-contraction coupling, or to reverse the phospholamban phosphorylation regarding serine16 damage.
The literature has largely investigated the benefits of aerobic exercise training for cardiac function and proteins related to myocardial intracellular Ca2+ handling in both healthy and pathological conditions [13,21,22,82]. However, the effects of RT on Ca2+ handling and its association with myocardial intracellular proteins have been poorly investigated. Although the literature reports that physical exercise favors Ca2+ transport and increased Ca2+ availability for cardiac contractility [83,84], in this study, RT did not modulate the protein expression of SERCA2a and PLB in obesity condition. SERCA2a and PLB proteins are highlighted by their important function in mediating Ca2+ recapture for sarcoplasmic reticulum [85], and PLB regulates SERCA2a activity by phosphorylation and dephosphorylation of domain sites, which are found in the transmembrane and cytosolic medium [86]. Elevated SR calcium reuptake may also be caused by higher SERCA2 pumping capacity without any change in the amount of SERCA2 or PLB. The unphosphorylated form of PLB inhibits SERCA2 activity, whereas the phosphorylated form of PLB dissociates from inhibitor leading to increased pumping activity. PLB is phosphorylated at the serine residue via β-adrenergic pathway and at the threonine residue primarily via calcium/calmodulin kinase II.
In this research, only the serine phosphorylation16 was evaluated, reported as the main mediator of the positive effect on cardiac contractility and prevalent in relation to the threonine17 [87]. Our results showed that obesity condition impaired pPLBser16, represented by lower cardiac expression of this protein in Ob group, but it was notable to affect the contractile function, since it improved relaxation. Nevertheless, RT approach was not able to reverse the pPLBser16 damage or to improve the cardiac function. Although we did not analyze all these possible mechanisms involved in Ca2+ handling, it is possible that the phospholamban phosphorylation at threonine-17 (PLB Thr17) was elevated in RT and this finding did not allow reversion of PLB at Ser16 downregulation. In this sense, an important feature of PLB phosphorylation at Ser16 is that this site is more physiologically important than Thr17 mainly due to the lower level of PLB Thr17 [87,88]. Phosphorylation of PLB at Thr17 must be potentiated by Ser16 phosphorylation, and Thr17 phosphorylation has a negligible effect after Ser16 has been phosphorylated [14,88]. Another possible explanation for the failure to prevent and/or reverse the damage to phosphorylation at Ser16 visualized in obese rats may be related to improvement in β-adrenergic system regulation.
Conclusion
Resistance training represents a relevant non-pharmacological treatment in improving body composition and obesity biomarkers, expressed by reduced visceral and epididymal fat pad and plasma leptin levels. However, positive alterations in cardiomyocyte contractile and Ca2+ handling, as well as in reversing the collagen accumulation and phospholamban phosphorylation regarding serine16 damage in obesity condition were not observed.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa e Inovação do Espírito Santo - FAPES (grant number: 74434489/16) and partially financed by the Coordination for Improvement of Higher Education Personnel - Brazil (CAPES) - Finance Code 001.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Nguyen DM, El-Serag HB. The Epidemiology of Obesity. Gastroenterol Clin North Am. 2010;39:1-7
2. Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv Exp Med Biol. 2017;960:1-17
3. Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Ver. 2008;88:389-419
4. Alpert MA, Lambert CR, Panayiotou H. et al. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol. 1995;76:1194-7
5. Ferron AJ, Jacobsen BB, Sant'Ana PG. et al. Cardiac dysfunction induced by obesity is not related to β-adrenergic system impairment at the receptor-signalling pathway. Plos One. 2015;10:e0138605
6. Karimian S, Stein J, Bauer B, Teupe C. Improvement of impaired diastolic left ventricular function after diet-induced weight reduction in severe obesity. Diabetes Metab Syndr Obes. 2017;10:19-25
7. Lima-Leopoldo AP, Leopoldo AS, Sugizaki MM. et al. Myocardial dysfunction and abnormalities in intracellular calcium handling in obese rats. Arq Bras Cardiol. 2011;97:232-40
8. Pascual M, Pascual DA, Soria F. et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152-6
9. Leopoldo AS, Sugizaki MM, Lima-Leopoldo AP. et al. Cardiac remodeling in a rat model of diet-induced obesity. Can J Cardiol. 2010;26:423-9
10. Leopoldo AS, Lima-Leopoldo AP, Sugizaki MM. et al. Involvement of L-type calcium channel and SERCA2A in myocardial dysfunction induced by obesity. J Cell Physiol. 2011;226:2934-42
11. Ren J, Zhu BH, Relling DP. et al. High-fat diet-induced obesity leads to resistance to leptin-induced cardiomyocyte contractile response. Obesity. 2008;16:2417-23
12. Relling DP, Esberg LB, Fang CX. et al. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J Hypertens. 2006;24:549-61
13. Paulino EC, Ferreira JC, Bechara LR. et al. Exercise training and caloric restriction prevent reduction in cardiac Ca2+-handling protein profile in obese rats. Hypertension. 2010;56:629-35
14. Ablorh NA, Miller T, Nitu F, Gruber SJ, Karim C, Thomas DD. Accurate quantitation of phospholamban phosphorylation by immunoblot. Anal Biochem. 2012;425:68-75
15. Sayadi M, Feig M. Role of conformational sampling of Ser16 and Thr17-phosphorylated phospholamban in interactions with SERCA. Biochim Biophys Acta. 2013;1828:577-85
16. Leite RD, Durigan RC, de Souza Lino AD. et al. Resistance training may concomitantly benefit body composition, blood pressure and muscle MMP-2 activity on the left ventricle of high-fat fed diet rats. Metabolism. 2013;62:1477-84
17. Medeiros C, Frederico MJ, da Luz G. et al. Exercise training reduces insulin resistance and upregulates the mTOR/p70S6k pathway in cardiac muscle of diet-induced obesity rats. J Cell Physiol. 2011;226:666-74
18. Silveira AC, Fernandes T, Soci ÚPR. et al. Exercise training restores cardiac microrna-1 and microrna-29c to nonpathological levels in obese rats. Oxid Med Cell Longev. 2018;2018:4818310
19. Melo SF, Barauna VG, Júnior MA. et al. Resistance training regulates cardiac function through modulation of miRNA-214. Int J Mol Sci. 2015;16:6855-67
20. Fernandes T, Soci UP, Oliveira EM. Eccentric and concentric cardiac hypertrophy induced by exercise training: microRNAs and molecular determinants. Braz J Med Biol Res. 2011;44:836-847
21. Locatelli J, de Assis LV, Isoldi MC. Calcium handling proteins: structure, function and modulation by exercise. Heart Fail Rev. 2014;19:207-25
22. Wisløff U, Støylen A, Loennechen JP. et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. Circulation. 2007;115:3086-94
23. Natali AJ. Efeitos do exercício crônico sobre os miócitos cardíacos: uma revisão das adaptações mecânicas. Braz J Sci Movement. 2004;12:91-6
24. Kemi OJ, Ellingsen O, Ceci M. et al. Aerobic interval training enhances cardiomyocyte contractility and Ca2+ cycling by phosphorylation of CaMKII and Thr-17 of phospholamban. J Mol Cell Cardiol. 2007;43:354-61
25. Ahmadiasl N, Najafipour H, Soufi FG. et al. Effect of short- and long-term strength exercise on cardiac oxidative stress and performance in rat. J Physiol Biochem. 2012;68:121-8
26. De Souza MR, Pimenta L, Pithon-Curi TC. et al. Effects of aerobic training, resistance training, or combined resistance-aerobic training on the left ventricular myocardium in a rat model. Microsc Res Tech. 2014;77:727-34
27. Alves JP, Nunes RB, Stefani GP. et al. Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: experimental model of heart failure. PLoS One. 2014;9:e110317
28. Mostarda CT, Rodrigues B, de Moraes OA. et al. Low intensity resistance training improves systolic function and cardiovascular autonomic control in diabetic rats. J Diabetes Complications. 2014;28:273-78
29. Van Der Heijden GJ, Wang ZJ, Chu Z. et al. Strength exercise improves muscle mass and hepatic insulin sensitivity in obese youth. Med Sci Sports Exerc. 2010;42:1973-80
30. Perreault K, Courchesne-Loyer A, Fortier M. et al. Sixteen weeks of resistance training decrease plasma heat shock protein 72 (eHSP72) and increase muscle mass without affecting high sensitivity inflammatory markers' levels in sarcopenic men. Aging Clin Exp Res. 2016;28:207-14
31. Panveloski-Costa AC, Pinto Júnior DAC, Brandão BB. et al. Resistive training reduces inflammation in skeletal muscle and improves the peripheral insulin sensitivity in obese rats induced by hyperlipidic diet. Arq Bras Endocrinol Metab. 2011;55:155-63
32. Benevenga NJ, Calvert C, Eckhert CD. et al. Nutrient requirements of the laboratory rat. DC Natl Acad Press: Washington. 1995
33. Hornberger TA Jr, Farrar RP. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can J Appl Physiol. 2004;29:16-31
34. Malkoff J. Non-invasive blood pressure for mice and rats. Animal Lab News. 2005:1-8
35. Sainas G, Milia R, Palazzolo G. et al. Mean blood pressure assessment during post-exercise: result from two different methods of calculation. J Sports Sci Med. 2016;15:424-33
36. Pitombo C, Araújo EP, Souza CT, Pareja JC, Geloneze B. Amelioration of diet-induced diabetes mellitus by removal of visceral fat. J Endocrinol. 2006;191:699-706
37. Carroll JF, Zenebe WJ, Strange TB. Cardiovascular function in a rat model of diet-induced obesity. Hypertension. 2006;48:65-72
38. Boustany-Kari CM, Gong M, Akers WS. et al. Enhanced vascular contractility and diminished coronary artery flow in rats made hypertensive from diet-induced obesity. Int J Obes. 2007;31:1652-9
39. Taylor BA, Phillips SJ. Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics. 1996;34:389-98
40. Matsubara LS, Matsubara BB, Okoshi MP. et al. Alterations in myocardial collagen content affect rat papillary muscle function. Am J Physiol Heart Circ Physiol. 2000;279:H1534-9
41. Louch WE, Sheehan KA, Wolska BM. Methods in cardiomyocyte isolation, culture, and gene transfer. J Mol Cell Cardiol. 2011;51:288-98
42. Gamelin FX, Aucouturier J, Iannotti FA. et al. Effects of chronic exercise on the endocannabinoid system in Wistar rats with high-fat diet-induced obesity. J Physiol Biochem. 2016;72:183-99
43. Martins F, Campos DH, Pagan LU. et al. High-fat diet promotes cardiac remodeling in an experimental model of obesity. Arq Bras Cardiol. 2015;105:479-86
44. da Silva VL, Lima-Leopoldo AP, Ferron AJT. et al. Moderate exercise training does not prevent the reduction in myocardial L-type Ca2+ channels protein expression at obese rats. Physiol Rep. 2017;5:e13466
45. Speretta GF, Rosante MC, Duarte FO. et al. The effects of exercise modalities on adiposity in obese rats. Clinics (Sao Paulo). 2012;67:1469-77
46. Cousin B, Casteilla L, Dani C. et al. Adipose tissues from various anatomical sites are characterized by different patterns of gene expression and regulation. Biochem J. 1993;292:873-6
47. Tchernof A, Bélanger C, Morisset AS. et al. Regional differences in adipose tissue metabolism in women minor: effect of obesity and body fat distribution. Diabetes. 2006;55:1353-60
48. Hauser C, Benetti M, Rebelo FPV. Weigth loss strategies. BrazJ Kinanthrop Human Performance. 2006;6:72-81
49. McGlory C, Devries MC, Phillips SM. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. J Appl Physiol. (1985). 2017;122:541-8
50. Hulston CJ, Woods RM, Dewhurst-Trigg R. et al. Resistance exercise stimulates mixed muscle protein synthesis in lean and obese young adults. Physiol Rep. 2018;6:e13799
51. Miller CT, Fraser SF, Levinger I. et al. The effects of exercise training in addition to energy restriction on functional capacities and body composition in obese adults during weight loss: a systematic review. PLoS One. 2013;8:e81692
52. Himes CL. Obesity, disease, and functional limitation in later life. Demography. 2000;37:73-82
53. Hulens M, Vansant G, Lysens R. et al. Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach. Int J Obes Relat Metab Disord. 2001;25:676-81
54. Maffiuletti NA, Jubeau M, Munzinger U. et al. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol. 2007;101:51-9
55. Bollinger LM. Potential contributions of skeletal muscle contractile dysfunction to altered biomechanics in obesity. Gait Posture. 2017;56:100-7
56. Capodaglio P, Vismara L, Menegoni F. et al. Strength characterization of knee flexor and extensor muscles in Prader-Willi and obese patients. BMC Musculoskelet Disord. 2009;10:47
57. Lafortuna CL, Fumagalli E, Vangeli V. et al. Lower limb alactic anaerobic power output assessed with different techniques in mordib obesità. J Endocrinol Invest. 2002;25:134-41
58. Grans CF, Feriani DJ, Abssamra ME. et al. Resistance training after myocardial infarction in rats: its role on cardiac and autonomic function. Arq Bras Cardiol. 2014;103:60-8
59. Nascimento V, Krause Neto W, Gonçalves L. et al. Morphoquantitative analysis revealed Triceps Brachialis muscle hypertrophy by specific Resistance training equipment in rats. J Morphol Sci. 2013;30:276-80
60. Philippe AG, Py G, Favier FB. et al. Modeling the responses to resistance training in an animal experiment study. Biomed Res Int. 2015;2015:914860
61. Garber CE, Blissmer B, Deschenes MR. et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334-59
62. Grgic J, Schoenfeld BJ. Are the Hypertrophic Adaptations to High and Low-Load Resistance Training Muscle Fiber Type Specific? Front Physiol. 2018;9:402
63. Krause Neto W, Gama EF. Strength training and anabolic steroid do not affect muscle capillarization of middle-aged rats. Rev Bras Med Esporte. 2017;23:137-41
64. Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med. 2007;37:145-68
65. Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798-808
66. Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270-99
67. Fan W, Evans RM. Exercise mimetics: impact on health and performance. Cell Metab. 2016;25:242-7
68. Al Saif A, Alsenany S. Aerobic and anaerobic exercise training in obese adults. J Phys Ther Sci. 2015;27:1697-700
69. Zanuso S, Sacchetti M, Sundberg CJ. et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. A review of the evidence. Br J Sports Med. 2017;51:1533-8
70. Sertié RAL, Paulino EC, Brum PC. et al. Exercise training and caloric restriction reduce adiposity index and hepatic lipids in obese rats. Immunoendocrinology. 2015;2:e1053
71. Speretta GF, Silva AA, Vendramini RC. et al. Resistance training prevents the cardiovascular changes caused by high-fat diet. Life Sci. 2016;146:154-62
72. Ihalainen JK, Inglis A, Mäkinen T. et al. Strength training improves metabolic health markers in older individual regardless of training frequency. Front Physiol. 2019;10:32
73. Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211-21
74. Kettunen J, Joensuu A, Hagnäs M. et al. Associations of increased physical performance and change in body composition with molecular pathways of heart disease and diabetes risk. Am J Physiol Endocrinol Metab. 2019;316:E221-9
75. Caponi PW, Lehnen AM, Pinto GH. et al. Aerobic exercise training induces metabolic benefits in rats with metabolic syndrome independent of dietary changes. Clinics (Sao Paulo). 2013;68:1010-7
76. Azevedo PS, Polegato BF, Minicucci MF. et al. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq Bras Cardiol. 2016;106:62-9
77. Mihl C, Dassen WR, Kuipers H. Cardiac remodelling: concentric versus eccentric hypertrophy in strength and endurance athletes. Neth Heart J. 2008;16:129-33
78. Pluim BM, Zwinderman AH, van der Laarse A. et al. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336-44
79. Cassilhas RC, Reis IT, Venâncio D. et al. Animal model for progressive resistance exercise: a detailed description of model and its implications for basic research in exercise. Motriz. 2013;19:178-84
80. Kemi OJ, Wisloff U. Mechanisms of exercise induced improvements in the contractile apparatus of the mammalian myocardium. Acta Physiol. 2010;199:425-39
81. Moore RL, Palmer BM. Exercise training and cellular adaptations of normal and diseased hearts. Exerc Sport Sci Rev. 1999;27:285-315
82. Sugizaki MM, Leopoldo AP, Conde SJ. et al. Upregulation of mRNA myocardium calcium handling in rats submitted to exercise and food restriction. Arq Bras Cardiol. 2011;97:46-52
83. Sordahl LA, Asimakis GK, Dowell RT. et al. Functions of selected biochemical systems from the exercised-trained dog heart. J Appl Physiol Respir Environ Exerc Physiol. 1977;42:426-31
84. Studer R, Reinecke H, Bilger J. et al. Gene expression of the cardiac Na(+)-Ca2+ exchanger in end-stage human heart failure. Circ Res. 1994;75:443-53
85. Katz AM. Excitation-contraction coupling: extracelular and intracellular calcium cycles. In: Katz AM (ed). Physiology of the heart. Philadelphia: Lippincott Williams & Wilkins. 2011:143-74
86. Wuytack F, Raeymaekers L, Missiaen L. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium. 2002;32:279-305
87. Calaghan SC, White E, Colyer J. Co-ordinated changes in cAMP,phosphorylated phospholamban, Ca2+ and contraction following betaadrenergic stimulation of rat heart. Pflugers Arch. 1998;436:948-56
88. Mayer EJ, Huckle W, Johnson RG Jr. et al. Characterization and quantitation of phospholamban and its phosphorylation state using antibodies. Biochem Biophys Res Commun. 2000;267:40-8
Author contact
![]() Corresponding author: André Soares Leopoldo, PhD, Department of Sports, Centre for Physical Education and Sports, UFES - Federal University of Espírito Santo, Vitória, Espírito Santo, Brazil. CEP: 29075-910 Tel: +55 (27) 4009-7882/ Fax: +55 (27) 4009-2620. E-mail address: andre.leopoldobr.
Corresponding author: André Soares Leopoldo, PhD, Department of Sports, Centre for Physical Education and Sports, UFES - Federal University of Espírito Santo, Vitória, Espírito Santo, Brazil. CEP: 29075-910 Tel: +55 (27) 4009-7882/ Fax: +55 (27) 4009-2620. E-mail address: andre.leopoldobr.

 Global reach, higher impact
Global reach, higher impact