3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2019; 16(5):751-756. doi:10.7150/ijms.32612 This issue Cite
Research Paper
Zoledronate induces cell cycle arrest and differentiation by upregulating p21 in mouse MC3T3-E1 preosteoblasts
1. Department of Orthopaedic Surgery, Chiayi Chang Gung Memorial Hospital, Chiayi County 61363, Taiwan
2. Chang Gung University College of Medicine, Taoyuan City 33302, Taiwan
3. Department of Medical Research and Development, Chiayi Chang Gung Memorial Hospital, Chiayi County 61363, Taiwan
Received 2018-12-27; Accepted 2019-3-23; Published 2019-5-10
Abstract
Background: Increasing research has recently been focused on the supplementary use of drugs such as bisphosphonates that are known to influence bone turnover to prevent and treat periprosthetic bone loss and subsequent implant loosening following total joint replacements. However, there are still concerns about the conflicting effects of bisphosphonate treatment on osteoblastic bone formation in the literature.
Methods: In this study, we investigate the role of zoledronate (ZOL) in regulating cell cycle distribution and differentiation in mouse MC3T3-E1 preosteoblasts and also explore the mechanism underlying this effect of ZOL. We examined the expression levels of osteocalcin (OCN) by quantitative polymerase chain reaction (qPCR), the total amount of CDK6, p21 and p27 proteins by Western blot analysis, and the cell cycle distribution by flow cytometric analysis in mouse MC3T3-E1 preosteoblasts to evaluate the effect of ZOL. Small interfering RNAs (siRNAs) were used to assess the individual contributions of genes to specific osteoblast phenotypes.
Results: In addition to increased OCN expression, we found that ZOL treatment induces the G0/G1 arrest and results in the increase of p21 and p27 expressions and decrease of CDK6 expression in mouse MC3T3-E1 preosteoblasts. Both p21 and p27 mediates ZOL-induced cell cycle exit; however, p21, but not p27, is responsible for the increase of ZOL-induced OCN expression in these cells.
Conclusions: These results endorse that ZOL might have an anabolic effect on osteoblasts. The CDK inhibitor p21 plays a key role in regulating osteoblast differentiation by controlling proliferation-related events in mouse MC3T3-E1 preosteoblasts.
Keywords: zoledronate, cell cycle arrest, osteogenic differentiation, p21, osteocalcin
Introduction
The primary use of most intramedullary implants in orthopaedics is to attain internal fixation into the cancellous bone bed by press-fit sealing [1]. Implants can be used as fixation devices to stabilize fractures until they heal, and for prosthetic joint replacements to provide desired persistent osseointegration [2]. Failure of prosthetic implant osseointegration will lead to early prosthetic implant migration, periprosthetic bone loss, localized bone lysis, and finally implant loosening, which may result in the need for revision [3,4]. Periprosthetic bone loss, osteolysis, and subsequent loosening is by far the most common cause for revising total joint replacements. The clinical outcome in such cases is poorer and has a higher cost factor than primary joint surgery [5,6]. By 2030 in the United States, the demand for primary total hip replacements is estimated to increase by 174% and that for primary total knee replacements is expected to increase by 673% [7]. Moreover, total hip and total knee revisions are expected to increase by 137% and 601%, respectively, between 2005 and 2030 [7]. If the expected trends hold true, the importance of acquiring prosthetic implant longevity will increase [8].
Periprosthetic bone loss and subsequent implant loosening is an active biological cascade process initiated in response to wear debris or mechanical instability [3,9]. The eventual response to this process is the activation of multiple cell types in the periprosthetic region resulting in an inflammatory process that ultimately leads to periprosthetic bone loss [3,10]. Activated macrophages, the key to the initiation of an osteolytic response, release various pro-inflammatory cytokines and then recruit osteoclasts and modulate their function [3,10,11]. Newly formed osteoclasts, the only cells capable of active bone resorption, polarize to form a ruffled membrane, adhere to the bone matrix, and secrete acid for hydroxyapatite dissolution and proteases for matrix protein digestion [12]. Although less importance has been placed on the role of osteoblasts in periprosthetic bone loss, they might be affected and contribute to the osteolytic process through the inhibition of their ability to secrete mineralized bone matrix [10].
To address the issue of periprosthetic bone loss, research has recently been focused on the supplementary use of drugs that are known to influence bone turnover [1,2,4-6,8,12-15]. Among these, bisphosphonates, through their antiresorptive properties on osteoclast activity, have demonstrated their ability to reduce the risk of hip and vertebral fractures and can have potential protective effects on implant survival [6,16]. Recent evidence from clinical studies indicate that bisphosphonates clearly reduce periprosthetic bone loss, and thus, improve fixation and durability of total joint replacement components [5,6,8,13-16]. Despite the beneficial effect of bisphosphonates in maintaining and improving periprosthetic bone quality, there are still concerns about the cumulative-dose effects on the survival and function of osteoblasts [17]. Some in vitro and in vivo evidence indicated that bisphosphonates may promote osteoblastic bone formation [18,19]. In contrast, others suggested that bisphosphonates could decrease the proliferation of and inhibit the differentiation and mineralization of osteoblasts [17,20,21]. Therefore, our aim in this study was to investigate the role of zoledronate (ZOL, a potent third-generation bisphosphonate) in regulating cell cycle distribution and differentiation in mouse MC3T3-E1 preosteoblasts and also to explore the mechanism underlying this effect of ZOL. The results of this study may be valuable in enhancing our understanding of the role of osteoblasts in ZOL-treated periprosthetic bone loss, thereby improving the prosthetic implant longevity and decreasing the cost burden of revision surgery.
Materials and methods
Materials. Mouse monoclonal antibodies (mAbs) against CDK6 (no. 3136) and p21 (no. 2946), and rabbit polyclonal antibodies (pAbs) against p27 (no. 2552) were obtained from Cell Signaling Technology (Beverly, MA, USA). The control small interfering RNA (siRNA) and specific siRNAs of p21 and p27 were obtained from Thermo (Waltham, MA, USA). ZOL was obtained from Novartis Pharma (Basel, Switzerland). All other chemicals of reagent grade were purchased from Sigma (St. Louis, MO, USA), unless otherwise noted.
Cell lines and cell cultures. Mouse MC3T3-E1 preosteoblasts were purchased from American Type Culture Collection (Manassas, VA, USA) and grown in the DMEM (Life Technologies Inc., Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS; Life Technologies Inc.) and 1% penicillin/streptomycin at 37 °C in a 5% CO2 humidified incubator. Before treatment with ZOL, the culture medium was replaced with an otherwise identical medium, containing only 0.5% FBS, and incubated for 24 h.
Flow cytometric analysis. The cells were collected, fixed with 70% cold ethanol for 30 min, and permeabilized with 0.1% Triton X-100 in PBS for 2 h. The cells were then stained with propidium iodide (50 µg/mL, Roche Diagnostics, Basel, Switzerland) and ribonuclease A (1 mg/mL) for 30 min. The stained cells were detected by the BD FACSC II flow cytometer and the data were analyzed using a mod-fit cell cycle analysis program.
RNA isolation and quantitative real-time PCR. Trizol reagent was used to purify the total RNA from MC3T3-E1 preosteoblasts, which was then converted to cDNA. The quantitative real-time PCR was performed by employing an ABI StepOnePlus (Applied Biosystems, Foster City, CA, USA) with SYBR Green kit (Applied Biosystems). Primers were as follows: OCN, sense: 5′-TTCTGCTCACTCTGCTGACCCT-3′ and antisense: 5′-CCTGCTTGGACATGAAGGCTT-3′; GAPDH, sense: 5′-GCATCTCCCTCACAATTTCCA-3′ and antisense: 5′-GTGCAGCGAACTTTATTGATGG-3′. The GAPDH gene expression served as the internal control. All gene expressions were assessed in duplicate. GAPDH gene expression was used to normalize the OCN gene expression in the same sample.
Western blot analysis. The collected cells were lysed with the lysis buffer containing protease and phosphatase inhibitors (phenylmethylsulfonyl-fluoride, aprotinin, and sodium orthovanadate). The total cell lysate (100 µg of protein) was separated by SDS-PAGE (10% running, 4% stacking) and transferred onto a nitrocellulose membrane (Immobilon P; 0.45-µm pore size). The membrane was then treated with the indicated antibodies. Western-Light chemiluminescent detection system (Applied Biosystems) was used to perform the immunodetection.
siRNA transfection. The transfection with 30 nM of indicated specific siRNA was done in 70-80% confluent mouse MC3T3-E1 preosteoblasts using the RNAiMAX transfection kit (Thermo) before the ZOL treatment.
Statistical analysis. Results are expressed as mean ± SEM. Statistical analysis was assessed by the independent Student's t test for two groups of data and ANOVA. The Scheffe's test was used for multiple comparisons. A P value < 0.05 was considered significant.
Results
ZOL induces OCN expression in mouse MC3T3-E1 preosteoblasts. Mouse MC3T3-E1 preosteoblasts were kept as controls or treated with ZOL (1, 10, and 100 µM) for 48 h, and their expression of OCN, a biochemical marker for osteogenic differentiation and subsequent bone formation, was examined. The treatment with ZOL resulted in significant dose-dependent increase in OCN gene expression in mouse MC3T3-E1 preosteoblasts (Fig. 1). These results suggest that ZOL induces differentiation of mouse MC3T3-E1 preosteoblasts.
Zoledronate (ZOL) induces OCN expression in mouse MC3T3-E1 preosteoblasts. Mouse MC3T3-E1 preosteoblasts were kept as controls or stimulated with ZOL (1, 10, and 100 µM) for 48 h, and their OCN mRNA expressions were determined by quantitative real-time PCR. Data are mean ± SEM from three to four independent experiments. *, P < 0.05 vs. unstimulated control cells.

ZOL induces the G0/G1 arrest and results in the increase of p21 and p27 expression levels, and the decrease of CDK6 expression in mouse MC3T3-E1 preosteoblasts. Mouse MC3T3-E1 preosteoblasts were kept as controls or treated with ZOL (1, 10, and 100 µM) for 48 h, and their cell cycle distributions were analyzed by flow cytometry. The treatment with ZOL caused significant dose-dependent increase of the percentage of cells in the G0/G1 phases and decrease of that in the S and G2/M phases in contrast to that of the untreated control cells for the same periods (Table 1). Additionally, we investigated the molecular basis of this ZOL effect on cell cycle distributions. The treatment of mouse MC3T3-E1 preosteoblasts with ZOL (100 µM) for 48 h resulted in increase in p21 and p27 expression levels in these cells (Fig. 2). In contrast, ZOL treatment resulted in the decrease of CDK6 expression in mouse MC3T3-E1 preosteoblasts. These results suggest that ZOL induces the G0/G1 arrest and results in the increase of p21 and p27 expressions and , but not p27, is involved in ZOL-induced OCN expression in mouse MC3T3-E1 preosteoblasts.
Zoledronate (ZOL) regulates cell cycle-regulatory protein expression in mouse MC3T3-E1 preosteoblasts. Mouse MC3T3-E1 preosteoblasts were kept as controls or stimulated with ZOL (100µM) for 24, 48, and 72 h. Expression of cell cycle-regulatory proteins were determined by Western blot analysis. Results are representative of three independent experiments with similar results. Data are mean ± SEM from three independent experiments. *, P < 0.05 vs. unstimulated control cells.

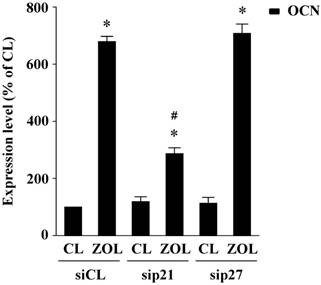
p21, but not p27, mediates zoledronate (ZOL)-induced OCN expression in mouse MC3T3-E1 preosteoblasts. Mouse MC3T3-E1 preosteoblasts were transfected with control siRNA (siCL) or a specific siRNA of p21 (sip21) or p27 (sip27) (30 nM for each) for 48 h, and then treated with ZOL for 48 h. Data are mean ± SEM from three to four independent experiments. *, P < 0.05 vs. unstimulated control cells. #, P < 0.05 vs. cells transfected with control siRNA.
Zoledronate (ZOL) induces a G0/G1 cell cycle arrest in mouse MC3T3-E1 osteoblasts.
| % of cells (mean ± SEM ) | |||
|---|---|---|---|
| G0/G1 | Synthetic | G2/M | |
| CL | 78.3 ± 4.2% | 10.3 ± 1.2% | 11.4 ± 2.1% |
| ZOL (1 µM) | 80.9 ± 3.5% | 9.2 ± 3.4% | 9.9 ± 2.2% |
| ZOL (10 µM) | 83.6 ± 3.2% | 9.0 ± 1.1% | 7.4 ± 2.3% |
| ZOL (100 µM) | 91.8 ± 2.6%* | 1.9 ± 0.5%* | 6.3 ± 0.8%* |
Mouse MC3T3-E1 osteoblasts were kept as control or treated with zoledronate (ZOL) for 48 h. The cells were stained with propidium iodide and analyzed for DNA content by flow cytometry to show percentages of cells in G0/G1, synthetic, or G2/M phases of the cell cycle. Data are mean ± SEM from three independent experiments. *P < 0.05 vs. untreated control cells.
Zoledronate (ZOL)-induced G0/G1 arrest in mouse MC3T3-E1 osteoblasts is mediated by p21.
| siRNA | Control (48 h) | ZOL (48 h) | ||||
|---|---|---|---|---|---|---|
| % of cells (mean ± SEM ) | % of cells (mean ± SEM ) | |||||
| G0/G1 | Synthetic | G2/M | G0/G1 | Synthetic | G2/M | |
| siCL | 75.9 ± 1.9% | 14.1±0.8% | 10.0 ± 1.3% | 90.1 ± 1.1%* | 6.3 ± 0.3%* | 3.6 ± 0.7%* |
| sip21 | 70.3 ± 2.1%# | 16.1±3.6%# | 13.6 ± 1.9%# | 72.5 ± 3.6%# | 14.1 ± 2.1%# # | 13.4 ± 1.0%# |
| sip27 | 77.3 ± 1.6% | 12.1 ± 1.5% | 10.6 ± 0.6% | 78.0 ± 3.8%# | 10.9 ± 0.8%# | 11.1 ± 1.4%# |
MC3T3-E1 osteoblasts were transfected with control siRNA (siCL) or specific siRNA of p21 (sip21) or p27 (sip27) (30 nM for each) for 48 h and then were kept as control or treated with zoledronate (ZOL) for 48 h. The cells were stained with propidium iodide and analyzed for DNA content by flow cytometry to show percentages of cells in G0/G1, synthetic, or G2/M phases of the cell cycle. Data are mean ± SEM from three independent experiments. *P < 0.05 vs. untreated control cells. #P < 0.05 vs. the cells transfected with control siRNA.
Discussion
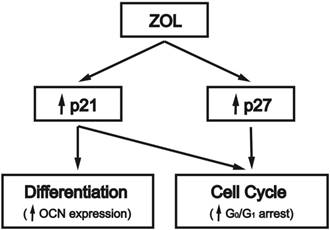
This study was aimed to elucidate the mechanism of the ZOL effect on inducing cell cycle arrest and differentiation in mouse MC3T3-E1 preosteoblasts (summarized in Fig. 4). Our investigation revealed the following observations. First, this study shows that ZOL can induce OCN expression in mouse MC3T3-E1 preosteoblasts. Second, ZOL induces G0/G1 arrest in these cells, with associated increase in the expression levels of CDK inhibitors p21 and p27. The induction of p21 and p27 expressions by ZOL is a prerequisite for the ZOL-induced G0/G1 arrest in these cells. Finally, p21, but not p27, is involved in ZOL-induced OCN expression in mouse MC3T3-E1 preosteoblasts. Our findings may help to explain the molecular mechanism used by ZOL to regulate cell cycle progression and differentiation in osteoblasts.
Schematic representation of the signaling pathways regulating zoledronate (ZOL)-induced cell cycle arrest and differentiation in mouse MC3T3-E1 preosteoblasts. ZOL induces G0/G1 arrest in osteoblasts by increasing their expressions of CDK inhibitors p21 and p27. CDK inhibitor p21, but not p27, is involved in ZOL-induced OCN expression. ↑, up-regulation by ZOL.
Osteoblast differentiation is regulated by physical stimuli, which ensures adequate bone formation for structural and functional support of the body [23]. In response to stimuli, preosteoblasts undergo differentiation in three stages, with the expression of certain specific osteoblast phenotype markers in each stage [24]. In the first stage, the cells proliferate and express fibronectin, collagen, TGFß receptor 1, and osteopontin. In the second stage, they exit the cell cycle and initiate differentiation, while promoting maturation of the extracellular matrix with alkaline phosphatase and collagen. In the third stage, OCN enriches the organic scaffold promoting the deposition of mineral substance, and thus, inducing matrix mineralization. In case of periprosthetic bone loss, osteoblast differentiation is affected through the inhibition of matrix secretion and mineralization [10]. Our data indicate that ZOL treatment resulted in a dose-dependent increase in OCN expression in mouse MC3T3-E1 preosteoblasts, with concomitant induction of cell cycle arrest in the G0/G1 phase. This is in agreement with results from another study [25] where ZOL selectively induced cytostasis and/or cell death in the precursor population of human osteoblast-like cells and concurrently promoted the differentiation and maturation of the remaining viable cells.
Regarding the mechanism underlying the ZOL effect, we found that CDK inhibitor p21 not only mediated cell cycle exit but also upregulated OCN expression in mouse MC3T3-E1 preosteoblasts. In contrast, p27 arrested cell cycle progression but had no effect on OCN expression in these cells. Owing to a high level of homology in their primary structure, p21 and p27 are believed to inhibit cyclin-CDK complexes and regulate cell cycle progression through similar mechanisms in the nucleus [26]. However, p21 and p27 might use different mechanisms to regulate cell death and to function as transcriptional cofactors in osteogenic differentiation [26-28]. Several studies have shown that p21 protects numerous cell types from apoptosis through regulating the activity of retinoblastoma protein (pRb), E2F family, NFκB, c-myc, STAT, and p300/CPB [26,27,29]. Additionally, p21 could interact with procaspase-3 on the mitochondria to inhibit caspase-3 activation and to resist Fas-mediated cell deaths [30]. As reported for the roles of p21, pRb and RUNX2 in osteogenesis, Yamato et al. demonstrated that BMP-2 activates p21 transcription by inducing a binding of Smad4 and Smad1 to the 29-base pair region of the promoter in HS-72 cells [31,32]. They also found that the enhanced expression of p21 might lead to the hypophosphorylation of pRb, which is responsible for BMP-2-mediated G0/G1 arrest, but not for BMP-2-induced apoptosis [33]. Moreover, Thomas et al. demonstrated that pRb might facilitate terminal osteoblast differentiation by activating transcription of differentiation-specific genes, such as OCN, in collaboration with RUNX2 [31]. These findings are consistent with those of Sciandra et al., showing high RUNX2 affinity to the promoter regions of p21 and of OCN genes, consistent with the terminally differentiated phenotype observed in osteoblast-like cells [34].
The functions of p27 in the apoptotic process and transcriptional regulation remain unclear [26,28]. Using a bone marrow cell model, from p27 null mice, Drissi et al. explored the mechanisms by which osteoblast growth is down-regulated at key transition points from proliferation and growth arrest to differentiation [35]. They found that p27-/- adherent marrow cells had increased p21 levels and proliferate faster, but retain their competency for differentiation when compared with wild-type. A recent study by Zhu et al. showed again that deletion of p27 in parathyroid hormone-related peptide 1-84 knock-in mice could partially rescue defects in skeletal growth and osteoblastic bone formation by reinforcing the endochondral bone formation and osteogenesis [36]. Our findings suggested that p21 controls events related to proliferation and plays a vital role in regulating the differentiation of osteoblasts in ZOL-treated bone cells.
There are some limitations to this study. First, this was an in vitro study, and its findings may not be used to accurately explain in vivo effects. However, use of a cell culture model provides a better understanding of the precise mechanisms underlying the effects of a drug on osteogenesis. Second, we use the mouse MC3T3-E1 preosteoblasts as the target cell line in this study as they are easily accessible and experiments can be repeated with reproducible results in contrast to that with primary bone-derived cells. Moreover, mouse MC3T3-E1 preosteoblasts were observed to have a cell proliferation rate similar to that of primary human osteoblasts and they were also found to generate a mineralized matrix in a similar manner [37], making it an ideal cell culture model for investigation of cell cycle regulation and osteogenesis, specifically in the later stages of osteogenic differentiation.
In summary, our findings might provide a clue to the conflicting effects of ZOL treatment on osteoblastic bone formation shown in previous literature [17-21]. Although ZOL treatment resulted in cell cycle exit in mouse MC3T3-E1 preosteoblasts, it did not affect their differentiation competency, such as the expression of OCN. Furthermore, we provide evidence for p21 as a key regulator in the transition from a proliferating osteoprogenitor to a post-proliferative osteoblast after ZOL treatment. Thus, these findings further support the concept that ZOL could have an anabolic effect on osteoblasts. Further investigation is required to compare these in vitro results to that of in vivo bone quality and bone turnover. The effects of ZOL on bone metabolism could significantly benefit numerous clinical indications, such as decreasing periprosthetic bone loss, and thus, lead to increased durability and longevity of the components of total joint replacement.
Acknowledgements
We would like to acknowledge the BD FACSC II flow cytometer service provided by the Precious Instrumentation Core Laboratory, Chiayi Chang Gung Memorial Hospital, Taiwan.
Funding
This work was supported by grants from the Chang Gung Memorial Hospital, Taiwan (Grant Nos. CMRPG 6B0531-3 and CMRPG 6G0061) and from the Taiwan National Science Council (Grant No. NSC95-2314-B-182A-087).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
KCH and SFC were involved in the study conception and design, data acquisition, analysis, interpretation, and drafting the manuscript. TWH, PYC, and TYY were involved in data collection, critical review and analysis of results, and editing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee (IACUC) of Chang Gung Memorial Hospital approved the animal use protocol (permit no. 2015122905), and all animal experiments followed the Animal Protection Law by the Council of Agriculture, Executive Yuan, ROC, and were performed according to the guidelines for the Care and Use of Laboratory Animals as promulgated by the Institute of Laboratory Animal Resources, National Research Council, USA.
Patient consent for publication
Not applicable.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cleemann R, Sorensen M, Bechtold JE, Soballe K, Baas J. Healing in peri-implant gap with BMP-2 and systemic bisphosphonate is dependent on BMP-2 dose - A canine study. J Orthop Res. 2018;36(5):1406-1414
2. Back DA, Pauly S, Rommel L, Haas NP, Schmidmaier G, Wildemann B. et al. Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet Disord. 2012;13:42
3. Ollivere B, Wimhurst JA, Clark IM, Donell ST. Current concepts in osteolysis. J Bone Joint Surg Br. 2012;94(1):10-15
4. Jakobsen T, Kold S, Shiguetomi-Medina J, Baas J, Soballe K, Rahbek O. Topical zoledronic acid decreases micromotion induced bone resorption in a sheep arthroplasty model. BMC Musculoskelet Disord. 2017;18(1):441
5. Friedl G, Radl R, Stihsen C, Rehak P, Aigner R, Windhager R. The effect of a single infusion of zoledronic acid on early implant migration in total hip arthroplasty. J Bone Joint Surg Am. 2009;91(2):274-281
6. Prietop-Alhambro D, Javaid MK, Judge A, Murray D, Carr A, Cooper C. et al. Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ. 2011;343:d7222
7. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785
8. Scott DF, Woltz JN, Smith RR. Effect of zoledronic acid on reducing femoral bone mineral density loss following total hip arthroplasty: preliminary results of a prospective randomized trial. J Arthroplasty. 2013;28(4):671-675
9. Amirhosseini M, Andersson G, Aspenberg P, Fahlgren A. Mechanical instability and titanium particles induce similar transcriptomic changes in a rat model for periprosthetic osteolysis and aseptic loosening. Bone Rep. 2017;7:17-25
10. O'Neill SC, Queally JM, Devitt BM, Doran PP, O'Byrne JM. The role of osteoblasts in periprosthetic osteolysis. Bone Joint J. 2013;95(8):1022-1026
11. Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2(2):102-113
12. Zaidi M, Blair HC, Moonga BS, Abe E, Huang CL. Osteoclastogenesis, bone resorption, and osteoclast-based therapeutics. J Bone Miner Res. 2003;18(4):599-609
13. Knusten AR, Ebramzadeh E, Longjohn DB, Sangiorgio SN. Systematic analysis of bisphosphonate intervention on periprosthetic BMD as a function of stem design. J Arthroplasty. 2014;29(6):1292-1297
14. Huang TW, Wang CJ, Shih HN, Chang Y, Huang KC, Peng KT. et al. Bone turnover and periprosthetic bone loss after cementless total hip arthroplasty can be restored by zoledronic acid: a prospective, randomized, open-label, controlled trial. BMC Musculoskelet Disord. 2017;18(1):209
15. Smith RL, Schwarz EM. Are biologic treatments a potential approach to wear- and corrosion-related problems? Clin Orthop Relat Res. 2014;472(12):3740-3746
16. Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2016;374(3):254-262
17. Huang KC, Cheng CC, Chuang PY, Yang TY. The effects of zoledronate on the survival and function of human osteoblast-like cells. BMC Musculoskelet Disord. 2015;16:355
18. Gou W, Wang X, Peng J, Lu Q, Wang Y, Wang A. et al. Controlled delivery of zoledronate improved bone formation locally in vivo. PLoS One. 2014;9(3):e91317
19. von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I. et al. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials. 2005;26(34):6941-6949
20. Idris AI, Rojas J, Greig IR, Van't Hof RJ, Ralston SH. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int. 2008;82(3):191-201
21. Pozzi S, Vallet S, Mukherjee S, Cirstea D, Vaghela N, Santo L. et al. High-dose zoledronic acid impacts bone remodeling with effects on osteoblastic lineage and bone mechanical properties. Clin Cancer Res. 2009;15(18):5829-5839
22. Zavitz KH, Zipursky SL. Controlling cell proliferation in differentiating tissues: genetic analysis of negative regulators of G1 -> S-phase progression. Curr Opin Cell Biol. 1997:9 (6); 773-781
23. Rutkovskiy A, Stensløkken KO, Vaage IJ. Osteoblast differentiation at a glance. Med Sci Monit Basic Res. 2016;22:95-106
24. Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4(13):3111-3123
25. Pan B, To LB, Farrugia AN, Findlay DM, Green J, Gronthos S. et al. The nitrogen-containing bisphosphonate, zoledronic acid, increases mineralization of human bone-derived cells in vitro. Bone. 2004;34(1):112-123
26. Coqueret O. New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol. 2003;13(2):65-70
27. Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst). 2016;42:63-71
28. Hnit SS, Xie C, Yao M, Holst J, Bensoussan A, De Souza P. et al. p27Kip1 signaling: Transcriptional and post-translational regulation. Int J Biochem Cell Biol. 2015;68:9-14
29. Soria G, Gottifredi V. PCNA-coupled p21 degradation after DNA damage: the exception that confirms the rule? DNA Repair (Amst). 2010;9(4):358-364
30. Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene. 1998;17(8):931-939
31. Thomas DM, Carty SA, Piscopo DM, Lee JS, Wang EF, Forrester WC. et al. The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell. 2001;8(2):303-316
32. Yamato K, Hashimoto S, Imamura T, Uchida H, Okahashi N, Koseki T. et al. Activation of the p21CIPI/WAF1 promoter by bone morphogenetic protein-2 in mouse B lineage cells. Oncogene. 2001;20(32):4383-4392
33. Yamato K, Hashimoto S, Okahashi N, Ishisaki A, Nonaka K, Koseki T. et al. Dissociation of bone morphogenetic protein-mediated growth arrest and apoptosis of mouse B cells by HPV-16 E6/E7. Exp Cell Res. 2000;257(1):198-205
34. Sciandra M, Marino MT, Manara MC, Guerzoni C, Grano M, Oranger A. et al. CD99 drives terminal differentiation of osteosarcoma cells by acting as a spatial regulator of ERK1/2. J Bone Miner Res. 2014;29(5):1295-1309
35. Drissi H, Hushka D, Aslam F, Nguyen Q, Buffone E, Koff A. et al. The cell cycle regulator p27kip1 contributes to growth and differentiation of osteoblasts. Cancer Res. 1999;59(15):3705-3711
36. Zhu M, Zhang J, Dong Z, Zhang Y, Wang R, Karaplis A. et al. The p27 pathway modulates the regulation of skeletal growth and osteoblastic bone formation by parathyroid hormone-related peptide. J Bone Miner Res. 2015;30(11):1969-1979
37. Czekanska EM, Stoddart MJ, Ralphs JR, Richards RG, Hayes JS. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J Biomed Mater Res A. 2014;102(8):2636-2643
Author contact
![]() Corresponding author: Kuo-Chin Huang, MD, PhD and Shun-Fu Chang, PhD. Chiayi Chang Gung Memorial Hospital, No. 6, West Sec., Chiapu Rd., Putz City, Chiayi County 61363, Taiwan. Tel: (+886)-5-362-1000 ext. 2855 E-mail: kc2672com and sfchangorg.tw
Corresponding author: Kuo-Chin Huang, MD, PhD and Shun-Fu Chang, PhD. Chiayi Chang Gung Memorial Hospital, No. 6, West Sec., Chiapu Rd., Putz City, Chiayi County 61363, Taiwan. Tel: (+886)-5-362-1000 ext. 2855 E-mail: kc2672com and sfchangorg.tw

 Global reach, higher impact
Global reach, higher impact