Impact Factor
ISSN: 1449-1907
Int J Med Sci 2019; 16(4):494-500. doi:10.7150/ijms.30380 This issue Cite
Research Paper
Anti-Cancer Effects of Sulfasalazine and Vitamin E Succinate in MDA-MB 231 Triple-Negative Breast Cancer Cells
1. Department of Nutrition, Master Program of Biomedical Nutrition, Hungkuang University, Taichung 433
2. Departments of Nursing, Hungkuang University, Taichung 433
3. Graduate Institute of Biomedical Sciences, China Medical University, Taichung 404
4. Drug Development Center, China Medical University, Taichung 404
5. Center for Molecular Medicine, China Medical University Hospital, Taichung 404
6. Department of Biotechnology, Asia University, Taichung 413
7. Department of Nursing, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei 231
8. Department of Emergency Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei 231
9. Department of Emergency Medicine, School of Medicine, Tzu Chi University, Hualien 970, Taiwan, R.O.C.
*The authors contributed equally to this study.
Received 2018-10-2; Accepted 2019-2-12; Published 2019-3-9
Abstract
Aim: Sulfasalazine (SSZ) displayed anti-cancer activities. Vitamin E succinate (VES) could inhibit cell growth in various cancer cells. However, chemical therapies were often not useful for triple-negative breast cancer cells (TNBCs) treatment. Here, this study investigated the anti-cancer effects and the mechanisms on TNBCs under combination treatment with SSZ and VES.
Methods: Cell viability was analyzed by using the MTT assay. The H2O2 levels were determined by using lucigenin-amplified chemiluminescence method. In addition, caspase and MAPs signals were studied by using western blotting.
Results: Low-dose VES antagonized the SSZ-induced cytotoxicity effects while high-dose VES promoted the SSZ-induced cytotoxicity effects on TNBCs. In addition, SSZ alone treatment activated both caspase-3 and ERK signals, however, VES alone treatment only activated JNK signals. On the other hand, activation of caspase-3, JNK, and ERK were found in SSZ plus VES-treated cells.
Conclusion: Combined SSZ and VES has synergistic or antagonistic cytotoxic effects depending on VES concentration. In addition, different cytotoxic signals are induced on SSZ-treated, VES-treated and SSZ plus VES-treated cells.
Keywords: vitamin E succinate, sulfasalazine, triple-negative breast cancer cells
Introduction
Triple-negative breast cancer cells (TNBCs) are estrogen receptors-deficient, progesterone receptors-deficient and epidermal growth factor receptor 2-deficient breast cancer, therefore, endocrine and targeted therapies do not applied for clinical TNBCs treatment [1, 2]. Today, to develop a potential therapy for TNBCs is important due to there are not useful clinical treatment for TNBCs [3, 4]. Sulfasalazine (SSZ), an anti-inflammatory drug, is commonly used as a first-line treatment for many rheumatic diseases [5, 6]. On the other hand, many studies has demonstrated that SSZ can inhibit cell proliferation on various cancers including primary brain tumors, lung adenocarcinoma cells, Hepatocellular carcinoma cells and glioma cells [7-11]. Previous studies also showed SSZ can inhibit cell proliferation of breast cancers including MCF-7 cells (ER-negative breast cancer) and MDA-MB-231 cells (TNBC) though many signal pathways remain to study [12, 13]. However, SSZ can cause adverse effects in human containing mitochondrial dysfunction and acute renal injury [14, 15]. In order to promote anti-cancer activity and decrease SSZ-induced adverse effects, many studies suggested SSZ in combination with other therapies may be a useful treatment for cancer treatment [9, 10].
Vitamin E succinate (VES) is the most useful form of vitamin E derivatives to inhibit cancer proliferation. VES has broad anti-cancer effects to suppress cell growth by inducing mitochondria dysfunction and apoptosis [16-18]. In addition, many studies showed VES can inhibit cell growth on various hormone-dependent breast cancer cells such as MCF-7 and MDA-MB-435 cells[19-21]. However only few study indicated VES can inhibit TNBCs proliferation [22, 23]. The studies showed that VES inhibit cell growth inefficiently on TNBCs, only high-dose VES can suppress TNBCs proliferation. In addition, VES can induce apoptosis and activate Fas signals on TNBCs while lots of mechanisms remained unclear.
The mitogen‑activated protein kinase (MAPK) signaling pathways majorly contain three phosphorylation signals: ERK, JNK and p38 phosphorylation[24-26]. Many studies demonstrated the MAPK signaling pathways control cell proliferation, cell death and differentiation[26-28]. SSZ is majorly used as a NF-κB inhibitor in many studies [11, 29]. Only few studies to investigate whether SSZ influences MAPK signals. Previous studies showed that SSZ can activate p38 phosphorylation in cholangiocarcinoma and melanocytes [30, 31]. However, whether SSZ can activate MAPK signals in TNBCs remained unclear. On the other hand, VES has anti-cancer effects on various cancers.[16-18]. Previous studies showed that VES-induced-apoptosis may activate ERK pathway on human gastric cancer cells [32, 33] and VES- induced-apoptosis mediated ERK and JNK pathways on hormone-dependent breast cancer cells [19]. However, whether VES can induce MAPK signals in TNBCs is unclear. In this study, the anti-cancer effects on SSZ-treated, VES-treated and SSZ/VES-treated TNBC cells were studied. Our study firstly showed VES has a synergistic or an antagonistic cytotoxic effect on SSZ-treated cells depending on the concentration of VES. In addition, different signal pathways were induced on SSZ-treated, VES-treated and SSZ/VES-treated TNBC cells.
Materials and methods
Materials
Vitamin E succinate, Luminol and Lucigenin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Anti-tubulin (1:1,000; cat. no. BS1699), anti-p38 (1:2000; cat. no. BS3567), anti-p-p38 (1:2000; cat. no. BS4766), anti-ERK (1:2000; cat. no. BS1112), anti-p-ERK (1:2000; cat. no. BS5016), anti-JNK (1:2000; cat. no. BS1544), and anti-p-JNK (1:2000; cat. no. BS4763) primary rabbit polyclonal antibodies were obtained from Bioworld (Louis Park, MN, USA). Anti-cleaved PARP (1:2000; cat. no. 9544) and anti-caspase-3 (1:1000; cat. no. 9965) primary rabbit polyclonal antibodies and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:2,000, cat. no. 7074) were obtained from Cell Signaling Technology (Danvers, MA, USA). The MTT assay kit was obtained from BIO-BASIC CANADA INC (Markham, OT, Canada). Fetal bovine serum, Dulbecco's modified Eagles medium (DMEM), non-essential amino acids, L-glutamine, and penicillin/streptomycin were obtained from GIBCO BRL (Invitrogen Life Technologies, Carlsbad, CA, USA). Sulfasalazine was kindly obtained from Dr. Chou PL (Division of Allergy-Immunology-Rheumatology, Department of Internal Medicine, Saint Mary's Hospital Luodong, Yilan, 265, Taiwan, R.O.C.).
Cell line and cell culture
MDA-MB-231 (Triple-negative breast cancer cell line) was obtained from the Bioresource Collection and Research Center (Shin Chu, Taiwan). MDA-MB-231 cells was cultured in a humidified atmosphere containing 5% CO2 at 37 ºC and supplies the cells with DMEM media containing 10% fetal bovine serum, 0.1 mM non-essential amino acids, 2 mM L-glutamine, and 100 IU/ml penicillin/streptomycin.
Cell viability assay
Cell viability was analyzed by using the MTT assay kit described in previous studies[26, 34]. In brief, MDA-MB-231cells were cultured into 96-well culture dish (1×104 cells/well). Every 24 hour, the MTT assay kit was added into the control and experimental groups. After incubation for 3 hours at 37ºC, the purple formazan products were measured at 570 nm (A570) using a Multiskan™ FC Microplate Photometer (Molecular Devices, Sunnyvale, CA, USA). The cell viability (%) was indicated as (A570 experimental group)/(A570 control group) × 100%.
Measurements of H2O2 levels
Intracellular H2O2 levels was determined using the lucigenin-amplified chemiluminescence method [26, 35]. The control and experimental groups (200 μl) were treated with 0.2 mmol/ml of luminol solution (100 μl). Next, all samples were observed and analyzed for 5 minutes using a chemiluminescence analyzing system (CLA-FSI; Tohoko Electronic Industrial Co., Ltd., Sendai, Japan).
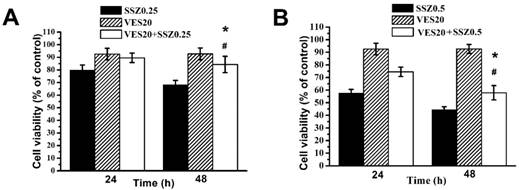
Cell viability of SSZ plus low-dose VES treatments. (A) MDA-MB-231 cells were treated with 0.25 mM SSZ, 20 μM VES, or 0.25 mM SSZ plus 20 μM VES. (B) MDA-MB-231 cells were treated with 0.5 mM SSZ, 20 μM VES, or 0.5 mM SSZ plus 20 μM VES. The 24- and 96-hour cell viability were determined by MTT assay and calculated as A570 experimental group/A570 control group × 100%. Results were obtained from four independent experiments and presented as mean ± SD. *P<0.05, compared to SSZ alone group. #P<0.05, compared to VES alone group.
SDS page and western blotting
Control and experimental cells were lysed in the radio-immunoprecipitation assay buffer (cat. no. 20-188; EMD Millipore, Billerica, MA, USA). Cellular proteins were obtained from the supernatant with centrifugation (16,000 × g; 4 ºC) for 20 min. The protein concentration was determined using a protein assay kit (cat. no. 23200; Thermo Fischer Scientific, Inc., Waltham, MA, USA). Equal quantities (40 μg) of protein were separated by SDS-PAGE (13.3% gels, 80 volts) and transferred onto polyvinylidene difluoride membranes (EMD Millipore). The membranes were treated with 5% non-fat milk at room temperature for 2 hours and washed with PBS buffer for 15 minutes (three times). Next membranes were incubated with primary antibodies at room temperature for 4 hours. After membranes were washed with PBS buffer for 15 minutes, the membranes were treated with anti-rabbit HRP-conjugated secondary antibodies at room temperature for 1 hour. Finally, the membranes were treated with Western Lightning® Chemiluminescence Plus reagent (PerkinElmer, Inc., Waltham, MA, USA) and observed with a Luminescence Image Analysis system (LAS-4000, FUJIFILM Electronic Materials Taiwan Co., Ltd., Tainan, Taiwan).
Statistical Analysis
All data were analyzed from four independent experiments. The values are presented as the mean ± standard error. Student's t‑test was used for the analysis of the data using Microsoft Excel (http://microsoft-excel-2010.updatestar.com/zh-tw). P value < 0.05 was considered statistically significant.
Results
Low-dose VES antagonizes SSZ-induced cytotoxic effects
Combination treatments of SSZ with low-dose VES (20 μM) on MDA-MB-231 cells was studied. Our data showed that the percentages of cell viability were about 70%, 93% and 80% with 0.25 mM SSZ, 20 μM VES and 0.25 mM SSZ plus 20 μM VES treatments at 48 hours respectively (Fig. 1A). The data indicated low-dose VES attenuated 0.25 mM SSZ-induced cytotoxicity on MDA-MB-231 cells. In addition, the percentages of cell viability were about 47%, 93% and 60% with 0. 5 mM SSZ, 20 μM VES and 0. 5 mM SSZ plus 20 μM VES treatments at 48 hours respectively (Fig. 1B). The date also suggested low-dose VES decreased 0.5 mM SSZ-induced cytotoxicity on MDA-MB-231 cells. These results demonstrated that low-dose VES antagonized SSZ-induced cytotoxic effects on MDA-MB-231 cells.
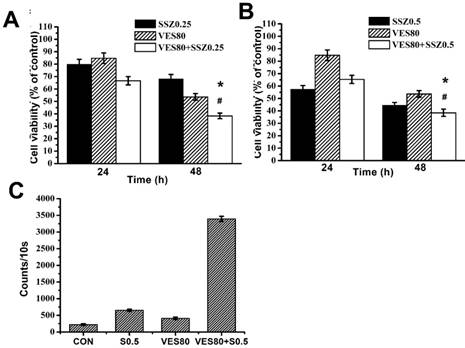
High-dose VES has synergistic effects on SSZ-induced cytotoxicity
Combination treatments of SSZ with high-dose VES (80 μM) on MDA-MB-231 cells was further determined. The percentages of cell viability were about 70%, 55% and 38.36% with 0.25 mM SSZ, 80 μM VES and 0.25 mM SSZ plus 80 μM VES treatments at 48 hours respectively (Fig. 2A). The data indicated high-dose VES promotes 0.25 mM SSZ-induced cytotoxicity. In addition, the percentages of cell viability were about 47%, 55% and 38.47% with 0.5 mM SSZ, 80 μM VES and 0.5 mM SSZ plus 80 μM VES treatments at 48 hours respectively (Fig. 2B). The data also indicated high-dose VES increased 0.5 mM SSZ-induced cytotoxicity on MDA-MB-231 cells. These results suggested high-dose VES has synergistic effects on SSZ-induced cytotoxicity on MDA-MB-231 cells. Next, intracellular H2O2 counts were determined and was increased in SSZ plus VES-treated group (Fig. 2C). The data indicated H2O2 might be related to synergistic cytotoxic effects on SSZ plus VES-treated MDA-MB-231 cells.
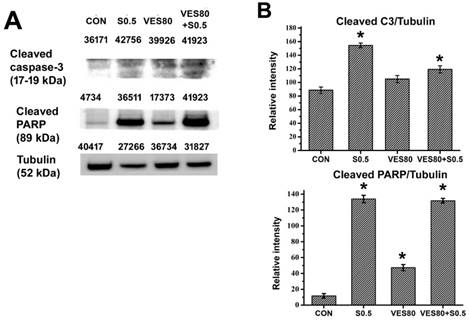
Caspase-3 activation is found in SSZ-treated and VES plus SSZ-treated cells
Whether caspase-3 signals related to SSZ-induced, VES-induced or SSZ plus VES-induced cytotoxicity was determined. Cleaved caspase-3 and caspase-3 were assayed by western blot, the data showed the ratio of cleaved casoase-3/caspase-3 was increased in SSZ-treated and VES plus SSZ-treated groups (Fig. 3A). PARP is a downstream substrate of caspase-3. PARP can be cleaved by activated caspase-3. Cleaved PARP was determined in SSZ-treated, VES-treated and VES plus SSZ-treated cells. Similar to the result of caspase-3 activity, cleaved PARP was increased in SSZ-treated and VES plus SSZ-treated groups (Fig. 3B). Taken together, these data indicated that SSZ- and VES plus SSZ-induced cell death were associated with caspase-3 signals.
Cell viability and Intracellular H2O2 levels of SSZ plus high-dose VES treatments. (A) MDA-MB-231 cells were treated with 0.25 mM SSZ, 80 μM VES, or 0.25 mM SSZ plus 80 μM VES. (B) MDA-MB-231 cells were treated with 0.5 mM SSZ, 80 μM VES, or 0.5 mM SSZ plus 80 μM VES. The 24- and 96-hour cell viability were determined by MTT assay and calculated as A570 experimental group/A570 control group × 100%. *P<0.05, compared to SSZ alone group. #P<0.05, compared to VES alone group. (C) H2O2 counts in the control group (CON), 0.5 mM SSZ group (S0.5), 80 μM VES group (VES80), and 0.5 mM SSZ plus 80 μM VES group (VES80+S0.5). Results were obtained from four independent experiments and presented as mean ± SD.
Caspase-3 activation and cleaved PARP. (A) Cleaved caspase-3, cleaved PARP and tubulin were assayed by western blot. The number indicated above the protein band was individual intensity value. (B) Cleaved caspase-3/tubulin and cleaved PARP/tubulin intensity ratios were calculated. These proteins were determined after 48 hours treatments in control (CON), 0.5 mM SSZ (S0.5), 80 μM VES (VES80) and 0.5 mM SSZ plus 80 μM VES (VES80+S0.5) groups. Results were obtained from three independent experiments and presented as mean ± SD. *P<0.05, compared to control group.
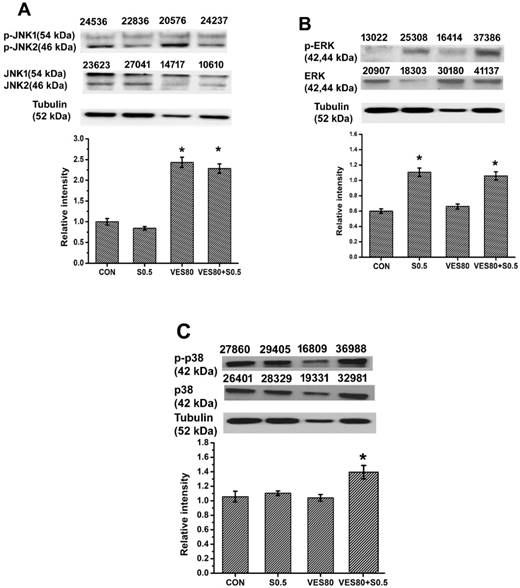
Different phosphorylated MAPKs are found in SSZ-treated, VES-treated and VES plus SSZ-treated cells
MAPKs contain JNK, ERK and p38 pathways. Activation of JNK, ERK or p38 was determined by western blot and the ratio of activated (phosphorylated) forms were calculated (Fig. 4). The ratio of p-JNK to JNK was increased in VES-treated and VES plus SSZ-treated cells (Fig. 4A). The data indicated JNK phosphorylation was majorly related to VES treatment. In addition, the ratio of p-ERK to ERK was increased in SSZ-treated and VES plus SSZ-treated cells (Fig. 4B). The data indicated ERK phosphorylation was majorly related to SSZ treatment. However, the ratio of p-p38 to p38 was only increased in VES plus SSZ-treated cells (Fig. 4C). The data indicated p38 phosphorylation was only induced by combination treatments of SSZ with VES. That is different phosphorylation of MAPKs was induced by different treatments (SSZ, VES, or VES plus SSZ) on MDA-MB-231 cells.
Discussion
In this study, combination treatment of high-dose VES with SSZ exerted a synergistic anti-cancer activity on MDA-MB-231 cell (Fig. 2). Interestingly, low-dose VES has an antagonistic effect on VES-treated MDA-MB-231 cells (Fig. 1). Recently, a study demonstrated high-dose sodium selenite and VES exerted cytotoxic effects on breast cancers while low-dose sodium selenite could antagonize VES-induced cytotoxic effect[20]. Our study has a similar result to this study. Therefore, these studies indicated synergistic or antagonistic effects might be a dose-dependent manner. In addition, our data showed that the cell viability was about 38% on 0.5 mM SSZ plus 80 μM VES-treated group (Fig. 2B) and the cell viability was also about 38% on 0.25 mM SSZ plus 80 μM VES-treated group (Fig. 2A). The data suggested combination of 0.25 mM SSZ with 80 μM VES might be a good choice for MDA-MB-231 treatment.
Previously, some studies indicated that oxidative stress might be involved in SSZ- and VES-induced cell cytotoxicity[36-39]. Whether SSZ and VES can induce oxidative stress on MDA-MB-231 cells has remained unclear. Both intracellular H2O2 and O2- belonging to ROS family can induce oxidative stress and cytotoxicity[26, 35]. SSZ, VES and SSZ plus VES treatments could induce cell cytotoxicity, however, H2O2 levels increased was only found in SSZ plus VES-treated group (Fig. 2C). We suggested intracellular H2O2 was not major factor in SSZ- and VES-induced cytotoxicity on MDA-MB-231cells while H2O2 levels increased might play an important role to induce synergistic cytotoxic effects on SSZ/VES-treated MDA-MB-231 cells. On the other hand, O2- levels were not significantly increased in SSZ-, VES- and SSZ plus VES-treated groups (data not show). These results were similar to the acetaminophen-induced and methotrexate-induced cytotoxicity as described in previous studies[26, 40].
MAPKs phosphorylation analysis. (A) JNK phosphorylation (containing JNK1 and JNK2) was assayed by western blot and phosphorylated JNK(1/2)/JNK(1/2) intensity ratio was calculated after 60 minutes treatments. (B) ERK phosphorylation was assayed by western blot and phosphorylated ERK/ERK intensity ratio was calculated after 60 minutes treatments. (C) p38 phosphorylation was assayed by western blot and phosphorylated p38/p38 intensity ratio was calculated after 30 minutes treatments. These proteins were determined in control (CON), 0.5 mM SSZ (S0.5), 80 μM VES (VES80) and 0.5 mM SSZ plus 80 μM VES (VES80+S0.5) groups. The number indicated above the protein band was individual intensity value. Results were obtained from three independent experiments and presented as mean ± SD. *P<0.05, compared to control group.
Caspases activation can induce cell death has been reported [26, 40]. Previous studies showed SSZ-induced cell death mediated caspase-3 activity [41, 42] as well as VES can induced caspase-3 activity resulting in cell death [21, 43]. Today our data showed that caspase-3 could be activated and PARP could be cleaved in SSV- and SSZ plus VES-treated groups. However, caspase-3 activation and PARP cleavage did not found in VES-treated group. These data suggested caspase-3 signal was involved in SSZ-induced cytotoxicity on MDA-MB-231 cells. However, caspase-3 signal was not activated in VES-induced cytotoxicity on MDA-MB-231 cells. A previous study showed VES-induced cytotoxicity can mediate caspase-3 independent pathways[44]. Similar to this study, our data indicated VES-induced cytotoxicity on MDA-MB-231 cells might mediate caspase-3-independent pathway.
MAPK signaling pathways mediated cellular biologic responses including growth, cell death, differentiation and inflammation have been reported [26-28]. Many studies found the cellular biologic responses might be through different MAPKs activation with distinct treatments. Previous studies showed SSZ could activate p38 signals in cholangiocarcinoma and melanocytes while VES could activate ERK and JNK signals in hormone-dependent breast cancer cells[30-33]. Unlike above studies, today, our data showed that ERK activation but not p38 activation was found in SSZ-treated MDA-MB-231 cells. In addition, only JNK activation was found in VES-treated MDA-MB-231 cells. Taken together, these studies suggested different MAPKs could be activated by using same drugs in different cancers. Furthermore, our data showed p38 activation only found in VES plus SSZ-treated MDA-MB-231 cells but not found in VES- and SSZ-treated groups. Our data also showed H2O2 levels increased was only found in SSZ plus VES-treated group (Fig. 2C). On the other hand, previous studies demonstrated oxidative stress was related to p38 activation [45, 46]. Therefore, we considered the H2O2 levels increased might be association with p38 activation on MDA-MB-231 cells with SSZ plus VES treatment.
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology, Taiwan (MOST106-2320-B-039-051-MY3; MOST107-2320-B-039-004); the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-112-015); the Taipei Tzu Chi Hospital, Taiwan (TCRD-TPE-106-35; TCRD-TPE-106-36; TCRD-TPE-105-20; TCRD-TPE-105-02) and the work was financially supported by the “Drug Development Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan, ROC.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Wu CW, Liu HC, Yu YL, Hung YT, Wei CW, Yiang GT. Combined treatment with vitamin C and methotrexate inhibits triple-negative breast cancer cell growth by increasing H2O2 accumulation and activating caspase-3 and p38 pathways. Oncology reports. 2017;37:2177-84
2. Robinson TJ, Liu JC, Vizeacoumar F, Sun T, Maclean N, Egan SE. et al. RB1 status in triple negative breast cancer cells dictates response to radiation treatment and selective therapeutic drugs. PloS one. 2013;8:e78641
3. Pelicano H, Zhang W, Liu J, Hammoudi N, Dai J, Xu RH. et al. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: role of mTOR pathway and therapeutic potential. Breast cancer research: BCR. 2014;16:434
4. Williams CB, Soloff AC, Ethier SP, Yeh ES. Perspectives on Epidermal Growth Factor Receptor Regulation in Triple-Negative Breast Cancer: Ligand-Mediated Mechanisms of Receptor Regulation and Potential for Clinical Targeting. Advances in cancer research. 2015;127:253-81
5. Cildag S, Senturk T. Sulfasalazine-Related Hypersensitivity Reactions in Patients With Rheumatic Diseases. Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases. 2017;23:77-9
6. Narayan N, Rigby S, Carlucci F. Sulfasalazine induced immune thrombocytopenia in a patient with rheumatoid arthritis. Clinical rheumatology. 2017;36:477-9
7. Chung WJ, Sontheimer H. Sulfasalazine inhibits the growth of primary brain tumors independent of nuclear factor-kappaB. Journal of neurochemistry. 2009;110:182-93
8. Lay JD, Hong CC, Huang JS, Yang YY, Pao CY, Liu CH. et al. Sulfasalazine suppresses drug resistance and invasiveness of lung adenocarcinoma cells expressing AXL. Cancer research. 2007;67:3878-87
9. Yerokun T, Winfield LL. Celecoxib and LLW-3-6 Reduce Survival of Human Glioma Cells Independently and Synergistically with Sulfasalazine. Anticancer research. 2015;35:6419-24
10. Song Y, Jang J, Shin TH, Bae SM, Kim JS, Kim KM. et al. Sulfasalazine attenuates evading anticancer response of CD133-positive hepatocellular carcinoma cells. Journal of experimental & clinical cancer research: CR. 2017;36:38
11. Su J, Liu F, Xia M, Xu Y, Li X, Kang J. et al. p62 participates in the inhibition of NF-kappaB signaling and apoptosis induced by sulfasalazine in human glioma U251 cells. Oncology reports. 2015;34:235-43
12. Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Suppression of cystine uptake by sulfasalazine inhibits proliferation of human mammary carcinoma cells. Anticancer research. 2003;23:4571-9
13. Narang VS, Pauletti GM, Gout PW, Buckley DJ, Buckley AR. Sulfasalazine-induced reduction of glutathione levels in breast cancer cells: enhancement of growth-inhibitory activity of Doxorubicin. Chemotherapy. 2007;53:210-7
14. Niknahad H, Heidari R, Mohammadzadeh R, Ommati MM, Khodaei F, Azarpira N. et al. Sulfasalazine induces mitochondrial dysfunction and renal injury. Renal failure. 2017;39:745-53
15. Durando M, Tiu H, Kim JS. Sulfasalazine-Induced Crystalluria Causing Severe Acute Kidney Injury. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2017;70:869-73
16. Suntharalingam K, Song Y, Lippard SJ. Conjugation of vitamin E analog alpha-TOS to Pt(IV) complexes for dual-targeting anticancer therapy. Chemical communications (Cambridge, England). 2014;50:2465-8
17. Gao Y, Qi X, Zheng Y, Ji H, Wu L, Zheng N. et al. Nanoemulsion enhances alpha-tocopherol succinate bioavailability in rats. International journal of pharmaceutics. 2016;515:506-14
18. Angulo-Molina A, Reyes-Leyva J, Lopez-Malo A, Hernandez J. The role of alpha tocopheryl succinate (alpha-TOS) as a potential anticancer agent. Nutrition and cancer. 2014;66:167-76
19. Yu W, Liao QY, Hantash FM, Sanders BG, Kline K. Activation of extracellular signal-regulated kinase and c-Jun-NH(2)-terminal kinase but not p38 mitogen-activated protein kinases is required for RRR-alpha-tocopheryl succinate-induced apoptosis of human breast cancer cells. Cancer research. 2001;61:6569-76
20. Badr DM, Hafez HF, Agha AM, Shouman SA. The Combination of alpha-Tocopheryl Succinate and Sodium Selenite on Breast Cancer: A Merit or a Demerit? Oxidative medicine and cellular longevity. 2016;2016:4741694
21. Wang XF, Xie Y, Wang HG, Zhang Y, Duan XC, Lu ZJ. alpha-Tocopheryl succinate induces apoptosis in erbB2-expressing breast cancer cell via NF-kappaB pathway. Acta pharmacologica Sinica. 2010;31:1604-10
22. Gagic Z, Ivkovic B, Srdic-Rajic T, Vucicevic J, Nikolic K, Agbaba D. Synthesis of the vitamin E amino acid esters with an enhanced anticancer activity and in silico screening for new antineoplastic drugs. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2016;88:59-69
23. Turley JM, Fu T, Ruscetti FW, Mikovits JA, Bertolette DC 3rd, Birchenall-Roberts MC. Vitamin E succinate induces Fas-mediated apoptosis in estrogen receptor-negative human breast cancer cells. Cancer research. 1997;57:881-90
24. Chen JY, Zhang L, Zhang H, Su L, Qin LP. Triggering of p38 MAPK and JNK signaling is important for oleanolic acid-induced apoptosis via the mitochondrial death pathway in hypertrophic scar fibroblasts. Phytotherapy research: PTR. 2014;28:1468-78
25. Liu X, Ye F, Xiong H, Hu D, Limb GA, Xie T. et al. IL-1beta Upregulates IL-8 Production in Human Muller Cells Through Activation of the p38 MAPK and ERK1/2 Signaling Pathways. Inflammation. 2014;37:1486-95
26. Yiang GT, Yu YL, Lin KT, Chen JN, Chang WJ, Wei CW. Acetaminophen induces JNK/p38 signaling and activates the caspase-9-3-dependent cell death pathway in human mesenchymal stem cells. International journal of molecular medicine. 2015;36:485-92
27. Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. Journal of receptor and signal transduction research. 2015;35:600-4
28. He Y, Jian CX, Zhang HY, Zhou Y, Wu X, Zhang G. et al. Hypoxia enhances periodontal ligament stem cell proliferation via the MAPK signaling pathway. Genetics and molecular research: GMR. 2016:15
29. Kim YG, Ohta T, Takahashi T, Kushiro A, Nomoto K, Yokokura T. et al. Probiotic Lactobacillus casei activates innate immunity via NF-kappaB and p38 MAP kinase signaling pathways. Microbes and infection. 2006;8:994-1005
30. Thanee M, Loilome W, Techasen A, Sugihara E, Okazaki S, Abe S. et al. CD44 variant-dependent redox status regulation in liver fluke-associated cholangiocarcinoma: A target for cholangiocarcinoma treatment. Cancer science. 2016;107:991-1000
31. Chen RS, Song YM, Zhou ZY, Tong T, Li Y, Fu M. et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/beta-catenin pathway. Oncogene. 2009;28:599-609
32. Zhao Y, Wu K, Yu Y, Li G. [Roles of ERK1/2 MAPK in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells]. Wei sheng yan jiu = Journal of hygiene research. 2003;32:573-5
33. Zhao Y, Zhao X, Yang B, Neuzil J, Wu K. alpha-Tocopheryl succinate-induced apoptosis in human gastric cancer cells is modulated by ERK1/2 and c-Jun N-terminal kinase in a biphasic manner. Cancer letters. 2007;247:345-52
34. Wu TK, Wei CW, Pan YR, Cherng SH, Chang WJ, Wang HF. et al. Vitamin C attenuates the toxic effect of aristolochic acid on renal tubular cells via decreasing oxidative stressmediated cell death pathways. Molecular medicine reports. 2015;12:6086-92
35. Yu YL, Yiang GT, Chou PL, Tseng HH, Wu TK, Hung YT. et al. Dual role of acetaminophen in promoting hepatoma cell apoptosis and kidney fibroblast proliferation. Molecular medicine reports. 2014;9:2077-84
36. Linares V, Alonso V, Domingo JL. Oxidative stress as a mechanism underlying sulfasalazine-induced toxicity. Expert opinion on drug safety. 2011;10:253-63
37. Wang YR, Tian FL, Yan MX, Fan JH, Wang LY, Kuang RG. et al. Sulfasalazine inhibits inflammation and fibrogenesis in pancreas via NF-kappaB signaling pathway in rats with oxidative stress-induced pancreatic injury. Drug design, development and therapy. 2016;10:1743-51
38. Hassoun EA, Vodhanel J, Abushaban A. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. Journal of biochemical and molecular toxicology. 2004;18:196-203
39. Hassoun EA, Walter AC, Alsharif NZ, Stohs SJ. Modulation of TCDD-induced fetotoxicity and oxidative stress in embryonic and placental tissues of C57BL/6J mice by vitamin E succinate and ellagic acid. Toxicology. 1997;124:27-37
40. Yiang GT, Chou PL, Hung YT, Chen JN, Chang WJ, Yu YL. et al. Vitamin C enhances anticancer activity in methotrexatetreated Hep3B hepatocellular carcinoma cells. Oncology reports. 2014;32:1057-63
41. Lee YM, Kang M, Hwang JM, Lee S, Cho H, Kim YS. Sulfasalazine induces apoptosis of HBx-expressing cells in an NF-kappaB-independent manner. Virus genes. 2010;40:37-43
42. Bertolotto M, Dallegri F, Dapino P, Quercioli A, Pende A, Ottonello L. et al. Sulphasalazine accelerates apoptosis in neutrophils exposed to immune complex: Role of caspase pathway. Clinical and experimental pharmacology & physiology. 2009;36:1132-5
43. Kulikov AV, Vdovin AS, Zhivotovsky B, Gogvadze V. Targeting mitochondria by alpha-tocopheryl succinate overcomes hypoxia-mediated tumor cell resistance to treatment. Cellular and molecular life sciences: CMLS. 2014;71:2325-33
44. Fujita H, Shiva D, Utsumi T, Ogino T, Ogawa T, Abe K. et al. Alpha-tocopheryl succinate induces rapid and reversible phosphatidylserine externalization in histiocytic lymphoma through the caspase-independent pathway. Molecular and cellular biochemistry. 2010;333:137-49
45. Lu H, Wang B, Cui N, Zhang Y. Artesunate suppresses oxidative and inflammatory processes by activating Nrf2 and ROSdependent p38 MAPK and protects against cerebral ischemiareperfusion injury. Molecular medicine reports. 2018
46. Zhang QL, Wang W, Jiang Y, Tuya A, Dongmei, Li LL. et al. GRGM-13 comprising 13 plant and animal products, inhibited oxidative stress induced apoptosis in retinal ganglion cells by inhibiting P2RX7/p38 MAPK signaling pathway. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018;101:494-500
Author contact
![]() Corresponding author: Department of Emergency Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei 231, Taiwan, R.O.C. E-mail address: jtyiangcom.tw (G.-T. Yiang).
Corresponding author: Department of Emergency Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei 231, Taiwan, R.O.C. E-mail address: jtyiangcom.tw (G.-T. Yiang).

 Global reach, higher impact
Global reach, higher impact