Impact Factor
ISSN: 1449-1907
Int J Med Sci 2020; 17(6):712-719. doi:10.7150/ijms.44088 This issue Cite
Research Paper
Expression of CD4+CD25+CD127Low regulatory T cells and cytokines in peripheral blood of patients with primary liver carcinoma
1. Clinical laboratory, the First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou 510080, China
2. Department of Endocrinology, Shenzhen Second People's Hospital, The First Affiliated Hospital of Shenzhen University, Health Science Center of Shenzhen University, Shenzhen 518035, People's Republic of China.
3. Clinical laboratory, Guangzhou Military Area Inspection Center, the General Hospital of Guangzhou Military Region, Guangzhou 510010, China
4. Clinical laboratory, the Hospital of Dongguan Renkang, Dongguan 523952, China
5. Institute of Biotherapy, Southern Medical University Guangzhou 510515, China
6. State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Department of Clinical Laboratory Medicine, Sun Yat-Sen University Cancer Center, Guangzhou 510060, China
*Contributed equally
Received 2020-1-8; Accepted 2020-2-16; Published 2020-2-24
Abstract
Objective: To assess the clinical utility of the ratio of CD4+CD25+CD127low regulatory T cells (Tregs) in subjects at high risk of HCC, investigate the relationship between the percentage of Tregs and the expression of transforming growth factor (TGF)-β1 and interleukin (IL)-10 in patients with hepatocellular carcinoma before and after treatment.
Methods: Peripheral venous blood was collected from patients with liver cancer before and after treatment. The proportion of CD4+CD25+CD127low Tregs was detected by flow cytometry. The levels of TGF-β1 and IL-10 in serum were detected by enzyme-linked immunosorbent assay, and were compared with healthy subjects as a control group.
Results: The proportion of CD4+CD25+CD127low to CD4+T lymphocytes in patients with hepatocellular carcinoma was significantly higher than that in healthy controls (P<0.01). The proportion of CD4+CD25+CD127lowTregs, whose AUC of ROC curve was 0.917, could effectively separate the HCC patients from the healthy subjects with a diagnostic sensitivity of 90%, specificity of 80%. The proportion of CD4+CD25+CD127low to CD4+T lymphocytes and the levels of TGF-β1 and IL-10 in patients with hepatocellular carcinoma after the operation and chemotherapy were significantly lower than those before treatment (P<0.05).The proportion of CD4+CD25+CD127lowTregs was positively correlated with the concentrations of TGF-β1 and IL-10 before and after treatment of primary liver cancer (P<0.05).
Conclusion: CD4+CD25+CD127lowTregs may be a significant predictor of HCC biopsy outcome and play an inhibitory role on effector T cells by regulating cytokines.
Keywords: CD4+CD25+CD127low regulatory T cells, cytokines, flow cytometry, peripheral blood, primary hepatic carcinoma
Introduction
The annual incidence of primary liver cancer ranks fifth among all malignant tumors in the world, and the mortality rate ranks third [1]. Surgical treatment is the only hope for long-term survival of patients with liver cancer; however, most patients with liver cancer in China are diagnosed at an advanced stage, and they have lost the chance for successful radical surgical resection [2]. CD4+CD25+ regulatory T cells (Tregs) are a subset of T lymphocytes with immunosuppressive properties that play an important role in the regulation of peripheral immune responses[3]. CD127 is a recently discovered antigen associated with Tregs that is weakly expressed on Tregs, while the self-activated memory T cell CD127 is strongly expressed; therefore, CD4+CD25+CD127 low/- is used to represent Tregs now[4, 5]. Yu et al. reported that CD127 with low or no expression on the CD4+CD25+ marker is more specific than the combined markers CD39, CD73, and CD25+ for recognizing Tregs more accurately. The CD4+CD25+CD127-T cell population has the most typical characteristics of Tregs. At the same time, the expression level of the intracellular Foxp3 protein is also significantly higher than that in other cell populations. We conclude that simultaneous labeling of CD4+CD25+CD127low on the cell surface is more accurate and more realistic for detecting Tregs than labeling of CD4+CD25+Foxp3.
The mechanism of action of CD4+CD25+Treg cells is unclear. It may be the secretion of immunosuppressive factors, such as transforming growth factor (TGF)-β (mainly TGF-β1), interleukin (IL)-10, and interferon (IFN)-γ into cytokines or on the cell surface. It plays an immunomodulatory role, and TGF-β promotes the development of regulatory T cells, maintains the expression of Foxp3 in CD4+CD25+Treg cells and, thus, regulatory T cells have a stable immunosuppressive function [6].
Tregs promote tumorigenesis and development by inhibiting the immune response of tumors. The role of Tregs in tumor immune escape is related to the secretion of inhibitory cytokines (mainly TGF-β and IL-10) by Tregs [7]. IL-10 has an immunosuppressive function, and TGF-β directly inhibits activation of CD8+ cytotoxic T lymphocytes, significantly reducing the effect of tumor immunity[8]. TGF-β1 is a potent immunosuppressive factor that affects the proliferation, activation, and differentiation of innate and acquired immune cells and inhibits the production of immunoregulatory cytokines. IL-10 is an inhibitory cytokine produced by helper T2 cell subsets. Regulatory T cells produce immunosuppressive effects by secreting cytokines, such as IL-10, which inhibit inflammation and reduce the number of antigen-specific T cells [9-11].
To date, there is still no report on the clinical utility of CD4+CD25+CD127LowTregs. The relationship between CD4+CD25+CD127LowTregs and TGF-β1 and IL-10 levels remains unclear. Therefore, in this study, we observed the proportion of CD4+CD25+CD127LowTregs in patients with primary hepatocellular carcinoma (HCC) before and after treatment and in healthy people, evaluate the clinical performance of CD4+CD25+CD127LowTregs in patients at high risk of HCC. Then, the concentrations of TGF-β1 and IL-10 in patients with HCC before and after treatment were examined. The relationship between CD4+CD25+CD127LowTregs and TGF-β1 and IL-10 levels, and the analysis of the differences in immune status of patients with liver cancer are expected to provide valuable and diagnostic reference data for clinical treatment, the disease course, and prognosis of liver cancer.
Materials and Methods
Clinical information
Sixty-nine patients (age, 31-68 years) confirmed by histopathological examination and diagnosed as the primary liver cancer were collected from the First Affiliated Hospital of Guangdong Pharmaceutical University. Patients were excluded if they had liver damage caused by drugs or ethanol and were treated with antiviral or immunosuppressive agents within 6 months. Peripheral blood of 87 healthy volunteers was collected as a control group. All liver functions, liver disease autoantibodies, routine blood scan, chest X-ray, abdominal B-ultrasound, electrocardiogram, and urine were normal.
This study was approved by the Institutional Ethics Review Board of the First Affiliated Hospital of Guangdong Pharmaceutical University and every subject signed a written informed consent.
Reagents
The reagents were CD4-FITC, CD25-PE, mouse anti-human CD127-PerCP-CyTM 5.5, mouse IgG1-PE, mouse IgG1-PerCP-Cy5.5, hemolytic agent, and sheath fluid (BD Biosciences, San Diego, CA, USA). TGF-β1 and IL-10 kits were purchased from IBL (IBL International GmbH, Hamburg, Germany).
Experimental method
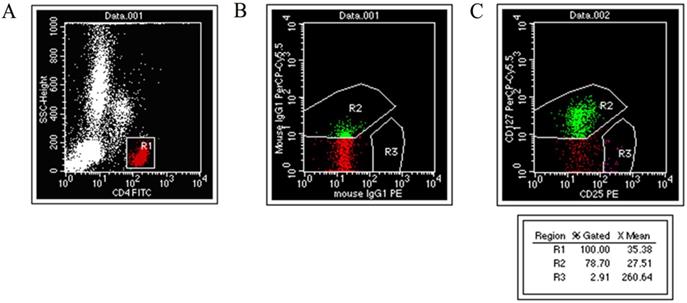
Two mL of venous blood was taken into EDTA-K3 coated tubes from fasting subjects in the morning. Each blood specimen was set up with two assay tubes and an isotype control. After adding 100 μL of anticoagulant to each tube, 10 μL of CD4-FITC, 10 μL of CD25-PE, PerCP-CyTM 5.5, and 2 μL of mouse anti-human CD127 were added to each assay tube. The same type of control was added to 10 μL of CD4-FITC and mouseIgG1-PE, along with 2.2 μL of mouseIgG1 percp-Cy5.5. The solutions were shaken and placed in the dark at room temperature for 15 min. One mL of hemolytic agent was added and vortexed in the dark at room temperature for 10 min. Then, the tube was centrifuged at 2,000 r/min for 2 min, the supernatant was discarded, 1 mL of sheath liquid was added and centrifuged at 2,000 r/min for 2 min, the supernatant was discarded, and 500 μL of sheath liquid was added, mixed well, and analyzed on the machine (Fig. 1).
Consistency analysis of CD127 and FOXP3
CD4+CD25+CD127LowTregs and CD4+CD25+Foxp3+Tregs were harvested from 10,000 cells/tube using CD4 gating. Cellquest software was applied to set the gate for CD4+FITC cells in the scatter diagram of the two CD4/SSC tubes, which is represented by a histogram and overlay histogram.
Statistical Analysis
The statistical analysis was performed using SPSS18.0 software (SPSS Inc. Chicago, IL, USA). The data are expressed as mean ± standard deviation. The t-test of two sample means was used between the two groups. A paired t-test was used before and after treatment. One-way analysis of variance was used for multiple groups. A correlation analysis was conducted using Pearson's method. A P-value < 0.05 was considered significant. The area under the receiver-operating characteristic (ROC) curves (AUC) was quantified to evaluate the diagnostic performance of CD4+CD25+CD127LowTregs ratio. The performance characteristics of CD4+CD25+CD127LowTregs ratios were evaluated by determining the diagnostic sensitivity, specificity and diagnostic odds ratio (DOR) with 95% CIs.
Results
Clinical Characteristics of the Study Cohort
The clinical characteristics of patients with HCC were summarized in Table 1. When the subjects are younger than 49 years' old, the age between the HCC patients and the healthy people has no significant difference (p>0.05), the gender distribution has significant difference between HCC patients and the healthy people (p<0.01), the AFP levels in the HCC were significantly higher than the controls (p<0.01); When the subjects are older than 50 years' old, the age between the HCC patients and the healthy people has no significant difference (p>0.05), the gender distribution has significant difference between HCC patients and the healthy people (p<0.01), the AFP levels in the HCC were significantly higher than the controls (p < 0.01).
Consistency between CD127 and Foxp3 expression
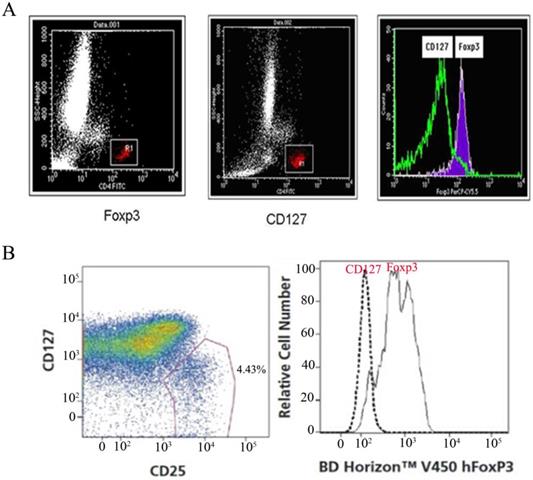
It has been reported that high expression of Foxp3 (Foxp3high) and low expression of CD127 (CD127low) may originate from the same group of cells [12]. CD4+CD25+Foxp3 and CD4+CD25+CD127 were used as phenotype markers to identify regulatory T cells in normal human peripheral blood whole blood specimens. The consistency analysis of CD127 and Foxp3 cells by BD FACS Calibur flow cytometry showed that the two groups of cells were almost in the same region (Fig. 2).
Experimental analysis of CD4+CD25+CD127low regulatory T cells. (A) CD4+ / SSC scatter diagram. (B) Isotype Control. (C) CD4+CD25+CD127low cell.
Clinical Characteristics of Study Population
| ≤ 49 years old | ≥ 50 years old | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Liver cancer | p | control | Liver cancer | p | |||
| Age | 41.29+7.96 | 42.12+5.82 | >0.05 | 61.06+8.20 | 60.25+6.65 | >0.05 | ||
| Sex | Male | 23 | 30 | 22 | 26 | |||
| Female | 22 | 7 | <0.01 | 20 | 6 | <0.01 | ||
| AFP(ng/mL) | 6.74±1.52 | 907.83±1172.70 | <0.01 | 7.79±2.95 | 1073.32±1565.13 | <0.01 | ||
Consistency of expression between CD127low and Foxp3+ regulatory T cells. (A) CD127+ and Foxp3+ regulatory T cells from one patient were investigated by FCS(BD FACS Calibur), consistency of expression between CD127low and Foxp3+ regulatory T cells were analyzed by overlaying the two histograms.(B) CD127+ and Foxp3+ regulatory T cells from one patient were also investigated by FCS(FACSCantoTM II), consistency of expression between CD127low and Foxp3+ Tregs were analyzed.
Comparison of CD4+CD25+CD127lowTregs in the peripheral blood of the primary liver cancer and healthy control groups
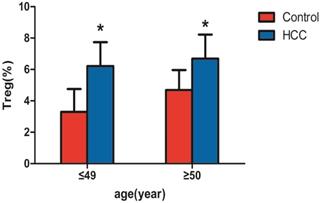
Among the 69 patients with liver cancer, 37 patients were ≤ 49 years old and 32 patients were ≥50 years old. Significant differences were observed between the two groups by the independent samples t-test (all P < 0.05). As shown in Table 2 and Figure 3, CD4+CD25+CD127lowTregs in peripheral blood of patients with liver cancer in those≤ 49 years old (6.21±1.53%) were significantly higher than those in health subjects with the same age (3.30±1.45%; P<0.05). CD4+CD25+CD127lowTregs in peripheral blood of patients with liver cancer in those≥50 years old (6.69±1.53%) were significantly higher than those in health subjects with the same age (4.69±1.27%; P<0.05).
Changes in CD4+CD25+CD127lowTregs before and after treatment of liver cancer
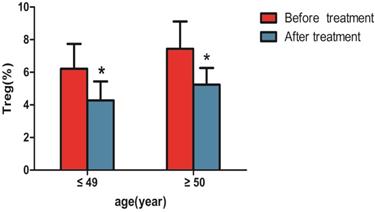
Changes in CD4+CD25+CD127lowTregs were observed before and after 1 month of treatment., as shown in Table 3 and Figure 4, the percentage of CD4+CD25+CD127lowTregs in patients aged ≤ 49 years (6.21±1.53%) before treatment were higher than that after treatment (4.27±1.17%; P<0.05). the percentage of CD4+CD25+CD127lowTregs in patients aged ≥50 years (7.44 ± 1.67%) before treatment were higher than that after treatment (5.24 ± 1.02%; P<0.05).
Comparison of CD4+CD25+CD127low regulatory T cells in peripheral blood of liver cancer patients and healthy control group.
| Group | N | CD4+CD25+CD127low regulatory T cells (%, x±s) |
|---|---|---|
| control group (aged ≤ 49 years) | 45 | 3.30±1.45 |
| Liver cancer group (aged ≤ 49 years) | 37 | 6.21±1.53* |
| control group (aged ≥50 years) | 42 | 4.69±1.27 |
| Liver cancer group (aged ≥50 years) | 32 | 6.69±1.53* |
Note: * indicates that the difference between the liver cancer group and the normal group at the same age is statistically significant (P<0.05)
Comparison of CD4+CD25+CD127low regulatory T cells in peripheral blood of liver cancer patients before and after treatment(%, x±s).
| Group | CD4+CD25+CD127low regulatory T cells (%, x±s) |
|---|---|
| before treatment (aged ≤ 49 years) | 6.21±1.53 |
| after treatment (aged ≤ 49 years) | 4.27±1.17* |
| before treatment (aged ≥50 years) | 7.44±1.67 |
| after treatment (aged ≥50 years) | 5.24±1.02* |
Note: * indicates statistically significant difference before and after treatment in the same age group (P<0.05)
Comparison of TGF-β1 and IL-10 concentrations before and after treatment of liver cancer
| group | TGF-β1(ng/L) | IL-10(ng/L) |
|---|---|---|
| Before treatment | 1260.80±359.87 | 577.49±161.74 |
| After treatment | 896.96±287.25* | 480.43±177.79** |
Note: * indicates that TGF-β1 is statistically significant before and after treatment (P<0.05); **IL-10 is statistically significant before and after treatment (P<0.01)
Comparison of CD4+CD25+CD127low regulatory T cells in peripheral blood of patients with liver cancer and the healthy control group. Note: * indicates that patients with liver cancer ≤ 49 years old (n=37) were significantly different compared with the healthy control group (n=45) (P < 0.05), and patients with liver cancer ≥ 50 years old (n=32) were significantly different compared with the healthy control group (n=42) (P < 0.05).
Comparison of CD4+CD25+CD127low regulatory T cells in peripheral blood of patients with liver cancer before and after treatment. Note: * indicates that with liver cancer patients ≤ 49 years old after treatment were significantly different compared with the patients before treatment (n=37, P < 0.05) , and patients with liver cancer ≥ 50 years old after treatment were significantly different compared with the patients before treatment (n=32, P < 0.05).
Clinical Performance Evaluation of CD4+CD25+CD127lowTregs
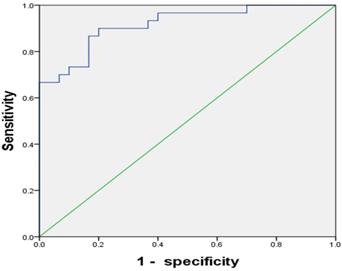
To test the clinical utility of CD4+CD25+CD127lowTregs in patients with HCC, the ROC curve was analyzed. The results showed the AUC for CD4+CD25+CD127lowTregs ratio was 0.917(95% CI, 0.848-0.986), with a diagnostic sensitivity of 90%, specificity of 80% (Figure 5).
Comparison of TGF-β1 and IL-10 concentrations before and after treatment in patients with primary liver cancer
As shown in Table 4, the TGF-β1 concentration before treatment of primary liver cancer (1,260.80± 359.87 ng/L) was higher than that after treatment (896.96± 287.25 ng/L; P < 0.05). The IL-10 concentration before treatment of primary liver cancer (577.49 ± 161.75 ng/L) was higher than that after treatment (480.43 ± 177.79 ng/L; P < 0.05).
The ROC curve of CD4+CD25+CD127lowTregs ratios whose AUC was 0.917. The best sensitivity and specificity at the best cutoff point were 90% and 80% respectively.
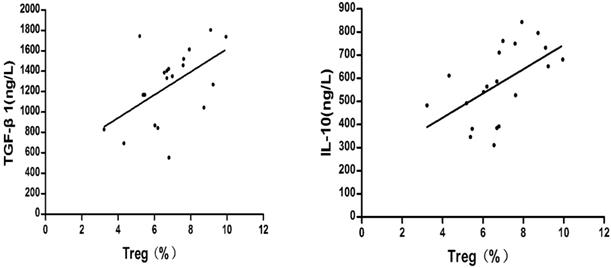
Correlation between the percentage of CD4+CD25+CD127lowTregs and the concentrations of TGF-β1 and IL-10
The relationships between the percentage of CD4+CD25+CD127lowTregs and the concentrations of TGF-β1 and IL-10 were compared. Pearson's method was used for the correlation analysis.
As shown in Fig.6, before liver cancer treatment, the TGF-β1 and IL-10 concentrations were positively correlated with the percentage of CD4+CD25+ CD127lowTregs in patients with HCC respectively ((r = 0.526, P = 0.017; r = 0.546, P = 0.013).
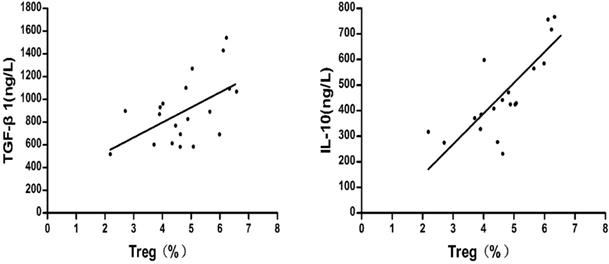
As shown in Fig.7, after liver cancer treatment, the TGF-β1 and IL-10 concentrations were positively correlated with the percentage of CD4+CD25+ CD127lowTregs in patients with HCC respectively (r = 0.543, P = 0.013; r = 0.789, P=0.000).
Discussion
Tregs inhibit the host's anti-tumor immune response, and have a clear correlation with the disease process. CD4+CD25+Tregs can be detected in peripheral blood of patients with gastric cancer, lung cancer, ovarian cancer, liver cancer, pancreatic cancer and breast cancer, local tumors, invasive lymph nodes, and drainage lymph nodes, and the number of CD4+CD25+Treg cells is negatively correlated with the disease course and the prognosis; the higher the number of Tregs, the worse the prognosis [13].
Liver cancer is a common tumor, as it has the third highest incidence in China, and the mortality rate is second among malignant tumors. The prognosis for liver cancer is poor. Tregs are a subset of CD4+ T cells with immunoregulatory functions that inhibit the activation and proliferation of anti-tumor effector cells and are associated with tumor immune escape[14]. Ormandy[15] et al. reported that the number of Tregs in peripheral blood of patients with liver cancer increases significantly. The number of Tregs in patients with liver cancer may be a prognostic indicator of HCC [16]. However, other studies have shown that the percentage of regulatory T cells in peripheral blood of patients with primary liver cancer is not different from that of a healthy control group [17]. Differences in the criteria for enrolling patients, different methods for detecting Tregs, and different cases are possible reasons for this difference.
At present, the technology for detecting Tregs by flow cytometry is insufficient. The main problem is the lack of specific markers. Previous studies on Tregs have mainly focused on Foxp3 in CD4+CD25+ cells. Foxp3 is considered a key marker molecule specifically expressed in CD4+CD25 +Tregs, which is closely related to cell differentiation, development, and functional maturation [18]. However, as Foxp3 is intracellular expressed, it is necessary to rupture the membrane during detection and analysis; the detection process will affect cell activity, thus affecting the accuracy of the test results [19]. Therefore, to study the role of Tregs in tumor diseases more effectively, it is necessary to find a more sensitive Tregs surface marker.
The CD127 molecule on the surface of the CD4+CD25+ cell population is also a specific marker for this group of cells. A good correlation has been observed between high expression of Foxp3 and low expression of CD127 in this group of cells; that is, high expression of Foxp3 (Foxp3high) and low expression of CD127 (CD127low) may be the same group of cells [12]. CD127 is mainly expressed on the surface of mature T cells in normal human peripheral blood, and in vitro experiments show that regulatory T cells with inhibitory activity express low levels of CD127 on the surface. Therefore, CD4+CD25+CD127Low is currently considered to be a more valuable molecular surface marker to detect regulatory T cells more specifically than CD4+CD25+Foxp3+. Our study showed that CD4+CD25+Foxp3 and CD4+CD25+CD127Low were phenotypic markers for identifying regulatory T cells in the same normal human peripheral blood sample. The two groups of cells are roughly in the same region, as shown in Figure 2. Foxp3 and CD127 are expressed by regulatory T cells.
Correlation between the percentage of regulatory T cells and the concentrations of TGF-β1 and IL-10 before treatment of liver cancer.
Correlation between the percentage of regulatory T cells and the concentrations of TGF-β1 and IL-10 after treatment of liver cancer.
In this study, the proportion of peripheral blood CD4+CD25+CD127lowTregs accounted for CD4+ T lymphocytes in patients with primary liver cancer (6.21 ± 1.53% for ≤ 49 years and 6.69 ± 1.53% for ≥ 50 years) and healthy control groups (3.30 ± 1.45% for ≤ 49 years and 4.69 ± 1.27% for ≥ 50 years) (P <0.05). These results suggest that the number of CD4+CD25+CD127low Treg regulatory T cells in the peripheral blood of patients with liver cancer is significantly higher than that of the control group. Regulatory T cells declined in patients with liver cancer treated for 1 month. Because the subjects of this study were patients with primary liver cancer who were initially diagnosed and not treated clinically, the effects of an immunomodulator and other factors, such as chemotherapy, were excluded. The results suggest that CD4+CD25+CD127lowTregs are closely related to the occurrence and development of tumors.
Though the role of CD4+CD25+CD127lowTregs on the cancers has been reported, its clinical utility has not been fully elucidated. In the present study, we retrospectively investigated the clinical performance of CD4+CD25+CD127lowTregs in the population undergoing initial or repeated biopsy and at high risk of HCC. The results showed the AUC for CD4+CD25+CD127lowTregs ratio was 0.917(95% CI, 0.848-0.986), with a diagnostic sensitivity of 90%, specificity of 80% (Figure 5). To our best knowledge, it is the first report on the clinical utility of CD4+CD25+CD127lowTregs.
The mechanism of action of Tregs may be that of secreted inhibitors, such as the cytokines TGF-β1, IL-10, and IFN-γ [20], which play an immunomodulatory role. CD127 is the IL-7 receptor alpha chain, and the specific response of T lymphocytes to IL-7 is mainly achieved through CD127. Studies have confirmed that the expression of TGF-β1 is closely related to the proliferation and differentiation of CD4+CD25+Tregs [21]. Overexpression of TGF-β1 in peripheral lymphoid organs increases peripheral Treg and Foxp3 expression, and blocking the T cell TGF-β1 signaling pathway produces the opposite effect, indicating that TGF-β1 plays an important role in regulating the peripheral blood CD4+CD25+ T cell pool and Foxp3 expression [22].
In a study of ulcerative colitis, CD4+CD25+ Tregs in rat peripheral blood were negatively correlated with IL-10 levels [23]. The level of CD4+CD25+CD127-Tregs in peripheral blood of patients with lung cancer is positively correlated with plasma IL-10 level [24]. CD4+CD25+ Foxp3+ Tregs are positively correlated with changes in IL-10 and TGF-β1 in patients with rheumatoid heart disease [25]. In the study of children with juvenile idiopathic arthritis (JIA), serum levels of IL-10 and TGF-β1 did not correlate with the proportion of regulatory T cells (CD4+CD25highFOXP3+) in peripheral blood from JIA patients and healthy controls [26].
The correlation between regulatory T cells and TGF-β1 and IL-10 has been widely reported, and the results of this study show that CD4+CD25+CD127low Tregs were positively correlated with TGF-β1 and IL-10 before and after treatment of liver cancer. These findings are consistent with other reports of TGF-β1 and IL-10 [25]. However, he specific mechanism is still unclear and further research is needed.
In present study, the proportion of CD4+CD25+CD127lowTregs was positively correlated with TGF-β1 and IL-10 concentrations, which was consistent with relevant reports. This study suggests that CD4+CD25+CD127lowTregs, TGF-β1, IL-10 are closely related to the occurrence and development of tumor. The increase of Tregs in patients with liver cancer may be related to immune escape by the liver cancer. This study used CD4+CD25+CD127low as a marker, consistent with the report of CD4+CD25+ Foxp3+-tagged regulatory T cells in liver cancer, which was higher than that of healthy control[27]. However, the production of CD4+CD25+CD127low Tregs and its related mechanisms in tumorigenesis remain unknown. Therefore, the correlation between the percentage of CD4+CD25+CD127lowTregs and the concentrations of TGF-β1 and IL-10 in this study will provide some insight for more in-depth studies on the tumorigenetic mechanism, and facilitate the development of more effective tumor treatment programs.
Acknowledgements
We gratefully thank National Science Foundation of China (NO. 81670759), Basic Research Foundation of Shenzhen (NO. JCYJ20170306092910641).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. Ca Cancer J Clin. 2016;66:115-32
2. Haug AR. Imaging of primary liver tumors with positron-emission tomography. The quarterly journal of nuclear medicine and molecular imaging: official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the Society of. 2017;61:292
3. Stephens LA, Barclay AN, Mason D. Phenotypic characterization of regulatory CD4+CD25+ T cells in rats. International Immunology. 2004;16:365
4. Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A. et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. Journal of Experimental Medicine. 2006;203:1693-700
5. Anna Maria W, Dominik W, Michael S, Guenther G, Eberhard G, Beatrix GL. Increase of regulatory T cells in the peripheral blood of cancer patients. Clinical Cancer Research An Official Journal of the American Association for Cancer Research. 2003;9:606
6. Liao H, Peng X, Gan L, Feng J, Gao Y, Yang S. et al. Protective Regulatory T Cell Immune Response Induced by Intranasal Immunization With the Live-Attenuated Pneumococcal Vaccine SPY1 via the Transforming Growth Factor-beta1-Smad2/3 Pathway. Frontiers in immunology. 2018;9:1754
7. Fantini MC, Becker C, Tubbe I, Nikolaev A, Lehr HA, Galle P. et al. Transforming growth factor beta induced FoxP3+ regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671-80
8. Juneja A, Sehgal A, Sharma S, Pandey A. Cervical cancer screening in India: strategies revisited. Indian Journal of Medical Sciences. 2007;61:34-47
9. Kan X, Zhang W, You R, Niu Y, Guo J, Xue J. Scutellaria barbata D. Don extract inhibits the tumor growth through down-regulating of Treg cells and manipulating Th1/Th17 immune response in hepatoma H22-bearing mice. Bmc Complementary & Alternative Medicine. 2017;17:41
10. Xing H, Liu S, Chen X, Fang F, Wu X, Zhu P. A specific immune tolerance toward offspring cells is to exist after the mother lymphocyte infusion. Immunobiology. 2017;222:658
11. Wong LL. Current status of liver transplantation for hepatocellular cancer. American Journal of Surgery. 2002;183:309-16
12. Hartigan-O'Connor DJ, Poon C, Sinclair E, Mccune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. Journal of Immunological Methods. 2007;319:41-52
13. Wolf D. Current status of immunotherapy for cancer treatment. memo - Magazine of European Medical Oncology. 2008;1:190-2
14. Xiaoli LI, Tongguo SI, Haipeng YU. Effect of Cryosurgery on Regulatory CD4~+CD25~+ T Lymphocytes in the Peripheral Blood of Patients with Hepatocellular Carcinoma. Chinese Journal of Clinical Oncology. 2008;36:227-9
15. Ormandy LA, Tina H, Heiner W, Manns MP, Greten TF, Firouzeh K. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Research. 2005;65:2457
16. Thakur S, Singla A, Chawla Y, Rajwanshi A, Kalra N, Arora SK. Expansion of peripheral and intratumoral regulatory T-cells in hepatocellular carcinoma: a case-control study. Indian Journal of Pathology & Microbiology. 2011;54:448
17. Erushbrook U. Compromised lymphocytes infiltrate hepatocellular carcinoma: The role of T-regulatory cells. Hepatology. 2005;41:722-30
18. Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S. et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. International Immunology. 2004;16:1643-56
19. Morgan ME, Bilsen JHMV, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC. et al. Expression of FOXP3 mRNA is not confined to CD4 + CD25 + T regulatory cells in humans. Human Immunology. 2005;66:13-20
20. Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunological Reviews. 2010;204:195-207
21. Xu L, Tanaka S, Bonno M, Ido M, Kawai M, Yamamoto H. et al. Cord blood CD4 + CD25 + regulatory T cells fail to inhibit cord blood NK cell functions due to insufficient production and expression of TGF-beta1. Cellular Immunology. 2014;290:89-95
22. Samuel H, Christoph S, Hans A L, Amrit M, Steffen S, Christoph B. et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. Journal of Immunology. 2004;173:6526-31
23. Holmén N, Lundgren A, Lundin S, Bergin AM, Rudin A, Sjövall H. et al. Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflammatory Bowel Diseases. 2010;12:447-56
24. Zhong AY, Xue P, Shi MH. Expression of PD-1 by CD4+CD25+CD127lowTreg cells in the peripheral blood of lung cancer patients. Oncotargets & Therapy. 2015;8:1831-3
25. Estradacapetillo L, Hernandezcastro B, Monsivaisurenda A, Alvarezquiroga C, Espinosa EL, Abudmendoza C. et al. Induction of Th17 lymphocytes and Treg cells by monocyte-derived dendritic cells in patients with rheumatoid arthritis and systemic lupus erythematosus. Clinical & Developmental Immunology. 2013;2013:584303
26. Sznurkowska K, Boćkowska M, Zieliński M, Plata-Nazar K, Trzonkowski P, Liberek A. et al. Peripheral regulatory T cells and anti-inflammatory cytokines in children with juvenile idiopathic arthritis. Acta Biochimica Polonica. 2018:65
27. Fenge L, Zhi G, Gregory L, Haipeng Y, Haitao W, Tongguo S. Clinical prognostic value of CD4+CD25+FOXP3+regulatory T cells in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma patients. Clinical Chemistry & Laboratory Medicine. 2014;52:1357-65
Author contact
![]() Corresponding authors: Jianpei Li, Email: lijporg.cn or Xiangsheng Cai, Email: xiangshengcaiedu.cn
Corresponding authors: Jianpei Li, Email: lijporg.cn or Xiangsheng Cai, Email: xiangshengcaiedu.cn

 Global reach, higher impact
Global reach, higher impact