3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(13):1530-1536. doi:10.7150/ijms.28470 This issue Cite
Research Paper
Effect of mistletoe on endometrial stromal cell survival and vascular endothelial growth factor expression in patients with endometriosis
1. Bom Women's cinic
2. Department of Obstetrics and Gynecology, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
* Jeong Min Moon and Youn-Jee Chung contributed equally to this work.
Received 2018-7-11; Accepted 2018-9-14; Published 2018-10-20
Abstract
Research Question: To evaluate the effect of mistletoe on the cell viability of patients with endometriosis, the expression levels of vascular endothelial growth factor (VEGF) were measured, and the change in the expression level of VEGF following mistletoe treatment was recorded.
Design: Forty reproductive-aged women with endometriosis (stage I/II [group 1, n=20], and stage III/IV [group 2, n=20]) were prospectively enrolled. Twenty women who underwent gynaecologic operations for benign conditions were selected as the control group. Both eutopic and ectopic endometrial tissues were obtained from the endometriosis patients. The endometrial tissues were cultured and the stromal cells were separated. The cells were cultured for 24 hours with peritoneal fluid from patients and controls with and without mistletoe supplementation (200 ng/mL), respectively. The MTT assay was used to assess cell viability, and VEGF expression was analysed by Western blotting and ELISA.
Results: Using peritoneal fluid from endometriosis patients treated with mistletoe, we found that both eutopic and ectopic endometrial stromal cell viability increased after treatment with peritoneal fluid from patients with early-stage (I and II) endometriosis. After mistletoe treatment, the cell viability was decreased, in both eutopic and ectopic endometrial stromal cells in all stages of endometriosis. These findings were verified consistently by evaluating the expression and concentration of VEGF, a marker of angiogenesis.
Conclusions: The present study showed that mistletoe can reduce the cell viability of endometrial stromal cells and the peritoneal fluid-induced elevation of VEGF in eutopic and ectopic endometrial stromal cells obtained from endometriosis patients, especially in the early stage. Mistletoe might have anti-angiogenic activity on endometrial stromal cells and thus is a potential candidate for the treatment of endometriosis.
Keywords: endometriosis, endometrial stromal cell, mistletoe, vascular endothelial growth factor
Introduction
Endometriosis, which is defined as the ectopic presence of endometrial glandular and stromal cells outside of the uterine cavity is one of the common benign gynaecologic disorders [1]. Endometriosis is often associated with chronic pelvic pain, dysmenorrhea, dyspareunia, and infertility. Endometriosis occurs in 10% of reproductive-age women, whereas the prevalence of endometriosis in women with chronic pelvic pain or infertility ranges from 20% to 90% [2]. Although the pathophysiology underlying endometriosis is not fully understood, the most accepted hypothesis is that endometrial cells pass through retrograde menstrual flow and implant in the abdominal cavity. On the basis of this hypothesis, it is thought that immunologic and biological factors influence the incidence of endometriosis. Retrograde menstrual flow is a common phenomenon in most women; however, there are several conditions that must be met to develop endometriosis. First, the endometrial cells in the abdominal cavity cannot be cleared or absorbed by immunological reactions. Second, the cells must evade apoptosis. Third, the cells must attach to the ectopic tissues of the peritoneal cavity, invade the interstitium, and be supplied by angiogenesis. Recently, the substances requisite for such conditions have been studied intensively, such as adhesion molecules, proteinases, growth factors, and angiogenic factors, and the expressions and activities of the substances have been compared [3-6]. To meet these conditions, peritoneal environment which is different from that in healthy women will be needed. Excessive cell proliferation and inadequate apoptosis have been considered two of the most abnormal features of endometriotic cells [7]. Increasing cell proliferation could result from inadequately activated ERK signaling in endometriotic tissues [8, 9]. Apoptosis, the process of programmed cell death, is precisely controlled for maintaining tissue homeostasis and removing senescent cells during the menstrual cycle from the human endometrium. It has been reported that changed apoptotic activity of endometriotic cells is related to the up-regulation of antiapoptotic factors. [10]. Some investigators suggested that endometriosis patients have a different peritoneum compared to healthy controls, which may help to attribute a pro-inflammatory environment, promoting the occurrence of endometriosis. In a study about molecular factors affecting endometriosis, various kinds of molecules such as IL-1ß, VEGF and IL-6 were related to the development and progression of endometriosis [11].
Mistletoe is a hemi-parasitic plant that grows attached to a wide range of host trees, and consists of mistletoe lectins, viscotoxins, vester protein, and Kuttan peptide. Mistletoe is known to stimulate and normalize the immune system so the growth of cancer cells is inhibited without killing normal cells [12-15]. Helixor®, the type of mistletoe used in the current study, is distinguished between A and M on the basis of trees upon which the mistletoe is parasitic. Helixor A®, which is hemi-parasitic on firs, is used clinically for treating skin allergies, breast cancer, and recto-sigmoid cancer. Moreover, Helixor A® has been reported to be effective in treating cancer, such as preventing the recurrence of cancer after surgery, inhibiting angiogenesis, stimulating bone marrow function, and curing endometriosis [16-20].
It has been reported that ectopic endometrial tissue transplantation is promoted by increased vascular endothelial growth factor (VEGF), uPA, and MMP-3 levels in the endometrium and abdominal cavity fluid of patients with endometriosis [21].
The current study evaluated the effect of mistletoe on cell viability of endometriosis, especially according to the early and advanced stage of endometriosis. To determine the anti-angiogenic effect of mistletoe, the expressions of VEGF was measured, and the change in expressions of VEGF following mistletoe treatment was recorded. Additionally, we determined if the anti-angiogenic effect of mistletoe could be used in the treatment of endometriosis, and if mistletoe could be used clinically.
Materials and Methods
Subjects and mistletoe
Forty women with endometriosis (stage I/II for group 1 [n=20], and stage III/IV for group 2 [n=20]) were designated as the experimental group. Twenty women with benign gynaecologic diseases, such as myomas (n=15) or ovarian dermoid cysts (n=5), were selected as the control group. During laparoscopic surgery for endometriosis, ectopic endometrial stromal cells from ovarian endometrioma tissue and eutopic endometrial stromal cells from the endometrium were collected. Forty samples of eutopic endometrial stromal cells were collected, and fifteen samples were used for the experiments. Twenty samples of ectopic endometrial stromal cells were collected, and ten samples were used for the experiments. Peritoneal fluid was also collected simultaneously. In the control group, peritoneal fluid was collected while laparoscopic surgery for other benign diseases. The stage of endometriosis was determined by the criteria of the American Society for Reproductive Medicine, which was revised after the development of laparoscopy [22]. The consent forms were signed by all patients, and the study was evaluated by the Council Committee of Seoul St. Mary's Hospital, Catholic University of Korea. Helixor A® (Boryung Co., Seoul, Korea) was used as the source of mistletoe.
Cell collection and culture from patients with endometriosis
Endometrial stromal cells were collected and cultured directly from both eutopic endometrium of the uterus (removed during endometriosis surgery) and ectopic endometrium of ovarian endometriomas. The endometrial tissue used in the current study was washed with phosphate-buffered saline (PBS), sliced under aseptic conditions, and treated with 0.25% collagenase I (Sigma, St. Louis, MO, USA) and 250 U/mL DNase I (15 U/mL; Takara Shuzo, Tokyo, Japan). After culturing for 30 minutes in an incubator at 37°C, the tissue was centrifuged for 3 minutes at 1,500 rpm. The precipitate was floated in Dulbecco's modified Eagle medium: a nutrient Mixture F-12 (DMEM/F12), which contained 10% foetal calf serum and 1% penicillin/streptomycin. Subsequently, eutopic endometrium was filtered through a 70-µm cell strainer, and ectopic endometrium was filtered through a 30-µm cell strainer. The filtered fluid was cultured in a 5% CO2 incubator at 37°C. After these processes, the morphologic characteristics were observed daily with an inverted microscope while the culture medium was changed every two days. If the cells were fully saturated, they were separated by trypsin-EDTA and sub-cultured three times, and endometrial stromal cells were acquired.
Analysis of cell viability
Endometrial stromal cells (1 × 104) were cultured in 96-well plates and treated with 10% control peritoneal fluid, or peritoneal fluid from patients with endometriosis stage I/II (group 1) or stage III/IV (group 2) for 24 hours; they were then treated with 200 ng/mL of mistletoe for 24 hours. The data were analysed at 450 nm and evaluated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT, Colorimetric assay kit; Chemicon Inc., CA, USA), which is a modification of the tetrazolium-based colorimetric assay.
Western blot
The cells (2 × 105) cultured on 100-mm2 dishes were collected and centrifuged for 5 minutes at 12,000 rpm. The cells extracted by centrifugation were lysed in 100 µL of lysis buffer at 4C for 30 minutes for Western blot analysis. Proteins were extracted from the lysed cells by centrifugation at 4C at 12,000 rpm, and the concentration of proteins was measured by the Bio-Rad Protein Assay (Bio-Rad, Philadelphia, PA, USA). Theproteins (50 µg) were boiled for 5 minutes and treated with electrophoresis for 2 hours at 100 V in a 10% sodium dodecyl sulphate (SDS)/polyacrylamide gel. The proteins were treated with electrophoresis for 1 hour with nitrocellulose paper at 360 mA, and the membrane was treated with 5% powdered skim milk and 0.05% Tween 20 for 1 hour to remove unnecessary factors. Additionally, membranes were reacted at 4°C overnight with adhesion molecule antibodies of VEGF and ß-actin (Cell Signaling Technology, Beverly, MA, USA), and washed with Tris-buffered saline Tween 20 (TBST) three times. Each membrane was left at room temperature for 1 hour with secondary antibody (Calbiochem, San Diego, CA, USA) and washed with TBST three times. Specific protein bands were acquired by an ECL Western blotting system (Amersham, Piscataway, NJ, USA).
Enzyme-linked immunosorbent assay (ELISA)
Endometrial stromal cells (1 x 104) were cultured in 96-well plates and treated with 10% control peritoneal fluid or peritoneal fluid from group 1 or group 2, then treated with 200 ng/mL of mistletoe for 24 hours. VEGF was identified using ELISA kits (R&D Systems, Minneapolis, MN, USA).
Statistical Analysis
The experiments (vide supra) were repeated five times to obtain the results, and the data were all recorded as mean ± standard deviation. The data were statistically calculated and analysed with one-way ANOVA and the Kruskal-Wallis test using SPSS 15 (SPSS, Inc., Chicago, IL, USA). The data were considered significant when the P-value was < 0.05.
Results
Analysis of endometrial stromal cell viability after treatment with peritoneal fluid
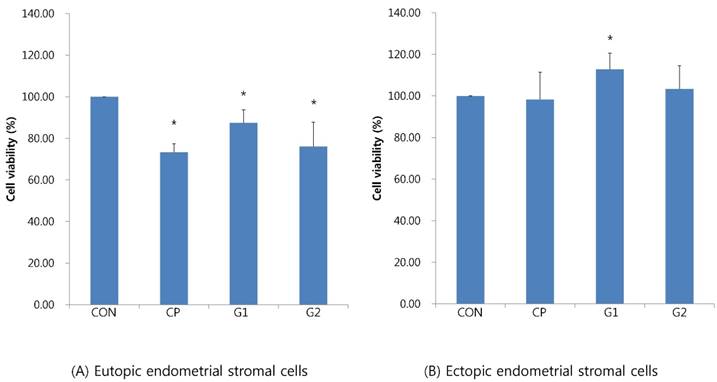
Four groups were compared with respect to cell viability and changes in cell viability, as follows: endometrial stromal cells; 24-hours of endometrial stromal cells with 10% control peritoneal fluid; 24-hours treatment of endometrial stromal cells with 10% peritoneal fluid from group 1; and 24-hours treatment of endometrial stromal cells with 10% peritoneal fluid from group 2. Changes in cell viability were analysed via MTT analysis.
In eutopic endometrial stromal cells, the cell viability upon treatment with 10% control peritoneal fluid decreased (73.29±40.7%), whereas the cell viability upon treatment with 10% peritoneal fluid from group 1 significantly increased (87.38±6.33%) compared to that in the control peritoneal fluid group (P < 0.05, Figure 1A).
In ectopic endometrial stromal cells, the cell viability treated with 10% control peritoneal fluid decreased (98.28±13.16%), whereas the cell viability treated with 10% peritoneal fluid from group 1 significantly increased (112.79±7.79%, P < 0.05, Figure 1B).
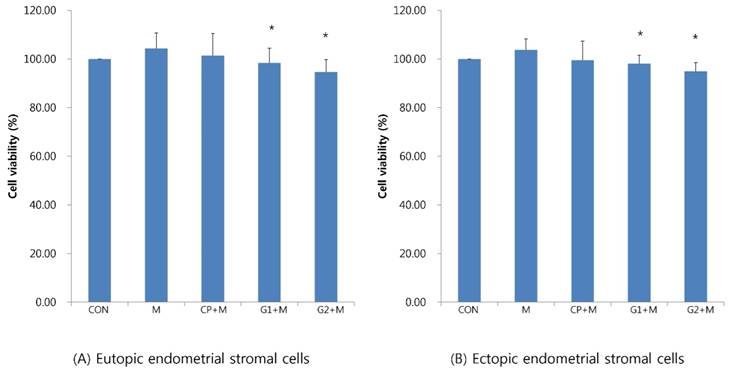
Analysis of endometrial stromal cell viability after mistletoe treatment
Endometrial stromal cells treated with peritoneal fluid, as above, were treated with mistletoe (200 ng/mL) for 24 hours. Cell viability was analysed with MTT analysis and compared.
In eutopic endometrial stromal cells, cell viability decreased in groups 1 and 2 compared to that in the control group (98.41±60.2% in group 1, 94.61±5.16% in group 2, P < 0.05, Figure 2A). Additionally, in ectopic endometrial stromal cells, cell viability decreased in groups 1 and 2 compared to that in the control group (98.13±3.45% in group 1, 95.01±3.51% in group 2, P < 0.05, Figure 2B).
Cell viability of endometrial stromal cells measured by MTT assay in eutopic endometrial stromal cells (A) and in ectopic endometrial stromal cells (B), treated for 24 hours with 10% peritoneal fluid (CON: media only for 24h, served as a standard, CP: 10% control peritoneal fluid for 24h, G1: 10% of peritoneal fluid from endometriosis stage I & II patients for 24h, G2: 10% of peritoneal fluid from endometriosis stage III & IV patients for 24h, * P < 0.05).
Cell viability of endometrial stromal cells measured by MTT assay in eutopic endometrial stromal cells (A) and in ectopic endometrial stromal cells (B), treated for 24 hours with 10% peritoneal fluid, and then treated with mistletoe (200 ng/mL) for 24 hours (CON: media only for 24h, served as a standard, M: 200 ng/mL of mistletoe for 24h, CP+M: 10% of control peritoneal fluid for 24h, then treated with 200 ng/mL mistletoe for 24h, G1+M: 10% of peritoneal fluid from endometriosis stage I & II patients for 24h, then treated with 200 ng/mL mistletoe for 24h, G2+M: 10% of peritoneal fluid from endometriosis stage III & IV patients for 24h, then treated with 200 ng/mL mistletoe for 24h * P < 0.05).
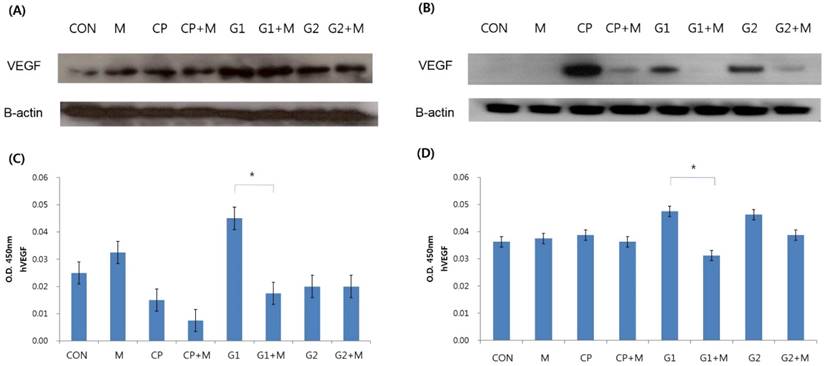
Verification of VEGF expression and VEGF concentration in endometrial stromal cells treated for 24 hours with 10% peritoneal fluid followed by 24-hour mistletoe treatment. VEGF expression verified by western blot analysis in eutopic endometrial stromal cells (A), and in ectopic endometrial stromal cells (B). VEGF concentration was measured by ELISA in eutopic endometrial stromal cells (C) and in ectopic endometrial stromal cells (D) (CON: media only for 24h, served as a standard, M: 200 ng/mL of mistletoe for 24h, CP: 10% control peritoneal fluid for 24h, CP+M: 10% of control peritoneal fluid for 24h, then treated with 200 ng/mL mistletoe for 24h, G1: 10% of peritoneal fluid from endometriosis stage I & II patients for 24h, G1+M: 10% of peritoneal fluid from endometriosis stage I & II patients for 24h, then treated with 200 ng/mL mistletoe for 24h, G2: 10% of peritoneal fluid from endometriosis stage III & IV patients for 24h, G2+M: 10% of peritoneal fluid from endometriosis stage III & IV patients for 24h, then treated with 200 ng/mL mistletoe for 24h * P < 0.05).
Verification of VEGF expression by Western blot and ELISA
Four groups were compared with respect to VEGF expression and VEGF concentration, as follows: endometrial stromal cells; 24-hours of endometrial stromal cells with 10% control peritoneal fluid; 24-hours treatment of endometrial stromal cells with 10% peritoneal fluid from group 1; and 24-hours treatment of endometrial stromal cells with 10% peritoneal fluid from group 2. Endometrial stromal cells treated with peritoneal fluid, as above, were then treated with mistletoe (200 ng/mL) for 24 hours. The expression of VEGF was determined using western blot analysis. The VEGF concentration was verified by ELISA.
In general, VEGF expression increased upon treatment with peritoneal fluid, and then decreased upon treatment with mistletoe, both in eutopic and ectopic endometrial stromal cells (Figure 3A, 3B). Especially in ectopic endometrial stromal cells, there was almost no VEGF expression in group 1 after the mistletoe treatment (Figure 3B). According to the VEGF concentration verified by ELISA, group 1 showed the highest VEGF concentration, but the VEGF concentration decreased significantly after mistletoe treatment, both in the eutopic and ectopic endometrial stromal cells (Figure 3C, 3D, P < 0.05).
Discussion
Using peritoneal fluid from endometriosis patients and mistletoe, we found that both the eutopic and ectopic endometrial stromal cell viability increased after treatment with peritoneal fluid from early-stage (I and II) endometriosis. In addition, after mistletoe treatment, cell viability decreased in, both eutopic and ectopic endometrial stromal cell in all stages of endometriosis. These findings were verified consistently by the expression and concentration of VEGF, a marker of angiogenesis. Thus, it is assumed that there is some factor in the peritoneal fluid of endometriosis patients that causes an increase in cell viability, which is suppressed by mistletoe. VEGF is a possible factor related to endometriosis, and VEGF expression is controlled by mistletoe. This is consistent with the results of an earlier study [23].
Furthermore, VEGF expression increased in group 1 and decreased more when treated with mistletoe compared to group 2. These results imply that an early stage of endometriosis is more active pathophysiologically, and it may yield better results. Moreover, the clinical symptoms do not correspond with the stage. Another study also reported that VEGF expression is higher in patients with stage I and II endometriosis than stage III and IV endometriosis [24].
Endometriosis is a benign disease, but the characteristics of the cells with endometriotic lesion resemble malignant tumour cells including cell proliferation, invasion, angiogenesis, and reduced apoptosis [25]. Peritoneal endometriosis is the most common type of endometriosis [26]. Peritoneal endometriosis may progress to the advanced stage with inflammatory cytokines and angiogenic factors [25].
There are several etiologic hypotheses to account for endometriosis, such as ectopic transplantation of endometrial cells, metaplasia of coelomic epithelium, and induction. Because no single theory can fully explain all locations of endometriotic lesions, the etiology of endometriosis is unclear. Even though endometrial cells pass through retrograde menstrual flow in most women, ectopic transplantation of endometrial cells does not always occur; the basis for this observation has not been proven. It has been reported that immunologic factors are abnormally high in endometriosis patients, and facilitate survival in the abdominal cavity, attachment to the peritoneum, proliferation, angiogenesis, and generation of inflammatory reactions. For example, endometrial cells from endometriosis patients shows resistance to apoptosis, the normal process of changing endometrial cells, which is regulated through the menstrual period. Moreover, the bcl-2/bax and Fas-ligand system is known to be involved in this resistance [27, 28]. It is assumed that due to the resistance to apoptosis, the viability of endometrial cells is increased in the abdominal cavity, and the retrograde menstrual flow shows resistance to the immunologic reactions of macrophages. Therefore, the cells survive and may cause endometriosis. Recurrent and recalcitrant endometriosis may be regarded as an immunologic disease, and studies of immune control treatment are necessary.
Because the incidence of endometriosis has shown a rapid increase in recent decades, the diagnosis tends to be delayed, and endometriosis may even relapse occur postoperatively, so long-term treatment methods that prevents relapse are needed. Clinically, mistletoe is used in some regions as an immune modulator because recurrent and recalcitrant endometriosis is regarded as an immunologic disease. However, studies focusing on the basic science of mistletoe are rare; thus, the present study was performed to establish a theoretical background for clinical application. In early stage endometriosis, such as pelvic endometriosis, endometriotic lesions are metabolically active and endometriosis is a disease with progressive and recurrent disease.
In conclusion, we found that peritoneal fluid in the early stage of endometriosis had specific factors increasing cell viability and VEGF expression, and this effect decreased after mistletoe treatment. Thus, mistletoe could be considered as an immune modulator for treatment of early or recurrent diseases.
Acknowledgements
We thank to Osuga Yutaka M.D., Ph.D. and his team from the department of Obstetrics and Gynecology of Tokyo University in Japan for helping us establish the methodology of setting up how to culture endometrial cells from ectopic endometrium.
Funding sources
This work was supported by the Korea Research Foundation(KRF) grant funded by the Korea government(MEST) (No. 2009-0073040) and Basic Science Research Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education, Science and Technology (No.2010-0002724, 2011-0005886).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ulukus M, Arici A. Immunology of endometriosis. Minerva Ginecol. 2005;57:237-48
2. Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55:759-65
3. Bilotas M, Meresman G, Buquet R, Sueldo C, Baranao RI. Effect of vascular endothelial growth factor and interleukin-1beta on apoptosis in endometrial cell cultures from patients with endometriosis and controls. J Reprod Immunol. 2010;84:193-8
4. Machado DE, Abrao MS, Berardo PT, Takiya CM, Nasciutti LE. Vascular density and distribution of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) are significantly higher in patients with deeply infiltrating endometriosis affecting the rectum. Fertil Steril. 2008;90:148-55
5. Wang Y, Yu J, Luo X. et al. Abnormal regulation of chemokine TECK and its receptor CCR9 in the endometriotic milieu is involved in pathogenesis of endometriosis by way of enhancing invasiveness of endometrial stromal cells. Cell Mol Immunol. 2010;7:51-60
6. Aghajanova L, Horcajadas JA, Weeks JL. et al. The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology. 2010;151:1341-55
7. Reis FM, Petraglia F, Taylor RN. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum Reprod Update. 2013;19:406-18
8. Ngo C, Nicco C, Leconte M. et al. Protein kinase inhibitors can control the progression of endometriosis in vitro and in vivo. J Pathol. 2010;222:148-57
9. Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum Reprod. 2011;26:885-97
10. Harada T, Kaponis A, Iwabe T. et al. Apoptosis in human endometrium and endometriosis. Hum Reprod Update. 2004;10:29-38
11. Kyama CM, Overbergh L, Mihalyi A. et al. Endometrial and peritoneal expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertil Steril. 2008;89:301-10
12. Becker H. Botany of European mistletoe (Viscum album L.). Oncology. 1986;43(Suppl 1):2-7
13. Hajto T. Immunomodulatory effects of iscador: a Viscum album preparation. Oncology. 1986;43(Suppl 1):51-65
14. Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111:765-72
15. Park WB, Lyu SY, Kim JH. et al. Inhibition of tumor growth and metastasis by Korean mistletoe lectin is associated with apoptosis and antiangiogenesis. Cancer Biother Radiopharm. 2001;16:439-47
16. Yoon TJ, Yoo YC, Choi OB. et al. Inhibitory effect of Korean mistletoe (Viscum album coloratum) extract on tumour angiogenesis and metastasis of haematogenous and non-haematogenous tumour cells in mice. Cancer Lett. 1995;97:83-91
17. Rentea R, Lyon E, Hunter R. Biologic properties of iscador: a Viscum album preparation I. Hyperplasia of the thymic cortex and accelerated regeneration of hematopoietic cells following X-irradiation. Lab Invest. 1981;44:43-8
18. Piao BK, Wang YX, Xie GR. et al. Impact of complementary mistletoe extract treatment on quality of life in breast, ovarian and non-small cell lung cancer patients. A prospective randomized controlled clinical trial. Anticancer Res. 2004;24:303-9
19. Park SH, Oh ST, Lee ES, Park SY. Treatment strategy for postoperative persistent pain of endometriosis. Korean J Obstet Gynecol. 2005;48:1934-41
20. Rim SY, Oh ST. The Effect of intraperitoneal instillation of Mistletoe extract during the diagnostic laparoscopy for pain of endometriosis. Korean J Obstet Gynecol. 2005;48:1004-8
21. Gilabert-Estelles J, Ramon LA, Espana F. et al. Expression of angiogenic factors in endometriosis: relationship to fibrinolytic and metalloproteinase systems. Hum Reprod. 2007;22:2120-7
22. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21
23. Hong S, Chang SY, Yeom DH, Kang JH, Hong KJ. Differential regulation of thrombospondin-1 expression and antiangiogenesis of ECV304 cells by trichostatin A and helixor A. Anticancer Drugs. 2007;18:1005-14
24. Pupo-Nogueira A, de Oliveira RM, Petta CA, Podgaec S, Dias JA Jr, Abrao MS. Vascular endothelial growth factor concentrations in the serum and peritoneal fluid of women with endometriosis. Int J Gynaecol Obstet. 2007;99:33-7
25. Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update. 2013;19:558-69
26. Mahmood TA, Templeton A. Prevalence and genesis of endometriosis. Hum Reprod. 1991;6:544-9
27. Garcia-Velasco JA, Arici A. Apoptosis and the pathogenesis of endometriosis. Semin Reprod Med. 2003;21:165-72
28. Huang FY, Lin QH, Fang XL. [Expression of Bcl-2 and Bax protein in endometriosis]. Hunan Yi Ke Da Xue Xue Bao. 2003;28:102-6
Author contact
![]() Corresponding author: Mee-Ran Kim, M.D., Ph.D., Professor, Division of Reproductive Endocrinology, Department of Obstetrics & Gynecology, College of Medicine, The Catholic university of Korea, 222 Banpo-Daero, Seocho-Gu, Seoul, 06591, Republic of Korea. Fax 82-2-595-1549 Tel 82-2-2258-6170; mrkimac.kr
Corresponding author: Mee-Ran Kim, M.D., Ph.D., Professor, Division of Reproductive Endocrinology, Department of Obstetrics & Gynecology, College of Medicine, The Catholic university of Korea, 222 Banpo-Daero, Seocho-Gu, Seoul, 06591, Republic of Korea. Fax 82-2-595-1549 Tel 82-2-2258-6170; mrkimac.kr

 Global reach, higher impact
Global reach, higher impact