3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2019; 16(5):766-773. doi:10.7150/ijms.29995 This issue Cite
Research Paper
Immunohistochemistry of YAP and dNp63 and survival analysis of patients bearing precancerous lesion and oral squamous cell carcinoma
Department of Oral Pathology and Medicine, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Okayama, Japan
Received 2018-9-17; Accepted 2019-3-21; Published 2019-5-28
Abstract
Background: Yes-associated protein (YAP) is a candidate oncogene in various human cancers, and recently, it has been reported that YAP expression and its activity was enhanced by ΔNp63. However, the role of YAP and ΔNp63 expression in carcinogenesis and progression of oral squamous cell carcinoma (OSCC) has been unknown. Therefore, we investigated how YAP and ΔNp63 influence carcinogenesis and progression of OSCC.
Methods: We performed immunohistochemical analyses in whole tissue samples to investigate YAP and ΔNp63 expression in normal oral mucosa, epithelial hyperplasia, oral epithelial dysplasia (OED; low/high grade), carcinoma in situ (CIS), and OSCC. Furthermore, in OSCC, we analyzed clinical significance by using Kaplan-Meier survival analysis.
Results: In normal oral mucosa and epithelial hyperplasia, YAP expression was primarily confined to the basal and parabasal layers, but YAP expression was elevated in OED, CIS, and OSCC. In OED, YAP and ΔNp63 expression levels were markedly higher in high grade than in low grade. In OSCC groups, YAP and ΔNp63 expression patterns tended to differ according to histopathological differentiation of OSCC. Furthermore, the YAP high expression group, which showed YAP staining in >50% positive cells with strong cytoplasmic staining or >10% positive cells with nuclear reactivity, showed a tendency to have a poor survival rate.
Conclusion: YAP and ΔNp63 expression levels correlated with grade of oral OED. Additionally, YAP expression was associated with OSCC survival rate. Our results suggested that YAP and ΔNp63 expression might serve as predictive markers to distinguish OSCC development and progression.
Keywords: YAP, ΔNp63, oral epithelial dysplasia, carcinoma in situ, oral squamous cell carcinoma
Introduction
Oral squamous cell carcinoma (OSCC) represents 90% of oral cancers. Alterations in the 11q22 amplicon are detected in 5-15% of OSCC [1]. The gene, Yes-associated protein (YAP), located in 11q22, is specifically amplified in 4 of 23 OSCC [2-3]. YAP is a transcription factor in the Hippo signaling pathway and implicate in the regulation of development, metabolism, organ size, and tumorigenesis [4-6]. YAP has also been proposed as a candidate oncogene in hepatocellular carcinoma, non-small cell lung carcinoma, esophageal squamous cell carcinoma, ovarian cancer, and gastric cancer [7-10].
p63 is an important cancer-related binding partner of YAP. p63 controls YAP activity in head and neck squamous cell carcinoma [11]. The p63 gene is expressed as two isoforms: one that contains an N-terminal p53-homologous transactivation domain (TAp63) or one that lacks this domain (ΔNp63) [12-13]. ΔNp63 isoforms were initially described as simple dominant-negative proteins with the ability to inhibit TAp63 and p53 activity. Furthermore, elevated expression of ΔNp63 in esophageal squamous cell carcinoma and laryngeal squamous cell carcinoma was reported. Recently, ΔNp63 was shown to not only directly bind to the region of YAP promoter and induce its expression but also enhance YAP activity in squamous cell carcinoma [14]. However, there are few studies that described the relationship between YAP and ΔNp63 in carcinogenesis and progression of OSCC of human tissue specimens. OSCC progresses from oral premalignant lesions to oral epithelial dysplasia (OED), turning into carcinoma in situ (CIS) and finally becoming invasive OSCC. Therefore, in this study, we focused on YAP and ΔNp63 expression in normal oral mucosa, epithelial hyperplasia, OED (low/high grade), CIS, and OSCC of human tissue specimens.
Material and Methods
Patients and Samples
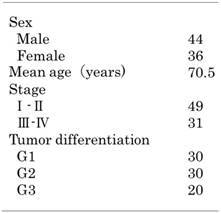
Patient samples were obtained from the oral pathology Department of Okayama University (Okayama, Japan) from August 2005 to January 2017. This study was approved by the Ethics Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (Approval number: 1608-018). A total of 270 cases were enrolled in the retrospective study, including 20 cases of normal oral mucosa, 20 cases of epithelial hyperplasia, 50 cases of low-grade OED, 50 cases of high- grade OED, 50 cases of CIS, and 80 cases of OSCC. Tissue samples from 270 patients were collected during clinical biopsy or excision. None of the patients received chemotherapy, radiotherapy or immunotherapy before sampling. All collected samples were histologically diagnosed by two pathologists and classified according to the World Health Organization criteria. Diagnostic WHO criteria of epithelial dysplasia include structural and cytological changes: the cut-off point between low-grade and high-grade dysplasia is four structural changes and five cytological changes. Based on the histologic categories of CIS, CIS cases were divided into two groups: differentiated type that marked atypical cells in the basal and parabasal layers while maintaining maturation and differentiation of the stratified squamous epithelium and basaloid type with atypia into the upper third of the epithelium. OSCC cases were divided into three groups: well-differentiated, moderately differentiated, and poorly differentiated. Tissue samples were fixed in 10% neutral formaldehyde and embedded in paraffin. Then, these samples were cut into 4-μm-thick sections for immunohistochemical (IHC) analyses and hematoxylin-eosin staining.
IHC Analysis
Paraffin-embedded tissue sections were deparaffinized and hydrated using routine techniques. Then, sections were reacted with 0.3% hydrogen peroxide methanol at room temperature for 30 min. Thereafter, sections were immersed in 0.01 M citrate buffer for antigen retrieval in a high-pressure cooker. Subsequent staining was performed using antibodies against YAP (1:100, R&D Systems, Minneapolis, MN, USA) and ΔNp63 (1:100, BioLogo, Kronshagen, Germany). For antibody detection, the Vectastain Elite ABC kit (Vector Laboratories, Inc., Burlingame, CA, USA) against YAP and Histofine, Simple Stain MAX-PO (MULTI) (Nichirei Bioscience, Tokyo, Japan) against ΔNp63 was used following the manufacturers' instructions. Finally, tissue sections were stained in 1:10000 diaminobenzidine tetrahydrochloride solution for visualization. Appropriate negative control sections were used in parallel in each run.
IHC Labeling Evaluation
According to a previous study [15], the intensity of YAP staining in IHC analyses was scored as follows: 0, complete absence of staining or positive cells only located in the basal layer or parabasal layer of oral squamous epithelium; 1, weak cytoplasmic staining; 2, <50% positive cells with strong cytoplasmic staining and <10% positive cells with nuclear staining. Additionally, we scored sections as a “3” if YAP staining was observed in >50% positive cells with strong cytoplasmic staining (type C) or >10% positive cells with nuclear reactivity (type N). ΔNp63 expression was considered positive if nuclear staining was present, and ΔNp63 staining was recorded as the percentage of ΔNp63-positive cells. The sections were blindly examined under the light microscope, and independently evaluated 100 cells/5HPF per sample and got dominant score by two pathologists.
Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics 24 (IBM, Chicago, IL, USA). Student's t-test with Bonferroni correction was used to analyze YAP and ΔNp63 expression levels in all samples. Kaplan-Meier survival analysis was used to analyze YAP and ΔNp63 expression levels in OSCC samples. The log-rank test was used to analyze the association between patient survival rate with YAP and ΔNp63 expression among different groups. P<0.05 was considered statistically significant.
Results
YAP and ΔNp63 expression in normal oral mucosa, epithelial hyperplasia, OED, and CIS
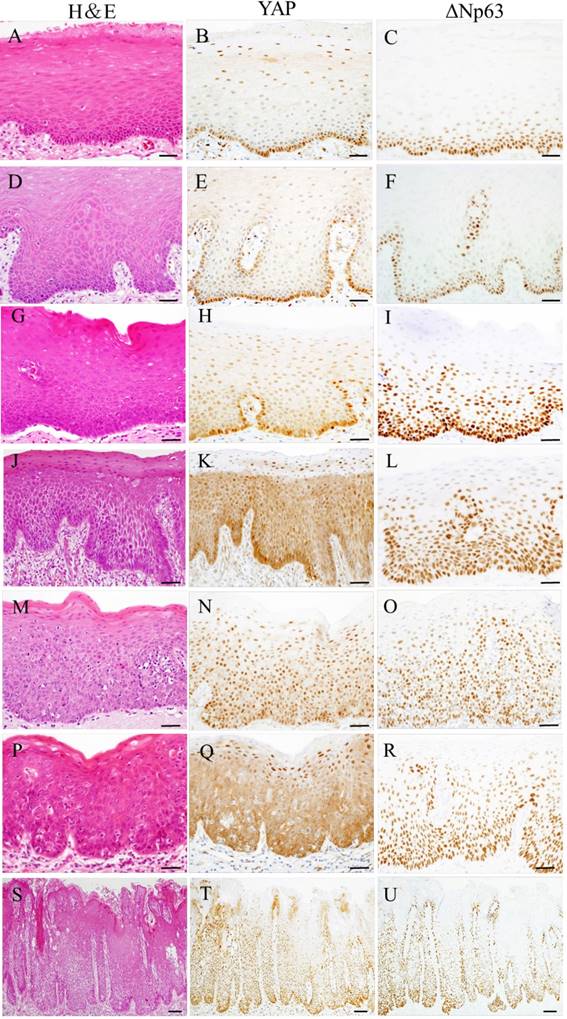
Representative examples of YAP and ΔNp63 expression in oral samples are shown in Fig 1. YAP and ΔNp63 expression were observed in all cases. In normal oral mucosa and epithelial hyperplasia, YAP was weakly observed in the cell cytoplasm and nuclei of the basal and parabasal layers, and ΔNp63 was observed in the cell nuclei of the basal and parabasal layers. In low-grade OED, within the lower third of squamous epithelium, YAP expression was weakly distributed in mainly the cell cytoplasm, and ΔNp63 expression was observed in cell nuclei. However, in high-grade OED and CIS, YAP and ΔNp63 expression was mainly distributed from the basal layer up to the surface of squamous epithelium. Strong YAP expression was observed in the cytoplasm (type C) and nuclei (type N) of neoplastic cells. In high-grade OED, type C was seen in the differentiated type of CIS. Conversely, type N was seen in the basaloid type of CIS.
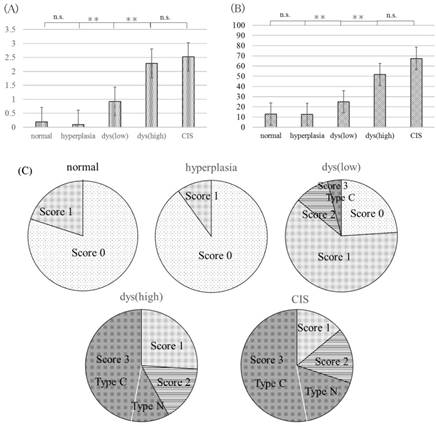
YAP immunolabeling scores and percentage of ΔNp63-positive cells in all samples are displayed in Fig 2. There was no significant difference in YAP immunolabeling scores and percentage of ΔNp63-positive cells between normal oral mucosa and epithelial hyperplasia (P>0.05). YAP and ΔNp63 expression in normal oral mucosa and epithelial hyperplasia was significantly lower than that in OED and CIS (P<0.05). Additionally, YAP and ΔNp63 expression in low-grade OED was significantly lower than that in high-grade OED and CIS (P<0.05). However, there was no significant difference in YAP and ΔNp63 expression between high-grade OED and CIS (P>0.05).
YAP immunolabeling scores are summarized in Fig 3. In normal oral mucosa and epithelial hyperplasia, no cases had scores of 2 or 3. In high-grade OED and CIS, no cases had a score of 0. Score 3 was noted in 2.0% (1/50) of low-grade OED, 54.0% (27/50) of high-grade OED, and 68.0% (34/50) of CIS. Among cases with score 3, type C was noted in 100.0% (1/1) of low-grade OED, 77.7% (21/27) of high-grade OED, and 70.5% (24/34) of CIS; type N was noted in no cases of low-grade OED, 22.2% (6/27) of high-grade OED, and 29.4% (10/34) of CIS.
YAP and ΔNp63 expression in OSCC samples
YAP and ΔNp63 expression was detected in all OSCC samples and tended to differ according to histopathological differentiation of OSCC (Fig 3).
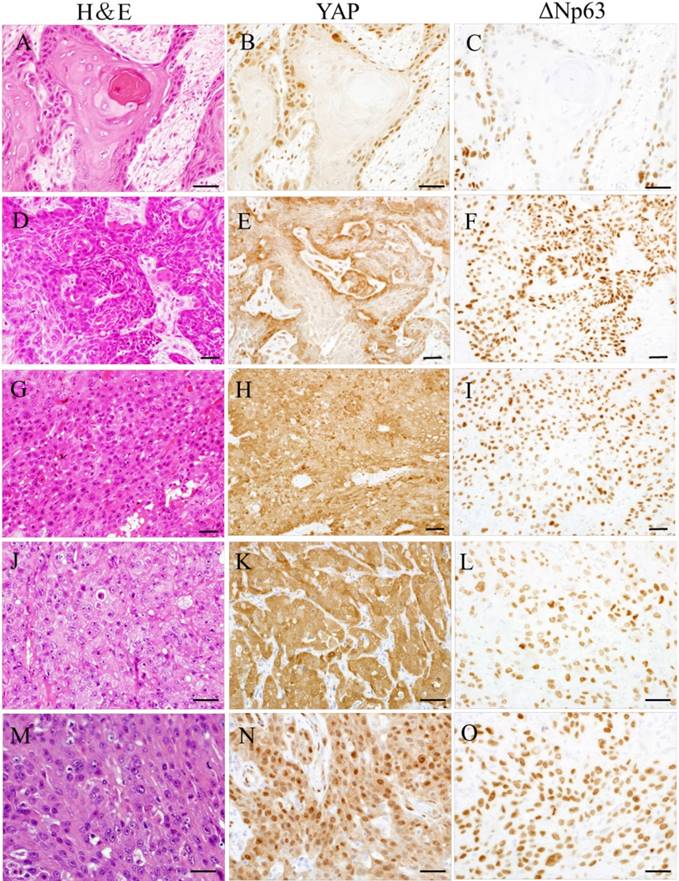
In well-differentiated OSCC, YAP and ΔNp63 expression were found in the tumor invasion front; YAP and ΔNp63 expression was observed in cell nuclei. Conversely, in poorly differentiated OSCC, YAP and ΔNp63 were expressed in nearly all malignant epithelial cells; YAP expression was strongly observed in cell nuclei or cytoplasm, and ΔNp63 expression was observed in cell nuclei. Moderately differentiated OSCC exhibited two patterns that tend to have maturation or less maturation of squamous epithelium. In the former, YAP and ΔNp63 expression patterns were similar to well-differentiated OSCC. In the latter, YAP and ΔNp63 expression patterns were similar to poorly differentiated OSCC.
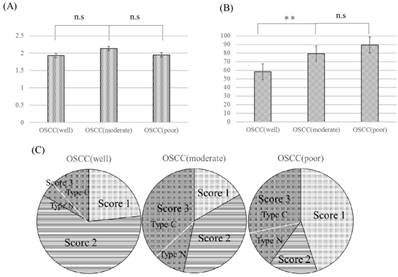
YAP immunolabeling scores and percentage of ΔNp63-positive cells in all samples are displayed in Fig 4. Both YAP immunolabeling scores and percentage of ΔNp63-positive cells in all samples were high, and there was no significant difference in YAP expression among the three OSCC groups (P>0.05). ΔNp63 expression in moderately and poorly differentiated OSCC was significantly higher than that in well-differentiated OSCC (P<0.05). As for YAP immunolabeling scores, no cases had a score of 0. Score 3 was noted in 16.6% (5/30) of well- differentiated OSCC, 46.6% (14/30) of moderately differentiated OSCC, and 40.0% (8/20) of poorly differentiated OSCC. For cases with a score of 3, type C was noted in 80.0% (4/5) of well-differentiated OSCC, 78.5% (11/14) of moderately differentiated OSCC, and 75.0% (6/8) of poorly differentiated OSCC.
YAP and ΔNp63 expression and survival rate in OSCC
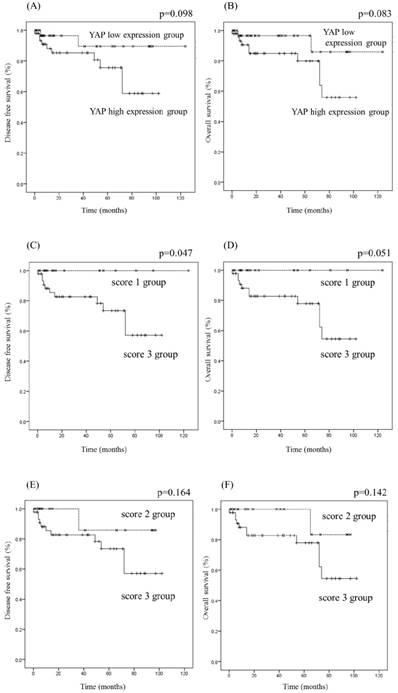
The clinical and pathological information of OSCC patients are arranged in Table 1. We evaluated the relationship between YAP expression and the survival rate of OSCC patients (Fig 5). OSCC patients were classified into two groups according to YAP expression: high (score 3; n=43) and low (scores 0-2; n=37). There was no significant difference in the survival rate between the YAP high and low expression groups. Additionally, based on YAP immunolabeling scores, OSCC patients were classified into two groups: scores 1 group vs. 3 group, and scores 2 group vs. 3 group. There was no significant difference in the survival rate between score 3 and score 2 groups (P>0.05), but the disease-free survival rate in score 3 group was significantly lower than that in score 1 group (P=0.047). These results suggest that YAP expression correlates with the survival rate.
We next investigated the relationship between ΔNp63 expression and the survival rate of OSCC patients. The average percentage of ΔNp63-positive cells was 72.9%. OSCC patients were classified into two groups as follows: ΔNp63 high expression group (>72.9% ΔNp63-positive cells; n=60) and ΔNp63 low expression group (<72.9% ΔNp63-positive cells; n=20). No statistically significant difference was observed in the survival rate between these two groups (P>0.05).
Representative photomicrographs of hematoxylin-eosin (H&E) staining (A, D, G, J, M, P, and S) and immunohistochemical staining for YAP (B, E, H, K, N, Q, and T) and ΔNp63 (C, F, I, L, O, R, and U) in oral samples. Oral samples were normal oral mucosa (A-C), epithelial hyperplasia (D-F), low-grade OED (G-I), high-grade OED (J-O) and CIS (P-U). In normal oral mucosa and epithelial hyperplasia, YAP was weakly observed in the cell cytoplasm and nuclei of the basal and parabasal layers, and ΔNp63 was observed in the cell nuclei of the basal and parabasal layers (A-F). In low-grade OED, within the lower third of squamous epithelium, YAP expression was weakly distributed mainly in the cell cytoplasm, and ΔNp63 was observed in cell nuclei (G-I). In high-grade OED and CIS, YAP and ΔNp63 expression was mainly distributed up to the whole thickness of squamous epithelium (J-U). Strong YAP expression was observed in the cell cytoplasm (type C) or nuclei (type N) of neoplastic cells (K, N, Q, and T). Bars: 20 μm.
Clinical features of cases of oral squamous cell carcinoma
Discussion
The present study focused on YAP and ΔNp63 expression in normal oral mucosa, epithelial hyperplasia, OED (low/high grade), CIS, and OSCC of human tissue specimens. IHC analyses showed that YAP was mainly distributed in atypical cells of oral OED (low/high grade) and CIS. YAP and ΔNp63 expression in normal oral mucosa and epithelial hyperplasia was significantly lower than that in oral OED and CIS (P<0.05). Additionally, YAP and ΔNp63 expression in high-grade OED, CIS, and OSCC was high. Our findings are consistent with a previous report that compared human precancerous lesion and cervical cancer with normal cervical mucosa, and observed that YAP expression was elevated in precancerous lesion and cervical cancer [15]. Additionally, it was reported that ΔNp63 is associated with the severity of oral OED [12], and ΔNp63 enhances YAP activity in OSCC [11]. We inferred that upregulation of YAP and ΔNp63 may play a role in human oral carcinogenesis. Furthermore, in analysis of YAP immunolabeling scores, score 3 was noted in 2.0% (1/50) of low-grade OED, 54.0% (27/50) of high-grade OED, and 68.0% (34/50) of CIS. Additionally, the following percentages of ΔNp63- positive cells were observed: 24.8% in low-grade OED, 51.5% in high-grade OED, and 67.4% in CIS. These results showed that the number of score 3 cases and percentage of ΔNp63-positive cells in high-grade OED and CIS were higher than those in low-grade OED. The fourth edition of the World Health Organization Classification of Tumours of the Head and Neck described a highly significant difference in the risk of malignant progression between low- and high-grade dysplasia, and high-grade dysplasia and CIS are associated with a higher risk of invasion [16]. Lam-Himlin et al. [17] reported that YAP was significantly correlated with a malignant phenotype in the esophagus and stomach. Matsubara et al. [18] found that high ΔNp63 expression was involved in malignant transformation in oral OED. Taken together, the results of these studies and our findings support our inference that score 3, in combination with the percentage of ΔNp63-positive cells, might facilitate the identification of precancerous oral lesions and prognostic value.
YAP immunolabeling scores (A) and percentage of ΔNp63-positive cells (B) in oral samples. YAP and ΔNp63 expression in low-grade OED was significantly lower than that in high-grade OED and CIS (P<0.05). There was no significant difference in YAP and ΔNp63 expression between in high-grade OED and CIS (P>0.05). YAP immunolabeling scores in oral samples (C). Normal: normal oral mucosa, hyperplasia: epithelial hyperplasia, dys (low): low-grade OED, dys (high): high-grade OED, CIS: carcinoma in situ. The numbers of score 3 cases in high-grade OED and CIS were higher than those in normal oral mucosa, epithelial hyperplasia and low-grade OED. n.s.: not significant, *P<0.05, **P<0.01.
Both YAP immunolabeling scores and percentage of ΔNp63- positive cells in all samples were high in OSCC. In previous study, ΔNp63 was shown to not only directly bind to the region of YAP promoter and induce its expression but also enhance YAP activity in SCC cell lines[14]. However, Ehsanian R et al show that ΔNp63 inhibits YAP expression, binds the YAP promoter, and suppresses cell death in SCC cell lines[19]. Our findings are consistent with the former study. Our study conducted on human tissue specimens and it might be strongly suggest the actual role of YAP and ΔNp63 in tumor tissue. Therefore, in vitro studies that reproduce the environment similar to the tumor tissue will be necessary to evaluate interactions of YAP and ΔNp63 in OSCC.
Strong YAP expression was observed in the cell cytoplasm (type C) or nuclei (type N) in high-grade OED and CIS. YAP plays different roles in the cytoplasm and nucleus. Furthermore, elevated nuclear YAP promotes proliferation, inhibits differentiation, and maintains an undifferentiated state both in vivo and in vitro in skin [17]. YAP cytoplasmic localization is crucial for differentiation of epithelial progenitors of adult airways [18]. Thus, it was suggested that type C is the differentiated state and type N is the undifferentiated state. In this study, YAP expression in high-grade OED and CIS was mainly distributed in the cell cytoplasm (type C). In contrast, Xiao et al. [15] found that precancerous cervical lesions and SCC groups, YAP labeling was predominately noted in nuclei of cells residing in squamous epithelium.
Representative photomicrographs of hematoxylin-eosin (H&E) staining (A, D, G, J, and M) and immunohistochemical staining for YAP (B, E, H, K, and N) and ΔNp63 (C, F, I, L, and O) in OSCC. OSCC cases were categorized as well- (A-C), moderately (D-I), and poorly (J-O) differentiated. In well-differentiated OSCC, YAP and ΔNp63 expression was found at tumor borders; YAP and ΔNp63 were observed in cell nuclei (A-C). Moderately differentiated OSCC displayed two patterns that tended to have maturation or less maturation (D and G). In the former, YAP and ΔNp63 expression levels were similar to those in well-differentiated OSCC (D-F). In the latter, YAP and ΔNp63 expression patterns were similar to those in poorly differentiated OSCC (G-I). In poorly differentiated OSCC, YAP and ΔNp63 were expressed in nearly all malignant epithelial cells; YAP was strongly observed in cell cytoplasm or nuclei, while ΔNp63 was seen in cell nuclei (J-O). Bars: 20 μm.
YAP immunolabeling scores (A) and percentage of ΔNp63-positive cells (B) in OSCC. OSCC: oral squamous cell carcinoma, OSCC (well): well-differentiated oral squamous cell carcinoma, OSCC (moderate): moderately differentiated oral squamous cell carcinoma, OSCC (poor): poorly differentiated oral squamous cell carcinoma. Both YAP immunolabeling scores and percentage of ΔNp63-positive cells in all samples were high, and there was no significant difference in YAP expression among the three groups (P>0.05). YAP immunolabeling scores in OSCC (C). OSCC (well): well-differentiated oral squamous cell carcinoma, OSCC (moderate): moderately differentiated oral squamous cell carcinoma, OSCC (poor): poorly differentiated oral squamous cell carcinoma. The numbers of score 3 cases in moderately and poorly differentiated OSCC were higher than that in well-differentiated OSCC. n.s.: not significant, *P<0.05, **P<0.01.
Human papillomavirus (HPV) plays a pivotal role in the pathogenesis of cervical cancer. However, tobacco and alcohol are the two most important known risk factors for the development of oral cancer, while HPV infection is considered a cofactor. Furthermore, in neoplastic lesions such as CIS, histologically features of oral mucosa differ from those of the cervix. CIS in oral mucosa is marked by atypical cells in the basal and parabasal layers while maintaining the maturation and differentiation of stratified squamous epithelium. Conversely, CIS in the cervix displays full-thickness atypia of the epithelium. Thus, the mechanism of cancerization and differentiation of the epithelium differ in oral mucosa versus the cervix and explain the differences in YAP expression between these sites. In OSCC, strong YAP expression was predominantly distributed in the cell cytoplasm (type C). Multiple groups reported that YAP nuclear localization was associated with development and progression of invasive cancer [3,6-9]. However, in a previous report [17], in primary or metastatic gastric adenocarcinoma and adenocarcinoma of the esophagus, there was a consistent finding of YAP nuclear and cytoplasmic localization. We suggest that an overabundance of YAP results from gene amplification or increased transcription, subsequently causing YAP nuclear or cytoplasmic expression in dysplastic and malignant cells.
In previous studies, YAP overexpression was an independent predictor of prognosis and might account for higher proliferation, metastasis, and poor survival outcome [20-25]. In the present study, the survival rate of OSCC tended to be lower in the YAP high expression group (score 3) than in the YAP low expression group (scores 0-2), and the disease-free survival rate was significantly lower in the score 3 group than in the score 1 group. Furthermore, the numbers of score 3 cases in moderately and poorly differentiated OSCC were higher than that in well-differentiated OSCC. Taken together, YAP expression, especially score 3, might contribute to accurate prediction of prognosis for patients following surgery.
Kaplan-Meier analysis of disease-free survival and overall survival rate of OSCC patients relative to YAP expression. OSCC patients were classified into two groups: YAP high expression group and YAP low expression group (A, B); score 3 group and score 1 group (C, D); or score 3 group and score 2 group (E, F). The YAP high expression group tended to have lower survival rates than the YAP low expression group (A, B), and the disease-free survival rate in the score 3 group was significantly lower than that in the score 1 group (P<0.05) (C).
In conclusion, varying YAP levels were observed in normal oral mucosa, epithelial hyperplasia, oral OED (low/high grade), CIS, and OSCC tissues. YAP and ΔNp63 expression was correlated with grade of oral OED, and YAP expression was associated with OSCC survival rate. Thus, YAP and ΔNp63 expression may serve as markers to distinguish development and progression of OSCC.
Acknowledgements
This study was funded by the Japan Society for Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (Nos. 16K11441, 18K09789, and 18K17224).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Baldwin C, Garnis C, Zhang L. et al. Multiple microalterations detected at high frequency in oral cancer. Cancer Res. 2005;65:7561-7567
2. Snijders AM, Schmidt BL, Fridlyand J. et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005;11:4232-4242
3. Overholtzer M, Zhang J, Smolen GA. et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci. 2006;103:12405-12410
4. Meng Z, Moroishi T, Guan, KL. Mechanisms of hippo pathway regulation. Genes Dev. 2016;30:1-17
5. Santinon G, Pocaterra A, Dupont S. Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. 2016;26:289-299
6. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783-803
7. Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246-257
8. Zhao B, Wei X, Li W, Udan RS. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747-2761
9. Steinhardt AA, Gayyed MF, Klein AP. et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582-1589
10. Zender L, Spector MS, Xue W. et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253-1267
11. Saladi SV, Ross K, Karaayvaz M. et al. ACTL6A is co-amplified with p63 in squamous cell carcinoma to drive YAP activation, regenerative proliferation, and poor prognosis. Cancer Cell. 2017;31:35-49
12. Mills AA. P63: onocogene or tumor suppressor? Curr Opin Genet Dev. 2006;16:38-44
13. Pruneri G, Pignataro L, Manzotti M. et al. p63 in laryngeal squamous cell carcinoma: evidence for a role of TA-p63 down-regulation in tumorigenesis and lack of prognostic implications of p63 immunoreactivity. Lab Invest. 2002;82:1327-1334
14. Li Y, Kong F, Shao Q, Wang R. et al. YAP expression and activity are suppressed by S100A7 via p65/NFκB-mediated repression of ⊿Np63. Mol Cancer Res. 2017;15:1752-1763
15. Xiao H, Wu L, Zheng H. et al. Expression of Yes-associated protein in cervical squamous epithelium lesions. Int J Gynecol Cancer. 2017;24:1575-1582
16. Warnakulasuriya S, Kovacevic T, Madden P. et al. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. J Oral Pathol Med. 2011;40:677-683
17. Lam-Himlin DM, Daniels JA, Gayyed MF. et al. The hippo pathway in human upper gastrointestinal dysplasia and carcinoma: a novel oncogenic pathway. Int J Gastrointest Cancer. 2006;37:103-109
18. Matubara R, Kawani S, Kiyosue T. et al. Increased ΔNp63 expression is predictive of malignant transformation in oral OED and poor prognosis in oral squamous cell carcinoma. Int J of Oncol. 2011;39:1391-1399
19. Ehsanian R, Brown M, Lu H. et al. YAP dysregulation by phosphorylation or ΔNp63-mediated gene repression promotes proliferation, survival and migration in head and neck cancer subsets. Oncogene. 2010;29:6160-6171
20. Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci USA. 2011;108:2270-2275
21. Mahoney JE, Mori M, Szymaniak AD. et al. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137-150
22. Song M, Cheong JH, Kim H. et al. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012;32:3827-3834
23. Muramatsu T, Imoto I, Matsui T. et al. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:89-98
24. Xu MZ, Yao TJ, Lee NP. et al. Yes-Associated Protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576-4585
25. Marti P, Stein C, Blumer T. et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology. 2015;62:1497-1510
Author contact
![]() Corresponding author: Sawako Ono, Department of Oral Pathology and Medicine, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, 2-5-1 Shikata-Cho, Okayama 700-8558, Japan; Tel: +81 86 235 6651; Fax: +81 86 235 6654; E-mail: de19008okayama-u.ac.jp
Corresponding author: Sawako Ono, Department of Oral Pathology and Medicine, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, 2-5-1 Shikata-Cho, Okayama 700-8558, Japan; Tel: +81 86 235 6651; Fax: +81 86 235 6654; E-mail: de19008okayama-u.ac.jp

 Global reach, higher impact
Global reach, higher impact