3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2019; 16(3):470-476. doi:10.7150/ijms.28139 This issue Cite
Research Paper
Hes3 Enhances the Malignant Phenotype of Lung Cancer through Upregulating Cyclin D1, Cyclin D3 and MMP7 Expression
Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences of China Medical University, 110001, Shenyang, China.
Received 2018-6-26; Accepted 2019-2-8; Published 2019-3-9
Abstract
Hes3 is a basic helix-loop-helix factor gene, which was found to be involved in neural cell differentiation. Expression and clinicopathological significance of Hes3 in non-small cell lung cancer was not clear. In this study, we used immunohistochemistry to examine Hes3 expression in normal human lung and non-small cell lung cancer tissues. Hes3 expression was detected in cytoplasm and nucleus. Hes3 expression in bronchial epithelial cells and epithelial cells of submucosal glands was relatively weak and the positive rate was of 30.3% (10/33). Hes3 expression in non-small cell lung cancer tissues (51.8% (58/112)) was significantly higher than that in normal lung tissues (p < 0.05). Hes3 expression in cancer tissues was significantly associated with poor differentiation, advanced TNM stages, lymph node metastasis, and a shorter patient survival time (p < 0.05). In vitro study showed that overexpression of Hes3 in A549 cells significantly promoted cancer cell proliferation and invasion, while inhibition of Hes3 expression significantly downregulated cancer cell proliferation and invasion (p < 0.05). Western blotting showed that overexpression of Hes3 significantly upregulated expression of Cyclin D1, Cyclin D3, and MMP7 in A549 cells, while inhibition of Hes3 expression in LK2 cells significantly downregulated the expression of these molecules (p < 0.05). These results indicated that Hes3 may contribute to the malignant phenotype of non-small cell lung cancer, possibly through regulation of Cyclin D1, Cyclin D3, and MMP7, and may be a promising cancer marker.
Keywords: Hes3, NSCLC, Cyclin D1, Cyclin D3, MMP7
Background
Hairy/enhancer of split 3 (Hes3) is a basic helix-loop-helix (bHLH) gene mapped to human chromosome 1p36.31 [1, 2]. The proteins of Hes family have similarities and also differences in their structures and functions [3]. The full length of Hes3 has a particular type of basic domain that binds to the N-box (CACNAG) [4]. Unlike other members of the family, Hes3 lacks a domain that combines with the E-box. However, it can affect gene transcription by indirectly interacting with the E-box [3]. Hes3 was found to play important roles in neural cell differentiation [2, 3]. Hiromi's study [2] indicated that concurrent Hes3 and Hes1 mutations led to abnormal brain development in mice. Hes3 was also found to have roles in promoting self-renewal of neural stem cells [5]. In the process, Hes3 transcription was upregulated by a phosphorylated form of STAT3-Ser in a non-canonical Notch pathway [5]. However, the functioning of Hes3 is not clear yet. As STAT3-Ser was revealed to play important roles in regulating tumor proliferation, Hes3 was presumed to be involved in regulation of cancer proliferation. Jimmy's study [6] showed that Hes3 expression in pancreatic islet MIN6 cells was stimulated under serum-free conditions. Deric's study [7] indicated that Hes3 may be an important regulator of stem cell numbers in glioblastoma multiforme. However, the expression and function of Hes3 in human cancers is largely unknown. In the current study, we investigated the expression of Hes3 in healthy human lung and non-small cell lung cancer (NSCLC) tissues, its function, and the possible molecular mechanism in cancer cells in vitro.
Materials and Methods
Tissue samples
Lung and NSCLC tissue samples were obtained from patients at the First Affiliated Hospital of China Medical University. The tumors were diagnosed according to the criteria for classification of lung cancer published by the World Health Organization [8]. There were 45 cases of squamous cell carcinomas (SCCs) and 67 cases of adenocarcinomas. This study was approved by the Institutional Review Board of China Medical University. Informed consent was obtained from all enrolled patients.
Immunohistochemistry
Immunohistochemistry staining was performed using SP-kit according to the manufacturer's instructions and as described previously [9]. The primary antibody against Hes3 was purchased from Santa Cruz (USA). The evaluation of Hes3 immunostaining was performed as described previously [9]. Evaluation of IHC staining was based on two parameters: the proportion of immunopositive cells and the intensity. The proportion of positive cells was categorized as follows: 0: <10%; 1: ≥10% to <25%; 2: ≥25% to <50%; 3: ≥50% to <75% and 4: ≥75%. The immunostaining intensity was categorized as follows: 0: no positivity; 1: weak (pale yellow, without obvious grains); 2: moderate (pale brown, with small grains) and 3: strong (dark brown, with obvious large grains). A final immunoreactivity score was calculated by multiplying the two individual scores. A final score less than 2 was considered as negative. Scores of 2 or more were considered as positive.
Western blotting
Western blotting was performed as described previously [9]. Primary antibody against Hes3 (sc-55587, Santa Cruz, USA, dilution 1:200), MMP7 (#71031, Cell signaling, USA, dilution 1:500), Cyclin D1 (sc-4074, Santa Cruz, USA, dilution 1:200), Cyclin D3 (sc-135875, Santa Cruz, USA, dilution 1:200), and GADPH (ab8245, Abcam, HK; 1:1000) were purchased.
Cell culture and transfection
Human bronchial epithelial cell (HBE) and carcinoma cell AGZY-83a, H292, A549, LK2, NCI-H1299, LTE, and NCI-H460 were cultured in RPMI 1640 tissue culture medium (Invitrogen, Carlsbad, CA, USA), containing 10% fetal calf serum (Invitrogen), 100 IU/mL penicillin (Sigma, St Louis, MO, USA), and 100 μg/mL streptomycin (Sigma) at 37 °C in a humidified atmosphere (5% CO2). Hes3 cDNA clone was purchased from Origene (USA). Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was used for transient transfection according to the manufacturer's instructions and as described previously [9].
MTT assay
MTT assay was performed as described previously [10].
Matrigel invasion assay
24-well Transwell with 8-μm pore polycarbonate membrane inserts (Corning, NY, USA) and matrigel (BD Bioscience) was used to examine the invasive ability of cancer cells according to the manufacturer's instructions and as described previously [9].
Statistical analysis
We used SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) to analyze the data. Pearson's chi-squared test was used to analyze the association between Hes3 expression and the clinicopathological features. McNemar's test was used for comparison of Hes3 expression in healthy and cancerous lung tissues. All data of the in vitro study is expressed as mean ± standard deviation (S.D.) and the experiment was repeated at least 3 times. p-values of < 0.05 were considered significant.
Results
Expression of Hes3 in healthy human lung and NSCLC tissues
We used immunohistochemistry to detect Hes3 expression in NSCLC and paired normal lung tissues. Hes3 expression was detected in cytoplasm and nucleus of normal and cancer epithelial cells (Figure 1). Hes3 immunostaining in bronchial epithelial cells and epithelial cells of submucosal glands was generally weak and the total positive rate was of 30.3% (10/33). Hes3 expression in NSCLC tissues including squamous cell carcinoma and adenocarcinoma was higher than that in the normal lung tissues (p < 0.05). The total positive rate of Hes3 in cancer tissues was 51.8% (58/112).
Clinicopathological significance of Hes3 expression in NSCLC
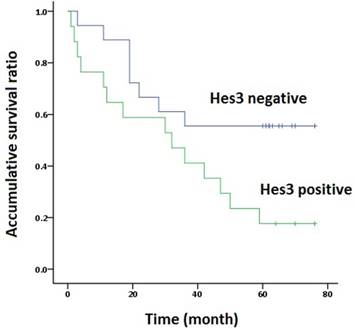
The association of Hes3 in NSCLC and the clinicopathological features is shown in Table 1. Hes3 expression was significantly associated with poor differentiation and advanced TNM stages (III + IV versus I + II) (p < 0.05). Hes3 expression in cancer tissues was also related with lymph node metastasis (p < 0.05). Survival time analysis revealed that the patients with Hes3 expression had shorter survival time (33.8 ± 6.4 m) than those without Hes3 expression (50.9 ± 6.8 m) (p < 0.05) (Figure 2).
Hes3 regulated cell proliferation and invasion in HBE, A549 and LK2 cells in vitro
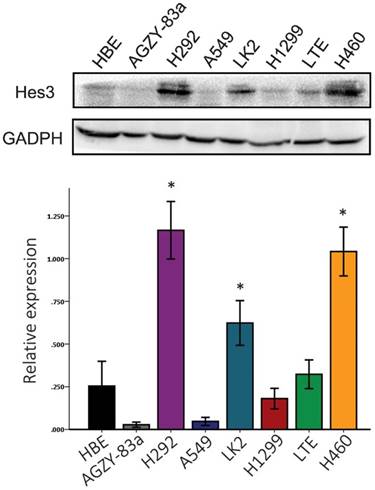
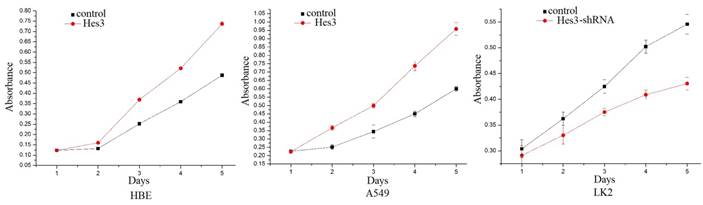
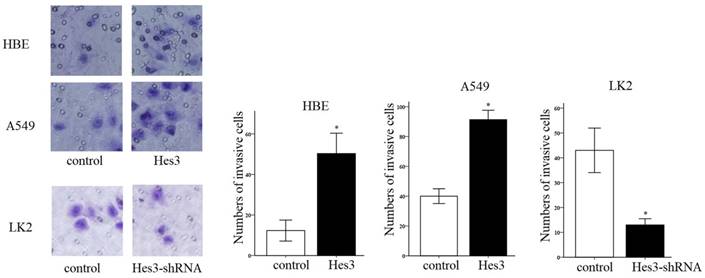
First, we examined the expression of Hes3 in HBE cells and carcinoma cells AGZY-83a, H292, A549, LK2, NCI-H1299, LTE, and NCI-H460 using western blotting. The results showed that Hes3 was expressed in all these cells in variable levels (Figure 3). Hes3 expression in HBE cells was relatively lower than that in lung cancer cells including H292, LK2, and NCI-H460 (p < 0.05). We selected A549 cells and HBE cells with lower Hes3 expression to investigate the function of Hes3 in epithelial and cancer cells. We transfected Hes3 cDNA in HBE and A549 cells to overexpress Hes3. MTT assay showed that overexpression of Hes3 in HBE and A549 cells significantly upregulated proliferation (Figure 4) (p < 0.05). Transwell study showed that upregulation of Hes3 in HBE and A549 cells significantly promoted the ability of cells to invade (Figure 5) (p < 0.05). We using transfection of Hes3 shRNA to inhibit Hes3 expression in LK2 cells. MTT assay showed that downregulation of Hes3 in LK2 cells significantly inhibited proliferation (figure 4) (p<0.05). Transwell study showed that downregulation of Hes3 expression in LK2 cells significantly inhibited the ability of invasion of cancer cells (figure 5) (p<0.05).
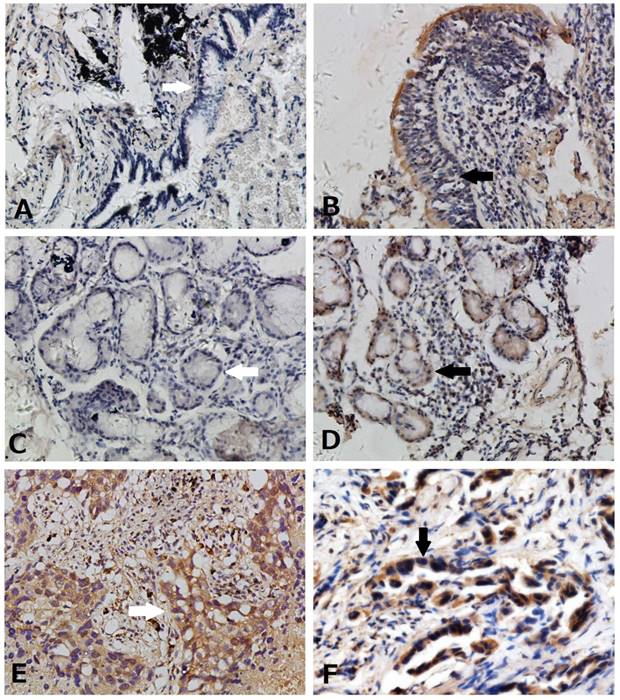
Expression of Hes3 in healthy lung and NSCLC tissues. (A) Negative (white arrow) and (B) weak expression (black arrow) of Hes3 in bronchial epithelial cells. (C) Negative (white arrow) and (D) weak expression (black arrow) of Hes3 in epithelial cells of the submucosal glands. Strong and diffused Hes3 expression was detected in cytoplasm and nuclei of cancer cells of (E) squamous cell carcinoma (white arrow) and (F) adenocarcinoma (black arrow) . (A, B, C, and D, 100X; E and F, 400X).
Survival analysis. The overall Kaplan-Meier survival curves demonstrated that the survival time of patients with Hes3 expression in NSCLC (33.8 ± 6.4 m) was significantly shorter than in those without Hes3 expression (50.9 ± 6.8 m) (Log rank analysis, p < 0.05).
Expression of Hes3 in cell lines in vitro. Hes3 expression was detected using western blotting in HBE and carcinoma cells AGZY-83a, H292, A549, LK2, NCI-H1299, LTE, and NCI-H460. Hes3 expression levels in H292, LK2, and H460 cells were significantly higher than those in HBE cells (*, p < 0.05).
Hes3 regulated Cyclin D1, Cyclin D3 and MMP7 expression in HBE, A549 and LK2 cells
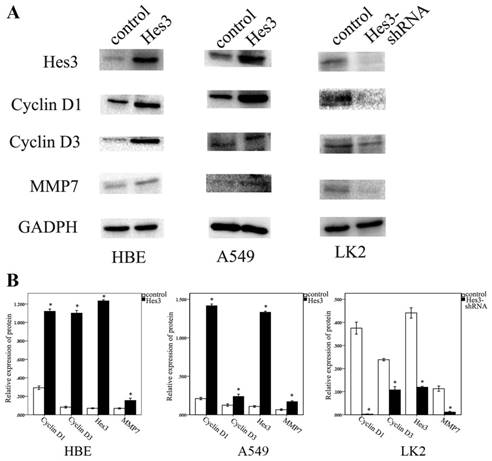
Next, we investigated the possible molecular mechanism underlying the function of Hes3 to promote cancer cell proliferation and invasion. Western blotting study showed that overexpression of Hes3 in HBE and A549 cells after transfection of Hes3 cDNA significantly upregulated the expression of Cyclin D1, Cyclin D3, and MMP7 (p < 0.05), while inhibition of Hes3 expression in LK2 cells significantly downregulated expression of Cyclin D1, Cyclin D3 and MMP7 (p<0.05) (figure 6 A, B).
Discussion
Currently, the research on the role of Hes3 is mainly focused on finding whether it is regulatory in certain special processes, but not on its effect on these processes. Corrinne's study [3] indicated that Hes3 played important roles in development of brain in the embryo. Hirata's research [2] showed that a mutation in Hes3 gene led to abnormal development of the brain in mice. Hes3 was also considered as a marker of neural endogenous putative progenitor cells of the brain [11]. It was found to be involved in the regulation of neural cell differentiation [11]. Hes3 expression was also found to be regulated during the process of demyelination and remyelination [12].
Hes3 was found to be a non-canonical Notch target gene [13]. In this signaling pathway, STAT3 gets phosphorylated at the ser site and leads to the transcription of Hes3 [5]. Hence, the pathway was also termed as STAT3-Hes3 signaling pathway, which was found to be involved in the regulation of neural cell differentiation [5, 14]. Transcription of Hes3 is regulated by STAT3 to decide the timing of neural cell differentiation [11], but the functioning of Hes3 is not clear yet.
Distribution of Hes3 status in non-small cell lung cancer according to clinicopathological features
| Characteristics | Numbers of patients | Hes3 immunostaining | p* | |
|---|---|---|---|---|
| negative | positive | |||
| Total | 112 | 54 | 58 | |
| Age(y) | ||||
| <55 | 27 | 14 | 13 | 0.664 |
| ≥55 | 85 | 40 | 45 | |
| Gender | ||||
| male | 66 | 29 | 37 | 0.278 |
| female | 46 | 25 | 21 | |
| Histological type | ||||
| squamous cell carcinoma | 45 | 21 | 24 | 0.788 |
| adenocarcinoma | 67 | 33 | 34 | |
| Grade | ||||
| well | 23 | 16 | 7 | 0.000 |
| Moderate and poor | 89 | 38 | 51 | |
| TNM stage | ||||
| Ⅰ and Ⅱ | 70 | 45 | 25 | 0.000 |
| Ⅲ and Ⅳ | 42 | 9 | 33 | |
| Lymph node metastasis | ||||
| No | 65 | 39 | 26 | 0.003 |
| Yes | 47 | 15 | 32 | |
* p values were obtained with the X2 test.
Regulation of cancer cell proliferation by Hes3. MTT study showed that overexpression of Hes3 in HBE and A549 cells significantly upregulated cancer cell proliferation and inhibition of Hes3 expression in LK2 cells significantly downregulated cancer cell proliferation (p < 0.05).
Regulation of cancer cell invasion by Hes3. Transwell study demonstrated that overexpression of Hes3 in HBE and A549 cells significantly upregulated cancer cell invasion and inhibition of Hes3 expression in LK2 cells significantly downregulated cancer cell invasion (*, p < 0.05).
Molecular mechanism behind the regulation by Hes3. Western blotting study showed that overexpression of Hes3 in HBE and A549 cells significantly upregulated Cyclin D1, Cyclin D3, and MMP7, while inhibition of Hes3 expression in LK2 cells significantly downregulated Cyclin D1, Cyclin D3, and MMP7 (*, p < 0.05)
There are few reports of Hes3 expression in healthy human tissues at present. Katoh's [15] integrative genomic analyses indicated expression of Hes3 in embryonic stem cells. Economopoulou [16] found that Hes3 expression was detected in human eye and pterygium, which indicated a possible function of STAT3-Hes3 signaling in these tissues. However, the exact function was unknown. In our current study, we found that Hes3 was also detected in healthy human lung tissues, though the immunostaining showed that its levels were generally low. On the contrary, we found that Hes3 was overexpressed in NSCLC tissues compared to healthy lung tissues. Moreover, the clinicopathological analysis showed that Hes3 may contribute to cancer development and poor clinical outcome. We also demonstrated that overexpression of Hes3 in A549 cells promoted cancer cell proliferation and invasion in vitro. Some studies have indicated a link between Hes3 and cell proliferation [6, 7, 17, 18]. Masjkur's study [6] showed that Hes3 may be an important regulator of growth of pancreatic islet cells and may affect insulin release. Their research showed that Hes3 can affect the number of specific cell groups in glioblastoma multiforme, namely the stem cells [7]. These studies mainly found that Hes3 was upregulated in these processes, but the functioning of Hes3 and the related molecular targets was not clear. In our study, we found that Hes3 could regulate the expression of Cyclin D1 and Cyclin D3, two novel Cyclins, which indicates that Hes3 may promote cancer cell proliferation through the regulation of cell cycle. Masjkur's study indicates that Hes3 regulates cell growth and gene expression including Golph3 in the adult pancreatic islet [6]. Wu's study proved that GOLPH3 promotes glioma progression through regulating Cyclin D1 and c-myc [19]. These studies may indicate a possible link between Hes3 and Cyclin Ds. However, the mechanism behind the regulation of these Cyclins needs to be further studied. Many proteins of the Matrix metalloproteinase (MMP) family have been found to be extracellular matrix remodeling endopeptidases involved in cancer invasion and metastasis [19]. We found in this study that MMP7, a member of this family, was upregulated by Hes3 in A549 cancer cells, which indicates that Hes3 may promote cancer cell invasion through the regulation of MMP7. Wang's study showed that GOLPH3 promotes Wnt/β‑catenin signal activation [21], which may indicate a possible link between Hes3 and MMP7, a downstream molecule of Wnt/β‑catenin signal. However, the detailed pathway is not clear yet.
Conclusion
Hes3 expression in human carcinomas was largely unknown. In the current study, we found that Hes3 was overexpressed in NSCLC compared to healthy lung tissues. Hes3 expression was significantly associated with cancer development. In vitro study showed that Hes3 could promote cancer cell proliferation and invasion, possibly through the regulation of Cyclin D1, Cyclin D3, and MMP7. These results indicated that Hes3 may be a promising cancer marker in NSCLC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 81472599 to Chuifeng Fan).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Katoh M, Katoh M. Identification and characterization of human HES2, HES3, and HES5 genes in silico. Int J Oncol. 2004;25(2):529-34
2. Hirata H, Tomita K, Bessho Y, Kageyama R. Hes1 and Hes3 regulate maintenance of the isthmic organizer and development of the mid/hindbrain. EMBO J. 2001;20(16):4454-66
3. Lobe CG. Expression of the helix-loop-helix factor, Hes3, during embryo development suggests a role in early midbrain-hindbrain patterning. Mech Dev. 1997;62(2):227-37
4. Hirata H, Ohtsuka T, Bessho Y, Kageyama R. Generation of structurally and functionally distinct factors from the basic helix-loop-helix gene Hes3 by alternative first exons. J Biol Chem. 2000;275(25):19083-9
5. Poser SW, Park DM, Androutsellis-Theotokis A. The STAT3-Ser/Hes3 signaling axis: an emerging regulator of endogenous regeneration and cancer growth. Front Physiol. 2013;4:273
6. Masjkur J, Arps-Forker C, Poser SW, Nikolakopoulou P, Toutouna L, Chenna R, Chavakis T, Chatzigeorgiou A, Chen LS, Dubrovska A, Choudhary P, Uphues I, Mark M, Bornstein SR, Androutsellis-Theotokis A. Hes3 is expressed in the adult pancreatic islet and regulates gene expression, cell growth, and insulin release. J Biol Chem. 2014;289(51):35503-16
7. Park DM, Jung J, Masjkur J, Makrogkikas S, Ebermann D, Saha S, Rogliano R, Paolillo N, Pacioni S, McKay RD, Poser S, Androutsellis-Theotokis A. Hes3 regulates cell number in cultures from glioblastoma multiforme with stem cell characteristics. Sci Rep. 2013;3:1095
8. Travis WD, Brambilla E, Burke AP, Marx A, Nicolson AG. WHO classification of tumors of the lung, pleura, thymus and heart. 2015. Lyon: IARC.
9. Chuifeng Fan, Yuan Miao, Xiupeng Zhang, Di Liu, Guiyang Jiang, Xuyong Lin, Qiang Han, Lan Luan, Zhonghai Xu, Enhua Wang. Btbd7 contributes to reduced E-cadherin expression and predicts poor prognosis in non-small cell lung cancer. BMC Cancer. 2014;14:704.c
10. Luan L, Zhao Y, Xu ZH, Jiang GY, Zhang XP, Fan CF, Liu D, Zhao HY, Xu K, Wang MH, Yu XM, Wang EH. Diversin increases the proliferation and invasion ability of non-small-cell lung cancer cells via JNK pathway. Cancer Lett. 2014;344(2):232-8
11. Pacioni S, Rueger MA, Nisticò G, Bornstein SR, Park DM, McKay RD, Androutsellis-Theotokis A. Fast, potent pharmacological expansion of endogenous hes3+/sox2+ cells in the adult mouse and rat hippocampus. PLoS One. 2012;7(12):e51630
12. Toutouna L, Nikolakopoulou P, Poser SW, Masjkur J, Arps-Forker C, Troullinaki M, Grossklaus S, Bosak V, Friedrich U, Ziemssen T, Bornstein SR, Chavakis T, Androutsellis-Theotokis A. Hes3 expression in the adult mouse brain is regulated during demyelination and remyelination. Brain Res. 2016;1642:124-130
13. Fukami M, Wada Y, Okada M, Kato F, Katsumata N, Baba T, Morohashi K, Laporte J, Kitagawa M, Ogata T. Mastermind-like domain-containing 1 (MAMLD1 or CXorf6) transactivates the Hes3 promoter, augments testosterone production, and contains the SF1 target sequence. J Biol Chem. 2008;283(9):5525-32
14. Nikolakopoulou P, Poser SW, Masjkur J, Fernandez Rubin de Celis M, Toutouna L, Andoniadou CL, McKay RD, Chrousos G, Ehrhart-Bornstein M, Bornstein SR, Androutsellis-Theotokis A. STAT3-Ser/Hes3 Signaling: A New Molecular Component of the Neuroendocrine System? Horm Metab Res. 2016;48(2):77-82
15. Katoh M, Katoh M. Integrative genomic analyses on HES/HEY family: Notch-independent HES1, HES3 transcription in undifferentiated ES cells, and Notch-dependent HES1, HES5, HEY1, HEY2, HEYL transcription in fetal tissues, adult tissues, or cancer. Int J Oncol. 2007;31(2):461-6
16. Economopoulou M, Masjkur J, Raiskup F, Ebermann D, Saha S, Karl MO, Funk R, Jaszai J, Chavakis T, Ehrhart-Bornstein M, Pillunat LE, Kunz-Schughart L, Kurth I, Dubrovska A, Androutsellis-Theotokis A. Expression of the transcription factor Hes3 in the mouse and human ocular surface, and in pterygium. Int J Radiat Biol. 2014;90(8):700-9
17. Parry PV, Engh JA. Hes3 regulates cell number in cultures from glioblastoma multiforme with stem cell characteristics. Neurosurgery. 2013;73(4):N15-6
18. Poser SW, Park DM, Androutsellis-Theotokis A. The STAT3-Ser/Hes3 signaling axis in cancer. Front Biosci (Landmark Ed). 2014;19:718-26
19. Wu S, Fu J, Dong Y, Yi Q, Lu D, Wang W, Qi Y, Yu R, Zhou X. GOLPH3 promotes glioma progression via facilitating JAK2-STAT3 pathway activation. J Neurooncol. 2018;139(2):269-79
20. Li G, Bu J, Zhu Y, Xiao X, Liang Z, Zhang R. Curcumin improves bone microarchitecture in glucocorticoid-induced secondary osteoporosis mice through the activation of microRNA-365 via regulating MMP-9. Int J Clin Exp Pathol. 2015;8(12):15684-95
21. Wang JH, Yuan LJ, Liang RX, Liu ZG, Li BH, Wen ZS, Huang ST, Zheng M. GOLPH3 promotes cell proliferation and tumorigenicity in esophageal squamous cell carcinoma via mTOR and Wnt/β-catenin signal activation. Mol Med Rep. 2017;16(5):7138-44
Author contact
![]() Corresponding author: Chuifeng Fan. Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences of China Medical University, 110001, Shenyang, China. E-mail: cffanedu.cn Tel.: +86 24 23261638; fax: +86 24 23261638.
Corresponding author: Chuifeng Fan. Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences of China Medical University, 110001, Shenyang, China. E-mail: cffanedu.cn Tel.: +86 24 23261638; fax: +86 24 23261638.

 Global reach, higher impact
Global reach, higher impact