3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(13):1502-1507. doi:10.7150/ijms.28055 This issue Cite
Research Paper
NDRG1 Downregulates ATF3 and Inhibits Cisplatin-Induced Cytotoxicity in Lung Cancer A549 Cells
Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences of China Medical University, Shenyang, China
Received 2018-6-22; Accepted 2018-8-30; Published 2018-10-20
Abstract
N-myc downstream regulated gene 1 (NDRG1) plays a variety of roles in human cancers. Our previous studies showed that NDRG1 expression is elevated in non-small cell lung cancer and contributes to cancer growth. However, its function in apoptosis and chemoresistance in malignant tumors, including lung cancer, is not yet fully understood. In this study, we investigated the roles of NDRG1 in chemoresistance to cisplatin in lung cancer cells. We found that overexpression of NDRG1 significantly reduced cisplatin-induced cytotoxicity in lung cancer A549 cells, while overexpression of activating transcription factor 3 (ATF3), a stress-inducible gene found to be associated with apoptosis in some human cancers, significantly promoted cytotoxicity (P < 0.05). Further investigation showed that overexpression of NDRG1 significantly downregulated ATF3 and P53 expression in A549 cells, while overexpression of ATF3 significantly upregulated P53 expression (P < 0.05). In addition, cisplatin significantly upregulated ATF3, phospho-P53(ser46), and cleaved caspase 3 expression in lung cancer cells, but overexpression of NDRG1 in the presence of cisplatin reduced the level of these proteins elevated by cisplatin (P < 0.05). While, overexpression of ATF3 significantly promoted the cytoxicity induced by cisplatin in 1299 cells (p<0.05) (Figure 4), but overexpression of NDRG1 didn't regulate the cytoxicity induced by cisplatin (p>0.05). These results indicate that NDRG1 may contribute to cisplatin-resistance in lung cancer, possibly due to its function in the regulation of ATF3 expression.
Keywords: ATF3, cisplatin, lung cancer, NDRG1
Introduction
The functions of N-myc downstream regulated gene 1 (NDRG1) in malignant tumors remain controversial [1-6]. It was identified as a metastasis suppressor in some cancers [4, 6], but other studies have found that it can promote cancer growth and metastasis [1, 3, 5]. A few studies have also indicated its possible association with cell apoptosis, but the mechanism involved remains unknown [7-12]. NDRG1 was also reported to be involved in the regulation of cancer cell cytotoxicity induced by some anticancer agents, but the functions reported were contradictory and the associated mechanism was not elucidated [7, 8, 10, 11]. ATF3, a stress-inducible gene, was also reported to play important roles in the regulation of cell apoptosis in some cancers and was associated with chemosensitivity [13-16]. A study by Bandyopadhyay et al. identified ATF3 as a downstream target of NDRG1 in prostate cancer cells [17]. However, the roles of these two proteins, and their interaction in cisplatin-chemoresistance in lung cancer cells, remain unclear. In this study, we investigated the role of NDRG1 in the regulation of chemosensitivity to cisplatin in lung cancer, and the possible role of ATF3.
Materials and Methods
Cell culture, transfection and cisplatin treatment
Human bronchial epithelial (HBE) cells and lung carcinoma cell lines were cultured in RPMI 1640 tissue culture medium (Invitrogen, Carlsbad, CA, USA), containing 10% fetal calf serum (Invitrogen), 100 IU/mL penicillin (Sigma, St Louis, MO, USA), and 100 μg/mL streptomycin (Sigma) at 37°C in a humidified atmosphere (5% CO2, 95% air). The cells were cultured in a 24-well plate for 24 h before transient transfection. Cells were then transfected with Lipofectamine 3000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The cells were harvested 48 h after transfection to measure the protein levels. To examine the effect of cisplatin on cancer cells, the cells were treated by cisplatin (50μM) for 24 h.
Western blotting
Cells were lysed in 10 volumes (w/v) of lysis buffer. After centrifugation, the supernatants were collected and quantified. The same amount of total protein was separated by 10% SDS-PAGE and then transferred to PVDF membranes. The membranes were then incubated overnight at 4°C with rabbit anti-human NDRG1 polyclonal antibody (ab63989, Abcam, HK; dilution 1:500), mouse anti-human ATF3 monoclonal antibody (sc-81189, Santa Cruz, USA; dilution 1:200), phospho-P53(ser46) antibody (2521, Millipore, CA; 1:500), P53 antibody (9282, Millipore, CA; 1:500), cleaved caspase 3 antibody (9664, Millipore, CA; 1:500), and mouse anti-human β-actin polyclonal antibody (ab20272, Abcam, HK; 1:500). After incubation with appropriate secondary antibodies labelled with HRP at room temperature for 2 h, protein bands were visualized using enhanced chemiluminescence (ECL) and detected using a BioImaging System.
Immunofluorescent staining
Cells were fixed with 4% paraformaldehyde and then blocked with 1% BSA, followed by incubation with rabbit anti-human NDRG1 polyclonal antibody (ab63989, Abcam, HK; dilution 1:100) and mouse anti-human ATF3 monoclonal antibody (sc-81189, Santa Cruz, USA; dilution 1:100) overnight at 4°C. The cells were then incubated with secondary antibodies conjugated to rhodamine/fluorescein isothiocyanate (FITC). The nuclei were counterstained with DAPI and the cells were examined with an Olympus IX51 fluorescence microscope (Olympus, Tokyo, Japan). Images were captured with a CoolPIX 5400 camera (Nikon, Japan).
Cell apoptosis and necrosis detection
Cell apoptosis and necrosis detection was performed with an AnnexinV-FITC/propidium iodide (PI) apoptosis detection kit (KeyGEN BioTECH Corp., Ltd, Jiangsu, China) according to the manufacturer's instructions. Cells were harvested using 0.25% trypsin 48 h after transfection and Annexin V/FITC and PI were added after washing twice with chilled PBS and resuspension in 250 μl of binding buffer. The cells were analyzed by FACSCalibur flow cytometer (Becton Dickinson, USA) after incubation for 30 min. Cells negative for both Annexin V and PI were considered viable. Cells showing Annexin V+/PI- were indicative of early apoptosis. Cells showing Annexin V+/PI+ were indicative of late apoptosis and necrosis.
Statistical analysis
SPSS statistical software package version 13.0 (SPSS Inc., Chicago, IL, USA) was used for the analyses. All data were expressed as means ± standard deviation (SD) of at least 3 in vitro experiments. Statistical significance was tested using an independent-samples t-test to compare data between two groups. P-values of <0.05 were considered statistically significant.
Results
NDRG1 and ATF3 show opposing effects on cisplatin-induced cytotoxicity in lung cancer cells
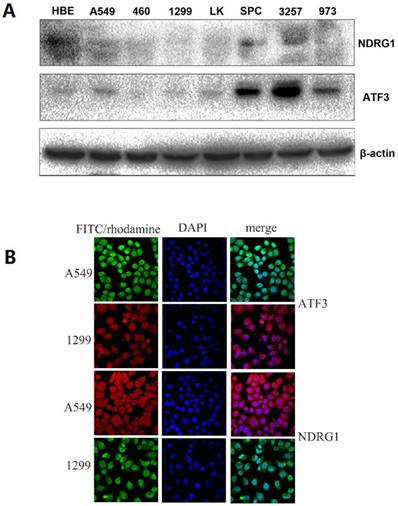
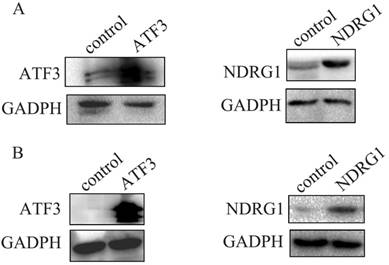
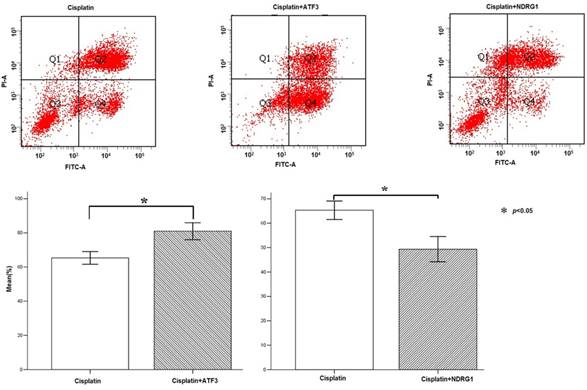
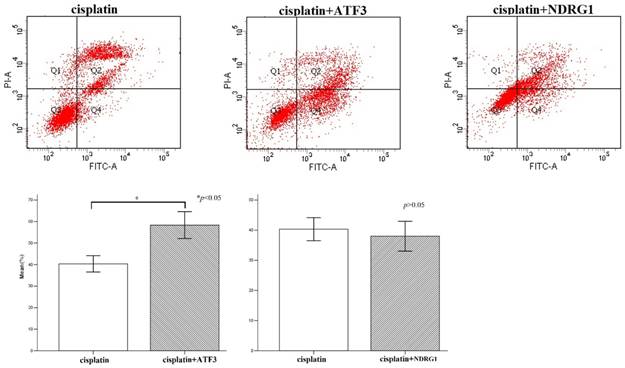
First, we examined NDRG1 and ATF3 expression using western blot and immunofluorescent staining in lung and lung cancer cell lines (Figure 1A). We selected A549 and H1299 cells to investigate the function of NDRG1 and ATF3 in vitro. Moderate levels of NDRG1 and ATF3 expression were observed in A549 cells and NDRG1 and ATF3 expression in H1299 cells was very low (Figure 1A). Immunofluorescent staining shows that ATF3 was located in cytoplasm and nuclear in both A549 and 1299 cells, while NDRG1 was located in cytoplasm and nuclear in 1299 cells, and mainly in cytoplasm with weak expression in nuclear in A549 cells (Figure 1B). Overexpression of NDRG1 and ATF3 in cancer cells by transfection of specific plasmids was confirmed by western blot (Figure 2). We examined cisplatin-induced cytotoxicity (cell apoptosis and necrosis) of lung cancer cells using Annexin V/PI double staining. Overexpression of NDRG1 significantly reduced the cytotoxicity to lung cancer A549 cells induced by cisplatin, while overexpression of ATF3 significantly promoted cytotoxicity (p<0.05) (Figure 3). While, overexpression of ATF3 significantly promoted the cytoxicity induced by cisplatin in 1299 cells (p<0.05) (Figure 4), but overexpression of NDRG1 didn't show a significant impact on the cytoxicity induced by cisplatin (p>0.05) (Figure 4).
NDRG1 and ATF3 expression using Western blot and immunofluorescent staining in lung and lung cancer cell lines. A549 and H1299 cells were selected to investigate the function of NDRG1 and ATF3 in vitro. There was a medium level of NDRG1 and ATF3 expression in A549 cells but very weak expression in 1299 cells (1A). ATF3 was located in cytoplasm and nuclear in both A549 and 1299 cells, while NDRG1 was located in cytoplasm and nuclear in 1299 cells, and mainly in cytoplasm with weak expression in nuclear in A549 cells (1B).
Overexpression of NDRG1 and ATF3 in A549 cancer cells (A) and H1299 cells (B) after transfection of specific plasmids was confirmed by western blot.
NDRG1 regulates cisplatin-induced expression of ATF3, phospho-P53 (ser46), and cleaved caspase 3 in lung cancer cells
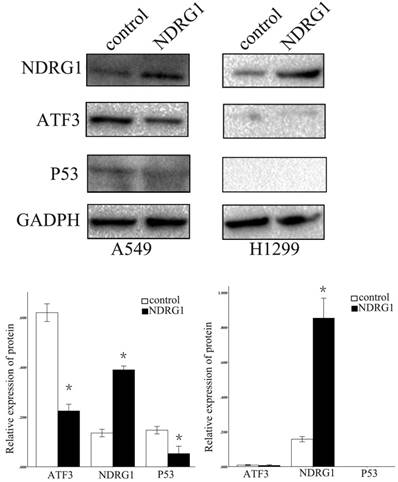
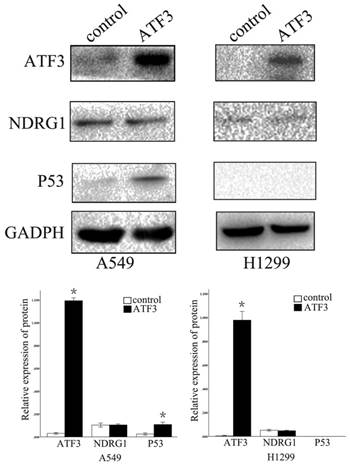
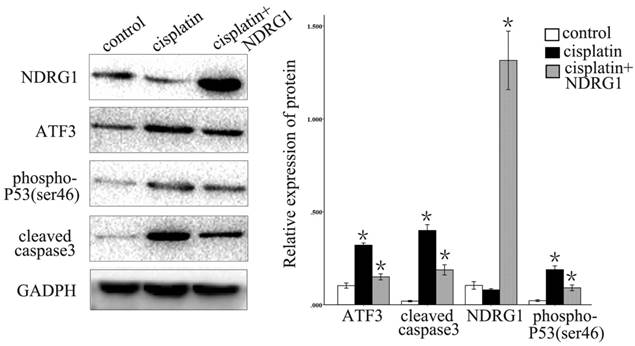
We further examined the function of NDRG1 in the regulation of ATF3 expression. Overexpression of NDRG1 significantly downregulated ATF3 and P53 expression in A549 cells (p < 0.05) (Figure 5). Overexpression of ATF3 significantly upregulated P53 expression (P < 0.05) but did not affect NDRG1 expression in A549 cells (P > 0.05) (Figure 5). While, in H1299 cells which has no P53 expression, overexpression of ATF3 didn't significantly regulate P53 and NDRG1 expression (p>0.05). These data indicate that the function of NDRG1 associated with chemoresistance is at least partly dependent on ATF3 in A549 cells. In H1299 cells, the function of ATF3 associated with chemoresistance is independent on P53. In addition, cisplatin significantly upregulated ATF3, phospho-P53(ser46), and cleaved caspase 3 expression in A549 cells, but overexpression of NDRG1 with addition of cisplatin reduced the elevated level of these proteins (Figure 6) (P < 0.05). These results indicate that NDRG1 may contribute to chemoresistance to cisplatin by lung cancer cells through regulation of ATF3 and P53 in A549 cells.
Discussion
NDRG1 is induced by hypoxia and cell differentiation signals [18]. It has also been found to suppress tumor metastasis in some human cancers [4, 6]. However, the role of NDRG1 in cancer cannot be generalized. There are also some studies show that it may play cancer-promoting roles, such as promotion of tumor growth and metastasis [1, 3, 5]. According to these data, NDRG1 may have a variety of functions under different conditions and in different tumors. NDRG1 has also been linked to apoptosis and chemosensitivity in some malignant tumors [7-12]. However, the mechanism involved was not fully elucidated. In this study, we found that overexpression of NDRG1 in lung cancer A549 cells significantly downregulated cisplatin-induced cytotoxicity. Our previous study showed that NDRG1 expression is elevated in non-small cell lung cancer and associated with cancer growth. Further studies have also shown that NDRG1 expression is increased in some other malignant tumors, such as malignant thyroid neoplasms [19] and hepatocellular carcinoma [20]. Thus, it is important to understand the mechanism and function of NDRG1 in chemotherapy-induced cytotoxicity of cancer cells to improve chemosensitivity.
ATF3 belongs to the ATF/cyclic AMP response element-binding (ATF/CREB) family [21]. It has been found to play a variety of functions including regulation of metastasis, apoptosis, and proliferation in human cancers [21, 22]. Bandyopadhyay et al. showed that ATF3 is a downstream target of NDRG1 in prostate cancer cells [17]. Our study shows that NDRG1 can also downregulate ATF3 expression in lung cancer A549 cells. But the regulation of ATF3 by NDRG1 in H1299 cells was not significant, which may be because the expression level of ATF3 in H1299 cells was very low. Here we found that overexpression of ATF3 significantly upregulated cisplatin-induced cytotoxicity in both A549 and H1299 lung cancer cells. But overexpression of NDRG1 significantly downregulated chemosensitivity to cisplatin in lung cancer A549 cells, but not in H1299 cells. It may be because NDRG1 does not significantly regulate ATF3 in H1299 cells. Further we found that NDRG1 could inhibit the promotion of cisplatin-induced cytotoxicity by ATF3 in lung cancer cells. These results indicate that ATF3 may be involved in the mechanism of NDRG1 regulation of chemosensitivity to cisplatin in lung cancer, although further investigation is still needed to clarify the mechanism including how NDRG1 regulates ATF3 and how ATF3 regulates the apoptosis pathway induced by cisplatin.
Flow cytometry analysis of cisplatin-induced cytotoxicity in A549 cells. Overexpression of NDRG1 significantly reduced the cytotoxicity to A549 lung cancer cells induced by cisplatin, while overexpression of ATF3 significantly promoted cytotoxicity (P < 0.05).
Flow cytometry analysis of cisplatin-induced cytotoxicity in H1299 cells. Overexpression of ATF3 significantly promoted the cytotoxicity induced by cisplatin in H1299 cells (p<0.05), while overexpression of NDRG1 didn't significantly impact on the cytotoxicity induced by cisplatin (p>0.05).
Regulation of ATF3 and NDRG1 expression by NDRG1 overexpression. NDRG1 significantly downregulated ATF3 and P53 expression in A549 cells (*p < 0.05), but not in H1299 cells (p>0.05).
Regulation of P53 expression by ATF3 overexpression. ATF3 significantly upregulated P53 expression (*p < 0.05) but did not affect NDRG1 expression in A549 cells (p > 0.05). ATF3 did not regulate P53 and NDRG1 expression in H1299 cells (p > 0.05).
Cisplatin significantly upregulated ATF3, phospho-P53(ser46), and cleaved caspase 3 expression in A549 cells, but overexpression of NDRG1 with addition of cisplatin reduced the level of elevation of these proteins (*P < 0.05).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81472599 to Chuifeng Fan, MD).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ureshino H, Murakami Y, Watari K, Izumi H, Kawahara A, Kage M, Arao T, Nishio K, Yanagihara K, Kinoshita H, Kuwano M, Ono M. N-myc downstream regulated gene 1 (NDRG1) promotes metastasis of human scirrhous gastric cancer cells through epithelial mesenchymal transition. PLoS One. 2012;7:e41312
2. Bae DH, Jansson PJ, Huang ML, Kovacevic Z, Kalinowski D, Lee CS, Sahni S, Richardson DR. The role of NDRG1 in the pathology and potential treatment of human cancers. J Clin Pathol. 2013;66:911-917
3. Wang Q, Li LH, Gao GD, Wang G, Qu L, Li JG, Wang CM. HIF-1α up-regulates NDRG1 expression through binding to NDRG1 promoter, leading to proliferation of lung cancer A549 cells. Mol Biol Rep. 2013;40:3723-3729
4. Fang BA, Kovačević Ž, Park KC, Kalinowski DS, Jansson PJ, Lane DJ, Sahni S, Richardson DR. Molecular functions of the iron-regulated metastasis suppressor, NDRG1, and its potential as a molecular target for cancer therapy. Biochim Biophys Acta. 2014;1845:1-19
5. Lu WJ, Chua MS, Wei W, So SK. NDRG1 promotes growth of hepatocellular carcinoma cells by directly interacting with GSK-3β and Nur77 to prevent β-catenin degradation. Oncotarget. 2015;6:29847-29859
6. Hu ZY, Xie WB, Yang F, Xiao LW, Wang XY, Chen SY, Li ZG. NDRG1 attenuates epithelial-mesenchymal transition of nasopharyngeal cancer cells via blocking Smad2 signaling. Biochim Biophys Acta. 2015;1852:1876-1886
7. Zheng Y, Wang LS, Xia L, Han YH, Liao SH, Wang XL, Cheng JK, Chen GQ. NDRG1 is down-regulated in the early apoptotic event induced by camptothecin analogs: the potential role in proteolytic activation of PKC delta and apoptosis. Proteomics. 2009;9:2064-2075
8. Lerner A, Grafi-Cohen M, Napso T, Azzam N, Fares F. The indolic diet-derivative, 3,3'-diindolylmethane, induced apoptosis in human colon cancer cells through upregulation of NDRG1. J Biomed Biotechnol. 2012;2012:256178
9. Luo EC, Chang YC, Sher YP, Huang WY, Chuang LL, Chiu YC, Tsai MH, Chuang EY, Lai LC. MicroRNA-769-3p down-regulates NDRG1 and enhances apoptosis in MCF-7 cells during reoxygenation. Sci Rep. 2014;4:5908
10. Wu F, Rom WN, Koshiji M, Mo Y, Hosomi Y, Tchou-Wong KM. Role of GLI1 and NDRG1 in Increased Resistance to Apoptosis Induction. J Environ Pathol Toxicol Oncol. 2015;34:213-225
11. Jung EU, Yoon JH, Lee YJ, Lee JH, Kim BH, Yu SJ, Myung SJ, Kim YJ, Lee HS. Hypoxia and retinoic acid-inducible NDRG1 expression is responsible for doxorubicin and retinoic acid resistance in hepatocellular carcinoma cells. Cancer Lett. 2010;298:9-15
12. Stein S, Thomas EK, Herzog B, Westfall MD, Rocheleau JV, Jackson RS 2nd, Wang M, Liang P. NDRG1 is necessary for p53-dependent apoptosis. J Biol Chem. 2004;279:48930-48940
13. Huang X, Li X, Guo B. KLF6 induces apoptosis in prostate cancer cells through up-regulation of ATF3. J Biol Chem. 2008;283:29795-29801
14. Kwon O, Soung NK, Thimmegowda NR, Jeong SJ, Jang JH, Moon DO, Chung JK, Lee KS, Kwon YT, Erikson RL, Ahn JS, Kim BY. Patulin induces colorectal cancer cells apoptosis through EGR-1 dependent ATF3 up-regulation. Cell Signal. 2012;24:943-950
15. Suk FM, Jou WJ, Lin RJ, Lin SY, Tzeng FY, Liang YC. 15,16-Dihydrotanshinone I-induced apoptosis in human colorectal cancer cells: involvement of ATF3. Anticancer Res. 2013;33:3225-3231
16. Sato A, Nakama K, Watanabe H, Satake A, Yamamoto A, Omi T, Hiramoto A, Masutani M, Wataya Y, Kim HS. Role of activating transcription factor 3 protein ATF3 in necrosis and apoptosis induced by 5-fluoro-2'-deoxyuridine. FEBS J. 2014;281:1892-1900
17. Bandyopadhyay S, Wang Y, Zhan R, Pai SK, Watabe M, Iiizumi M, Furuta E, Mohinta S, Liu W, Hirota S, Hosobe S, Tsukada T, Miura K, Takano Y, Saito K, Commes T, Piquemal D, Hai T, Watabe K. The tumor metastasis suppressor gene Drg-1 down-regulates the expression of activating transcription factor 3 in prostate cancer. Cancer Res. 2006;66:11983-11990
18. Ellen TP, Ke Q, Zhang P, Costa M. NDRG1, a growth and cancer related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis. 2008;29:2-8
19. Chua MS, Sun H, Cheung ST, Mason V, Higgins J, Ross DT, Fan ST, So S. Overexpression of NDRG1 is an indicator of poor prognosis in hepatocellular carcinoma. Mod Pathol. 2007;20:76-83
20. Gerhard R, Nonogaki S, Fregnani JH, Soares FA, Nagai MA. NDRG1 protein overexpression in malignant thyroid neoplasms. Clinics (Sao Paulo). 2010;65:757-762
21. Thompson MR, Xu D, Williams BR. ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med (Berl). 2009;87:1053-1060
22. Wang CM, Yang WH. Loss of SUMOylation on ATF3 inhibits proliferation of prostate cancer cells by modulating CCND1/2 activity. Int J Mol Sci. 2013;14:8367-8380
Author contact
![]() Corresponding author: Chuifeng Fan, Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences of China Medical University, Shenyang 110001, China. Tel: +86 24 23261638; Fax: +86 24 23261638; E-mail: cffanedu.cn
Corresponding author: Chuifeng Fan, Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences of China Medical University, Shenyang 110001, China. Tel: +86 24 23261638; Fax: +86 24 23261638; E-mail: cffanedu.cn

 Global reach, higher impact
Global reach, higher impact