3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(13):1486-1501. doi:10.7150/ijms.27181 This issue Cite
Research Paper
Comparison of Oxidative Stress Effects on Senescence Patterning of Human Adult and Perinatal Tissue-Derived Stem Cells in Short and Long-term Cultures
1. Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Via Massarenti 9, 40138 Bologna, Italy;
2. National Laboratory of Molecular Biology and Stem Cell Bioengineering of the National Institute of Biostructures and Biosystems (NIBB) - Eldor Lab, at the Innovation Accelerator, CNR, Via Piero Gobetti 101, 40129 Bologna, Italy;
3. Department of Biomedical Sciences, University of Sassari, Viale San Pietro 43/B, 07100 Sassari, Italy;
4. Istituto di Ricerca Genetica e Biomedica, Consiglio Nazionale delle Ricerche (CNR), Monserrato, 09042 Cagliari, Italy.
*These Authors equally contributed to this work
Received 2018-5-9; Accepted 2018-8-27; Published 2018-10-20
Abstract
Human Mesenchymal Stem Cells (hMSCs) undergo senescence in lifespan. In most clinical trials, hMSCs experience long-term expansion ex vivo to increase cell number prior to transplantation, which unfortunately leads to cell senescence, hampering post-transplant outcomes.
Hydrogen peroxide (H2O2) in vitro represents a rapid, time and cost-effective tool, commonly used as oxidative stress tantalizing the stem cell ability to cope with a hostile environment, recapitulating the onset and progression of cellular senescence.
Here, H2O2 at different concentrations (ranging from 50 to 400 μM) and time exposures (1 or 2 hours - h), was used for the first time to compare the behavior of human Adipose tissue-derived Stem Cells (hASCs) and human Wharton's Jelly-derived MSCs (hWJ-MSCs), as representative of adult and perinatal tissue-derived stem cells, respectively. We showed timely different responses of hASCs and hWJ-MSCs at low and high subculture passages, concerning the cell proliferation, the cell senescence-associated β-Galactosidase activity, the capability of these cells to undergo passages, the morphological changes and the gene expression of tumor protein p53 (TP53, alias p53) and cyclin dependent kinase inhibitor 1A (CDKN1A, alias p21) post H2O2 treatments.
The comparison between the hASC and hWJ-MSC response to oxidative stress induced by H2O2 is a useful tool to assess the biological mechanisms at the basis of hMSC senescence, but it could also provide two models amenable to test in vitro putative anti-senescence modulators and develop anti-senescence strategies.
Keywords: human mesenchymal stem cells, cell senescence, oxidative stress-induced premature senescence, hydrogen peroxide, Resazurin-based assay, senescence-associated β-galactosidase activity
Introduction
The human body continuously repairs damaged tissues and opposes senescence-related processes due to the peculiar properties of its resident stem cells. Human mesenchymal stem cells (hMSCs) in fact are able of self-renewal and multi-lineage differentiation and the equilibrium between these two events determines the stem cell fate and their roles in the human body [1]. They can be isolated and expanded in vitro from virtually all adult tissues [2], including bone marrow [3], adipose tissue [4], peripheral blood [3], and also from several fetal and perinatal sources, as well as placenta [5], umbilical cord [6] and cord blood [7]. MSCs obtained from various sources differ in their biological characteristics [8,9], and their proteome and transcriptome profiles revealed source specific markers [10]. Moreover, diversity in multi-lineage differentiation potency and paracrine functions [8,9,11,12] determine different clinical applications of hMSCs [13]. Recently, hMSCs have been utilized for cell-based therapy in regenerative medicine to treat several injury and degenerative disorders, like Crohn's disease, diabetes mellitus, multiple sclerosis, myocardial infarction, liver failure, and rejection after liver transplant [14-21].
Since cell-based therapy procedures usually require hundreds of million hMSCs for each treatment (http://www.clinicaltrials.gov), cells isolated from donors need to be expanded ex vivo for several culture passages to obtain a large amount of cells prior to transplantation [13,22].
Unfortunately, as the function of hMSCs decreases with age in vivo [23,24], hMSCs, as well as all cultured primary cells [25], undergo cellular senescence along culture passages, with substantial decay in differentiation and self-renewal potential [22-24]. Premature senescence is a continuous process where cells share many molecular and functional characteristics, including changes in morphology, enhanced Senescence-Associated β-Galactosidase (SA β-Gal) activity, and permanent cell cycle arrest [22,26].
Oxidative stress, defined as an imbalance between the production of free radicals/Reactive Oxygen Species (ROS), and antioxidants [27], is thought to contribute significantly to DNA damage and cellular senescence [28-30]. According to the free radical or oxidative stress theory of aging [31], oxidative stress incurs when the cellular antioxidant defense systems fail to counteract ROS bringing them back to their basal levels.
For this reason, hydrogen peroxide (H2O2) treatment is commonly used as a model for assessing cellular susceptibility to oxidative stress. Although hMSCs appear to efficiently handle oxidative stress, nevertheless they undergo premature senescence in vitro when exposed to H2O2 [32,33]. Understanding hMSC behavior in oxidative stress would be important to study how to postpone, anticipate or revert Oxidative Stress-Induced Premature Senescence (OSIPS) in hMSC cultures.
It has been recently shown that OSIPS is a common feature in bone marrow hMSCs, the stem cell population that has been first isolated and characterized, with evidence ranging from morphological traits and SA β-Gal positivity to differential proteomic/metabolomic signatures in H2O2 exposed cells, as compared with untreated controls [34-37]. In hMSCs isolated from adipose tissue (hASCs), H2O2 was found to increase intracellular ROS production and to reduce antioxidant defenses (i.e. superoxide dismutase - SOD and glutathione synthetase - GSH) [38], hampering cell viability in a dose- and exposure time- dependent manner [38,39]. It has been recently shown that SOD2 overexpression in ASCs promotes cell resistance to oxidative stress [40]. Moreover, H2O2 treatment provokes DNA breaks [41], raises SA β-Gal positive cells [42], alters the expression of senescent marker genes, as well as p53, p21, mitogen-activated protein kinase 14 (MAPK14, alias p38) and sirtuin 1 (SIRT1) [38,39,42], and increases apoptosis with a decline of pro-survival gene expression [38]. It has been recently shown that also human Wharton's Jelly-derived MSCs (hWJ-MSCs) treated with H2O2 undergo premature senescence at early culture passages: these cells show typical changes in morphology [43], slow their proliferation [44,45], result positive for SA β-Gal [43,44], express typical senescence [43] and pro-apoptotic gene markers, while displaying a down-regulation of survival genes [44,46].
The aim of the present study was to investigate and compare the effects of H2O2 on morphology, proliferation and senescence in hASCs and hWJ-MSCs, as representative of adult and perinatal tissues derived hMSCs, respectively. In particular, we investigated along time the effects of H2O2 supplied to hMSCs at different concentrations (ranging from 50 to 400 μM), for 1 or 2 hours (h), at low and high subculture passages.
The hASCs are commonly used in cell-based therapy since their isolation is minimally invasive and because they are abundant and rapid in proliferation. On the other hand, in the human body, these cells, as well as all other adult MSCs, exhibit an age-dependent decline in their repairing capacity, increasing their susceptibility to degenerative diseases, cell death and senescence processes. In fact, multiple studies showed that the age of tissue donors affects several properties of the cells [47-49]. In particular, aged hASCs are significantly compromised in their ability to support the vascular network formation, owing to alterations in angiogenic properties [50], and their genes, normally related to senescence, act as positive regulators of apoptosis [51].
On the other hand, neonatal MSCs such as hWJ-MSCs, in their short prenatal life are not so influenced by age [52,53]. These cells express mesenchymal but not endothelial and hematopoietic markers [54,55] and display several features of embryonic stem cells (ESCs) although with a minor expression of pluripotency genes [56-58], explaining the lack of tumorigenicity of hWJ-MSCs [59,60].
The high efficiency of hWJ-MSC recovery compared with hASCs (1 to 5 × 104 cells/cm of umbilical cord, while 1 g of adipose tissue yields approximately 5 × 103 stem cells) [61,62], the minimal ethical concerns associated with their use, their ability to modulate immunological responses and the fact that they are from young donors make them an ideal source of MSCs for therapeutic applications in allogeneic settings. Moreover, hWJ-MSCs are preferable to other stem cells isolated from perinatal tissues (i.e. fetal membranes) because their isolation guarantees the absence of contaminating maternal cells [52].
Therefore, the comparison between the hASC and hWJ-MSC response to oxidative stress can be useful to study the biological mechanisms at the basis of hMSC senescence and could provide two OSIPS models amenable to test putative anti-senescence modulators and develop anti-senescence strategies.
Materials and Methods
A comprehensive overview of the experimental procedures that have been used in this study was described in Figure 1.
Comprehensive overview of the experimental procedures.
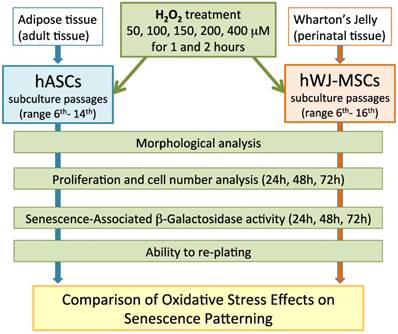
hASCs and hWJ-MSCs: harvesting and culture
All tissue samples were obtained from subjects that gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the local Ethical Committees (CE) (S.Orsola-Malpighi University Hospital - project identification code: n.1645/2014, ref. 35/2014/U/Tess and Villalba Hospital - project identification code: 16076 of Bologna, Italy). hASCs have been isolated by Lipogems device (PCT/IB2011/052204) and characterized according to standard procedures and with ethical clearance, as previously described [63]. hASCs were cultured in alfa-Minimal Essential Medium (α-MEM, Carlo Erba Reagents, Milano, Italy) supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS) (Gibco, Waltham, MA, USA), 1% Penicillin-Streptomycin Solution, 1% L-Glutamine 200 mM (Carlo Erba Reagents) [64]. hWJ-MSCs have been isolated from umbilical cords from healthy donor mothers and characterized as previously described [65,66]; cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) low glucose (BioWhittaker Cambrex, Walkersville, MD, USA) supplemented with 10% FBS (Gibco) and 1% Penicillin-Streptomycin Solution. Both hASCs and hWJ-MSCs were maintained at standard culture conditions of 37°C with 5% carbon dioxide in a humidified atmosphere. The non-adherent cells were removed, medium was changed twice a week and at 80% confluency cells were detached by treatment with trypsin-EDTA (Sigma-Aldrich Co., St. Louis, MO, USA), maintained and expanded until desired experimental culture passages. Both hASCs and hWJ-MSCs were derived from four healthy donors.
Hydrogen peroxide treatment
In order to test hydrogen peroxide (H2O2, Sigma-Aldrich Co.) capacity to induce cell senescence, hASCs and hWJ-MSCs were treated with different H2O2 concentrations and then submitted to a Resazurin-based proliferation (Sigma-Aldrich Co.) or to a SA β-Gal (Sigma Aldrich Co.) assays. Cells were incubated at 37°C in complete cell culture medium containing H2O2 for 1 or 2 h. Untreated cells were considered as controls. In preliminary experiments, cells to be used for the cell counts and proliferation assay were exposed to five H2O2 concentrations (50, 100, 150, 200 or 400 μM), while cells to be used for SA β-Gal assay, were treated with the same H2O2 concentrations, except for the 50 μM. In the following experiment settings, H2O2 400 μM was excluded. Moreover, a gene expression analysis was performed after 48 h from the end of the H2O2 stimulus: hASCs and hWJ-MSCs were treated for 2 h with H2O2 150 or 200 μM respectively.
Cell count
hASCs and hWJ-MSCs, both obtained from one healthy subject (subculture passages 9th and 8th, respectively), were used in three different experiments: they were seeded in 24-well plates at the density of 4000 and 3500 cells/cm2, respectively. After 24 h in standard conditions, cells were exposed for 2 h at H2O2 50, 100, 150, 200 and 400 μM or unexposed (control cells) in technical duplicate. At the end of the treatment, fresh medium was added in all the wells and culture plates were incubated in standard conditions until the count test. At 24, 48 and 72 h from the end of the stimulus, cells were detached by trypsin-EDTA and resuspended in a medium with 50% Eritrosyn B dye 0.2% in PBS (Sigma-Aldrich); counts were done under a light microscope at least twice with a Neubauer chamber (BRAND GmbH, Wertheim, Germany) and the cell number was calculated following the manufacturer's specifications.
Cell metabolic activity and proliferation
To evaluate the proliferation as function of metabolic activity of the cells, the “In vitro toxicology assay kit - Resazurin based” (Sigma-Aldrich Co.) was used. In this assay, metabolically active cells reduce Resazurin (not-fluorescent and blue) to Resorufin (highly fluorescent and red). Resorufin is a water-soluble compound and its intrinsic fluorescence can be measured avoiding the cell lysis (necessary with tetrazolium-salt based assays, i.e. MTT test). This allows to monitor cell proliferation of the same sample over time [67]. A preliminary proliferation study was conducted in order to determine the hASC and hWJ-MSC adequate cell density for seeding (data not shown). On the basis of results, hASCs and hWJ-MSCs were seeded in quadruplicates in a 96-well plate (BD Biosciences, Milano, Italy) at 4000 cells/cm2 or 3500 cells/cm2 respectively, in each experiment.
To determine the hASC and hWJ-MSC growth curves and to compare their basal proliferation, both hASCs and hWJ-MSCs were recovered from 4 healthy subjects (passages of subculture spanning from 6th to 14th and from 6th to 16th, respectively). Then, in order to preliminarily evaluate the toxicity of H2O2 treatment, both hASCs and hWJ-MSCs (9th and 8th subculture passage, respectively) were obtained from one subject and cells were treated in a technical quadruplicate with H2O2 at 5 different concentrations (50, 100, 150, 200 and 400 μM) for 1 and 2 h. Later, in order to evaluate the effect of selected H2O2 concentrations on hASC and on hWJ-MSC proliferation, experiments (in technical quadruplicate) were performed with cells obtained from 4 healthy subjects (passages of subculture spanning from 6th to 14th and from 6th to 16th, respectively). Finally, to perform a comparative analysis of cell proliferation throughout four different culture passages in hASCs (6th, 9th, 11th and 14th) and in hWJ-MSCs (6th, 8th, 11th and 16th), three different experiments were performed with cells derived from the same subject at each studied culture passage.
In every experimental test, after 24 h from the cell seeding in standard conditions, treated cells were exposed to H2O2 as described above and control cells were cultured in complete medium. At the end of treatment time, fresh complete medium with Resazurin reagent (at the ratio of 10:1 respectively) was added to each well and cells were incubated at 37°C. As a negative control Resazurin solution was added to the medium without cells; we also included the totally reduced Resazurin to the medium without cells as a positive control. The fluorescence signal was measured with the Wallac 1420 Victor2 Multilabel Counter (Perkin Elmer, Waltham, MA, USA) at wavelength of 590 nm using an excitation wavelength of 560 nm. Fluorescence was measured after 2, 4, 24, 48 and 72 h from the end of the treatment. The number of viable cells correlating with the magnitude of dye reduction was expressed as percentage of Resazurin reduction according to this formula: (FI 590 of test agent - FI 590 of negative control)/(FI 590 of 100% reduced of Resazurin - FI 590 negative control) × 100 where FI is Fluorescence Intensity.
Senescence-Associated β-Galactosidase assay
To evaluate the SA β-Gal activity, the "Senescence Cells Histochemical Staining Kit" (Sigma-Aldrich Co.) was used. hASCs and hWJ-MSCs were seeded in 24-well plates (BD Biosciences) at the density of 4000 and 3400 cells/well, respectively. These specific cell densities were determined after a preliminary growth curve analysis (data not shown). After 24 h in standard conditions, cells were exposed to H2O2 as described above. A preliminary study was performed in triplicate for each treatment on hASCs and hWJ-MSCs derived from one subject (9th and 8th culture passage, respectively), and SA β-Gal assay was carried out after 24, 48 or 72 h from the end of the exposure to H2O2. Then SA β-Gal staining was investigated at 48 h using cells from three different subjects (n=3, culture passages ranging from 6th and 9th) for each cell type. The assessment of SA β-Gal activity was carried out according to the manufacturer's instructions and the positive blue staining was used as a biomarker of cellular senescence. The number of positive (blue) and negative (not colored) cells was counted in each sample in at least three random fields under a light microscope (at 200× magnification and bright field illumination) [68]. To avoid staining due to cell confluence rather than to proliferative senescence, assay was performed in sub-confluent cultures displaying comparable cell density.
Capacity of cells to undergo passages post H2O2 treatments
In order to test the remaining adhesion cell capacity after H2O2 treatment, when cultures reached 80% confluency, cells were re-seeded in 24-well plates (BD Biosciences). Control cells were splitted with 1:3 ratio, while H2O2-treated cells were seeded with 1:1 ratio. Cells were analyzed the following days in order to identify the proliferation cell capacity and the complete growth arrest.
Morphological analysis of senescent H2O2-treated cells
Cells were analyzed for morphological characteristics and changes under a light microscope (at 40× and 200× magnification) before treatment with H2O2, after 1 o 2 h of exposure, after 48 h from the end of the treatment and after their re-seeding. Cell images were detected under bright field illumination with the Leica MC170 HD Imaging System.
RNA extraction and RT-PCR
Cells obtained from one individual healthy subject for hASCs or hWJ-MSCs were seeded in T75 flasks at the density of 3500 cells/cm2. After 24 h in standard conditions, cells were exposed to H2O2 150 μM (hASCs) or 200 μM (hWJ-MSCs) for 2 h. After 48 h from the end of the stimulus, total RNA was extracted from treated or untreated hMSCs using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA) and digested with RNase-free Deoxyribonuclease I (DNase I) (RNase-free DNase set, QIAGEN) following the manufacturer's instructions. RNAs were reverse-transcribed as previously described [69], except for the temperature of the reaction that was 37°C instead of 42°C. The success of the reaction was verified with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene amplification as described in [70], except for 25 cycles instead of 45; GAPDH amplicon detection was performed by gel agarose electrophoresis, as described by Beraudi and coll. [71]. The experiment was repeated three times.
Real time PCR
For each experimental condition, 25 ng of cDNA were amplified using the SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) in technical triplicates in a Bio-Rad CFX96 real-time thermal cycler (Bio-Rad Laboratories), as previously described [70]. Specific primers for p53 and p21 genes were designed by Bio-Rad and used following the manufacturer's instructions (TP53 and CDKN1A, 20×, Bio-Rad Laboratories). Relative gene expression was determined by CFX Manager Software version 3.1 (Bio-Rad Laboratories) using hypoxanthine phosphoribosyl transferase 1 (HPRT1), TATA box binding protein (TBP), GAPDH (20×, Bio-Rad Laboratories) as reference genes with the “delta-delta CT method”[72,73].
Statistical analysis
Data obtained from the in vitro toxicology assay were analyzed using one-way ANOVA followed by the Tukey HSD and Student's T-test. Data obtained from cell count assay were analyzed using one-way ANOVA followed by the Tukey HSD. Data obtained from real time PCR were analyzed by the CFX Manager Software version 3.1 (Bio-Rad Laboratories). Results were considered statistically significant with a p-value < 0.05 and highly significant with a p-value < 0.01.
Results
Basal cell proliferation and senescence: a comparison between human ASCs and WJ-MSCs
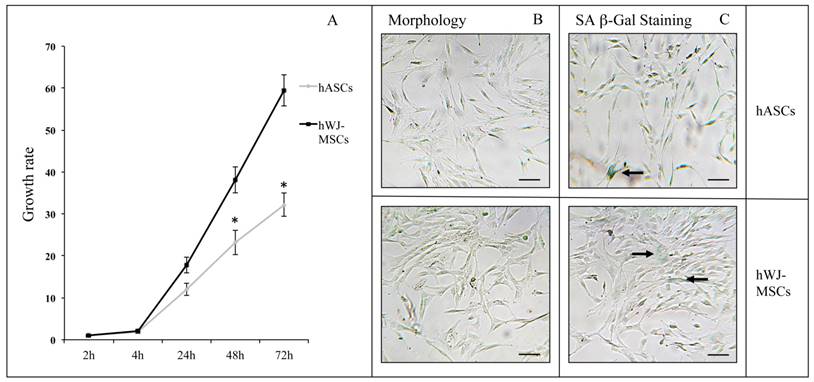
As previously described, the Resazurin-based assay allowed a cell proliferation monitoring over- and real- time, due to the Resorufin atoxicity [67]. On the basis of preliminary evaluations on hASC and hWJ-MSC growth curves (data not shown), cells were seeded at different concentrations (4000 cells/cm2 and 3500 cells/cm2, respectively) to perform proliferation experiments. In order to compare the cell metabolism of hASCs and hWJ-MSCs, results were expressed as growth rates, calculated as the percentage of Resazurin reduction at each time point divided by the percentage of reduction at the 2 h from the Resazurin inoculation. As shown in Figure 2A, the hWJ-MSC growth rate was significantly greater (at 48 h and 72 h time points, p<0.05) than the one detected for hASCs (approximately twice). Cells derived from four subjects were used in each experiment for both hASCs and hWJ-MSCs related assays (passages of subculture spanning from 6th to 14th and from 6th to 16th, respectively). Since basal metabolism was higher in hWJ-MSCs than in hASCs, in the following experiments we decided to analyze hASC and hWJ-MSC proliferation starting from 4 and 2 h from Resazurin administration, respectively. hMSC senescence in basal conditions was evaluated by SA β-Gal assay. Figure 2B shows the characteristic fibroblast-like morphology of both hASCs and hWJ-MSCs, cultured at the 9th and the 8th passage, respectively. After SA β-Gal staining, in both cell types only a small percentage of blue colored cells were appreciable (Figure 2C, black arrows).
(A) Cell proliferation and senescence in hASCs and hWJ-MSCs: growth rate of hASCs (n=4) and hWJ-MSCs (n=4) expressed as mean ± standard error of the mean (SEM; *p<0.05). Typical hASC and hWJ-MSC morphology at 9th - and 8th culture passages respectively (B), and SA β-Gal staining in hASCs and hWJ-MSCs (C). Cells were analyzed under a light microscope and cell images were detected under bright field illumination with the Leica MC170 HD Imaging System at 200× magnification. Scale bars: 100 μm. Representative blue cells, as markers of senescence, are indicated by black arrows.
Preliminary screening of H2O2 treated cells: proliferation analysis
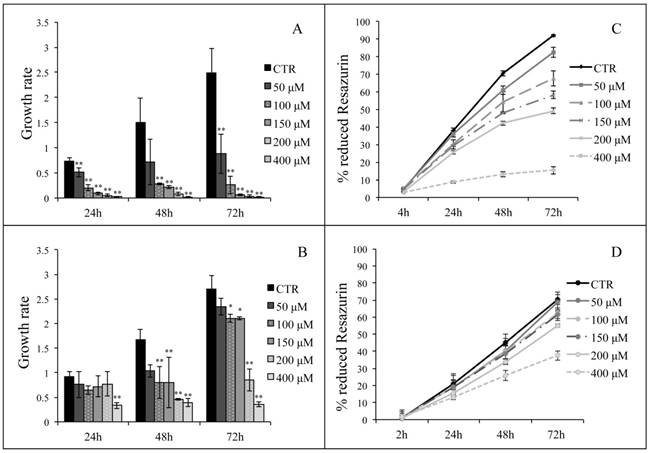
hASCs and hWJ-MSCs cell count was performed after 24, 48 and 72 h from the end of H2O2 treatment (50, 100, 150, 200 and 400 μM) for 2h. Data shown in Figure 3 (Panels A and B) were expressed as growth rates, calculated as the cell number at each time point divided by the cell number of seeded cells. Results underline that, in each experimental point, both hASCs and hWJ-MSCs treated with H2O2 were less in number comparing with untreated cells in a dose-dependent manner.
Moreover, a preliminary Resazurin based assay was performed to evaluate the toxicity of H2O2 on hASCs and hWJ-MSCs proliferation (derived from one subject at 9th and 8th subculture passage, respectively). The assay was performed after treatment with H2O2 at 5 different concentrations (50, 100, 150, 200 and 400 μM) for 1 and 2 h. In Figure 3 (Panels C and D) we show the percentage of Resazurin reduction in presence or absence of a 2-h H2O2 treatment until 72 h post-treatment, expressed as the mean of technical quadruplicates. H2O2 treatment at 50, 100, 150 and 200 μM seems to act in a dose-dependent manner on cell metabolic activity in both the hMSC types, while 400 μM H2O2 induced an evident cell proliferation arrest in hASCs (Figure 3C). Similar results were obtained when H2O2 was administered for 1 h (data not shown).
In order to compare hASCs and hWJ-MSCs behavior, we decided to exclude the 400 μM higher concentration from the subsequent experimental plan.
Effect of H2O2 on hASC proliferation
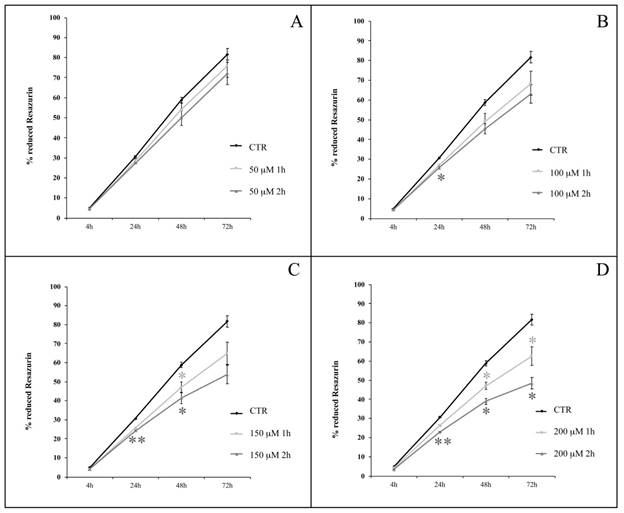
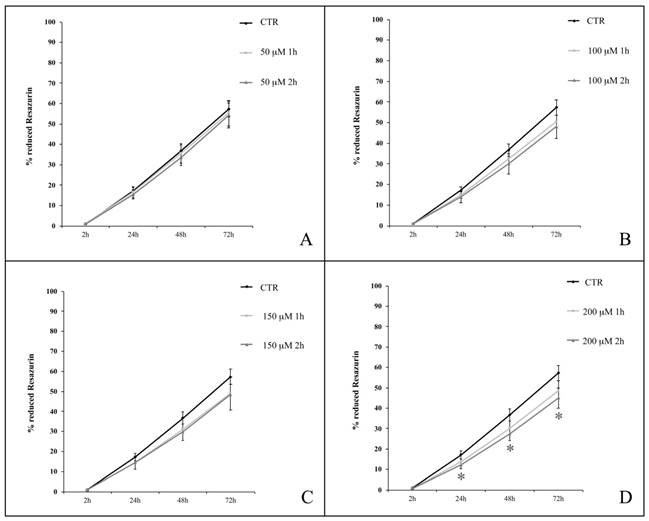
We analyzed hASC proliferation up to 72 h following the termination of 1- or 2-h treatment in the presence of H2O2 at concentrations ranging from 50 to 200 μM. Cells were obtained from four healthy subjects (passages of subculture spanning from 6th to 14th). We observed a decrease in the percentage of Resazurin reduction in H2O2-treated cells compared with untreated cells (Figure 4). After 1 h of H2O2 treatment (Figure 4, light grey lines), the differences between treated and untreated (control) cells were statistically significant (p<0.05) at 48 h when treatment was in the presence of 150 μM H2O2 (Figure 4C), or at both 48 and 72 h when 200 μM H2O2 was used (Figure 4D). Cell exposure to H2O2 for 2 h (Figure 4, dark grey lines), further enhanced the significance of experimental outcomes. In particular, while among the lower concentrations (50 and 100 μM), 100 μM H2O2 affected proliferation (p<0.05) after 24 h (Figure 4A and 4B), a 2-h treatment with higher concentrations (150 and 200 μM) of H2O2 significantly decreased cell proliferation at multiple time points: after 24 (p<0.01) and 48 (p<0.05) h from the treatment with both 150 μM and 200 μM H2O2, and even after 72 h (p<0.05) in cells exposed to this latter concentration (Figure 4C and 4D).
Panels A and B: hASCs (A) and hWJ-MSCs (B) cell counts, expressed as growth rates, calculated as the cell number at each time point divided by the cell number of seeded cells. Data are expressed as mean of growth rates ± SD (n=3, *p<0.05, **p<0.01). Panels C and D: percentage of reduction of Resazurin (as indicator of cell proliferation) after treatment of hASCs (C) and hWJ-MSCs (D) for 2 h with H2O2 (50, 100, 150, 200 or 400 μM). Analysis was conducted on a technical quadruplicate at 4, 24, 48, 72 h (C) or at 2, 24, 48, 72 h (D) from the end of the treatment and data are expressed as mean ± SD.
hASC proliferation curves after treatments with H2O2 at concentrations of 50 μM (A), 100 μM (B), 150 μM (C) and 200 μM (D) for 1 or 2 h. Analysis was conducted at 4, 24, 48, 72 h from the end of the treatment; data are expressed as mean of percentage of reduction of Resazurin (n=4, culture passages 6th- 14th) ± SEM, *p<0.05, **p<0.01. CTR is control.
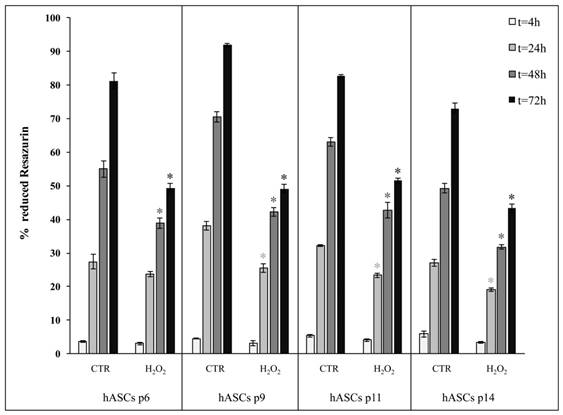
Since the presence of 200 μM H2O2 for 2-h treatment induced in hASCs the most evident reduction of cell proliferation, we also decided to perform comparative analysis of cell proliferation throughout four different culture passages (p6, p9, p11 and p14), in which these cells may intrinsically differ in senescence susceptibility, following a 2-h treatment in the presence of 200 μM H2O2. As shown in Figure 5, we observed that, irrespective of the time spent in culture at different passages, H2O2-exposed hASCs shared a similar proliferation decrease in comparison with the related control cells.
Comparison of cell proliferation between hASCs cultured in the absence (CTR) and presence of H2O2 at four subculture passages (p6, p9, p11 and p14). Treated cells were incubated with 200 μM H2O2 for 2 h. Data were expressed as mean of percentage of reduction of Resazurin (technical triplicate) ± SD; *p<0.05.
Effect of H2O2 on hWJ-MSC proliferation
In Figure 6, we show the proliferation curves of hWJ-MSCs treated for 1 and 2 h with H2O2 at concentrations ranging from 50 to 200 μM. Cells were obtained from four healthy subjects (passages of subculture spanning from 6th to 16th). H2O2 at 50, 100 and 150 μM had a weak effect on hWJ-MSCs (Figure 6A, 6B and 6C), while a 2-h exposure to the higher concentration of H2O2 (200 μM) resulted in a significant decrease in the percentage of Resazurin reduction, as compared with control cells. This was evident at 24, 48 and 72 h following termination of the H2O2 treatment (p<0.05) (Figure 6D).
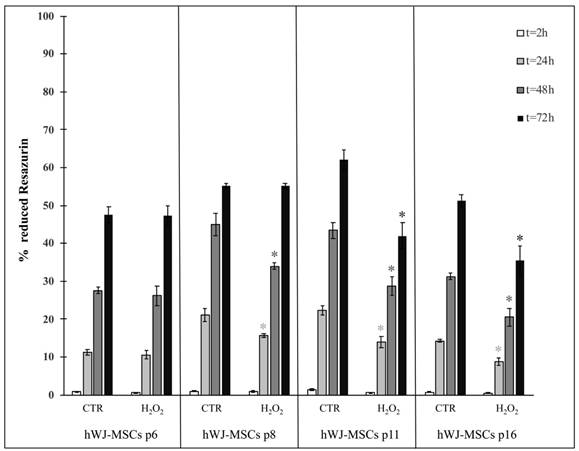
As for hASCs, we analyzed cell proliferation in hWJ-MSCs throughout four distinct subculture passages (p6, p8, p11 and p16): it was evident that exposed and unexposed cells exhibited a differential response as a function of the time spent in culture (Figure 7). In fact, in early passages (i.e. p6) proliferation was not affected from the treatment at any time point. In contrast, in late passages (i.e. p11 and p16) proliferation always decreased in treated cells compared with control cells (Figure 7).
Effect of H2O2 on hASC SA β-Galactosidase activity
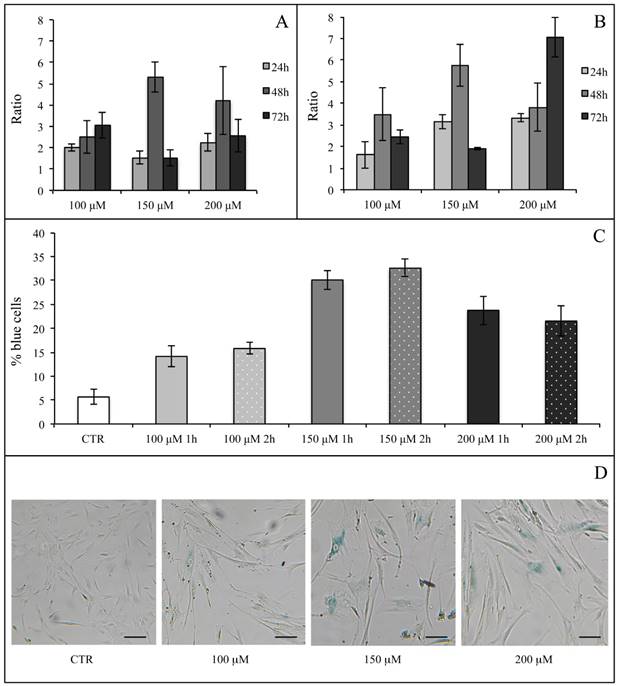
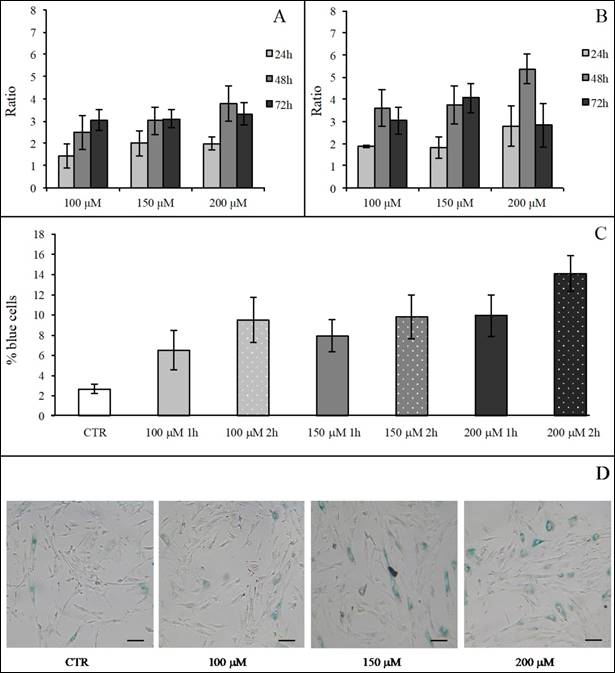
We first evaluated the H2O2 effect on SA β-Gal activity in hASCs (at subculture passage 9th) recovered from one healthy subject (in technical triplicate). H2O2 was used at the final concentrations of 100, 150, 200 and 400 μM for 1 and 2 h, and the assay was performed after 24, 48 and 72 h from the termination of the stimuli. At each experimental time point, 400 μM H2O2 produced a toxic effect and for this reason such a high concentration was not used for the assessment of SA β-Gal expression (data not shown). In all investigated conditions, H2O2 increased the enzyme activity in comparison with the related controls (considered as a value of 1) (Figure 8A and Figure 8B). We arbitrarily defined a ratio greater than 3 in the percentage of blue cells in treated versus control hASCs as a proof for the effectiveness of the treatment. Under these experimental conditions, after 1 h of exposure only 150 or 200 μM H2O2 raised SA β-Gal staining at 48 h from the termination of the treatment (Figure 7A). On the contrary, after a 2-h treatment all the H2O2 concentrations tested efficaciously increased SA β-Gal activity at 48 h from the end of treatment, with a more prolonged effect lasting up to 72 h when a concentration of 200 μM H2O2 was used (Figure 7B).
After a critical analysis of this preliminary test, we further explored SA β-Gal activity after 48 h from the end of H2O2 treatment in cells that had been recovered from three healthy subjects (at subculture passages 6th-9th): in Figure 8 we also show results from a representative experiment (Figure 8C) and the corresponding culture images of H2O2 2-h exposure (Figure 8D). We found that the percentage of blue cells after each treatment was greater than in controls (Figure 8C) and that it reached a peak after the H2O2 150 μM treatment.
hWJ-MSC proliferation curves after treatment with H2O2 at concentrations of 50 μM (A), 100 μM (B), 150 μM (C) and 200 μM (D) for 1 or 2 h. Analysis was conducted at 2, 24, 48, 72 h from the end of the treatment; data are expressed as mean of percentage of reduction of Resazurin (n=4, culture passages 6th- 16th) ± SEM, *p<0.05. CTR is control.
Comparison of cell proliferation between hWJ-MSCs cultured in the absence (CTR) and presence of H2O2 at four subculture passages (p6, p8, p11 and p16). Treated cells were incubated with 200 μM H2O2 for 2 h. Data were expressed as mean of percentage of reduction of Resazurin (technical triplicate) ± SD; *p<0.05.
hASC SA β-Gal activity after H2O2 treatment. Effect of H2O2 treatment expressed in term of ratio between the percentage of blue cells in treated sample and the relative percentage of blue cells in untreated sample after 1 h (Panel A) and 2 h (Panel B) of treatment. In each panel the effect of every H2O2 concentration was presented after 24, 48 and 72 h from the end of the stimulus. Data were obtained in triplicate from one subject and expressed as ratio (n=3) ± SD. Control value is 1 (not shown) and treatment was arbitrarily considered effective when ratio was >3. In Panels (C) and (D) the effects of different H2O2 concentrations on hASC SA β-Gal activity tested at 48 h from the end of the treatment are shown. In Panel (C), a graph representative of three (obtained from three different subjects) showing the percentage of blue cells in untreated (CTR) and treated cells after 1 and 2 h of H2O2 treatment. Data were expressed as percentage of blue cells ± SD. Panel (D): representative SA β-Gal staining images of hASCs untreated (CTR) and treated with H2O2 at final concentrations of 100 μM, 150 μM and 200 μM after 2-h treatment. Cells were analyzed under a light microscope (at 200× magnification) and cell images were detected under bright field illumination with the Leica MC170 HD Imaging System. Blue staining indicates senescent cells and scale bars correspond to 100 μm.
Effect of H2O2 on hWJ-MSCs SA β-Galactosidase activity
A preliminary SA β-Gal activity assay was performed in technical triplicate also on hWJ-MSCs (recovered from one healthy subject and at subculture passage 8th) after 24, 48 and 72 h from termination of the H2O2 treatment at concentrations of 100, 150, 200 and 400 μM for 1 and 2 h. As seen for hASCs, cells treated with H2O2 400 μM were not analyzed due to the cytotoxicity of the treatment (data not shown).
In all experimental conditions, H2O2 treatments (as compared with controls, corresponding to a value 1) increased the enzyme activity, and consequently the percentage of blue stained senescent cells (Figure 9). The effectiveness of treatment was arbitrarily defined as reported in the paragraph “Effect of H2O2 on hASCs SA β-Galactosidase activity”. We found that the most pronounced effect was detected at 48 h from the end of the treatments: after 1 h of exposure, an increase in the number of positive blue cells could only be observed in the presence of 200 μM H2O2 (Figure 9A), while when cells were treated for 2 h, all H2O2 concentrations were effective (Figure 9B). Based upon these results, SA β-Gal activity was assessed after 48 h from the end of the H2O2 treatments in hWJ-MSCs harvested from three healthy subjects (at subculture passages 6th-9th): in Figure 9, we also show results from a representative experiment (Figure 9C) and the corresponding culture images of H2O2 2-h exposure (Figure 9D). H2O2 administration gave an increased percentage of blue cells in comparison with control hWJ-MSCs (Figure 9C). This augmentation was dependent upon both the concentration used and the duration of treatment.
Capacity of cells to undergo passages and morphological changes post H2O2 treatments
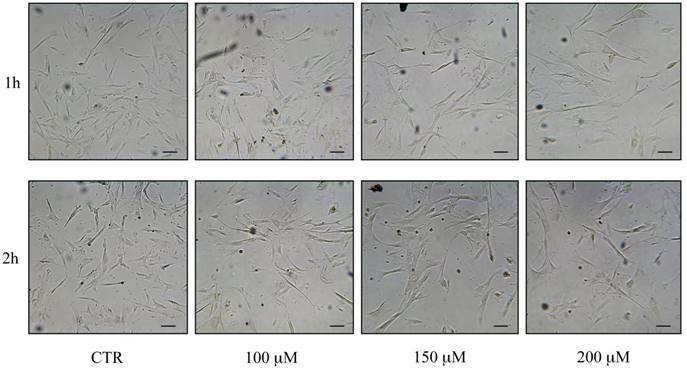
All investigated cell types were able to undergo culture passages post-H2O2 administration, revealing differences related to H2O2 concentrations. In fact, while the hMSC growth was found to be slowed down after treatment with H2O2 400 μM, and the cells were not further viable on re-plating, cells that had been treated with 100, 150 and 200 μM H2O2 were still able to undergo at least 2 passages. Furthermore, after increasing H2O2 concentration and time of exposure, hASCs and hWJ-MSCs changed their morphology at the end of the treatment, at 48 h post treatment and after the re-seeding (Figure 10 and 11). In particular, hASCs become thinner and longer (Figure 10), while hWJ-MSCs enlarged and ramified (Figure 11).
hWJ-MSC SA β-Gal activity after H2O2 treatment. Effect of H2O2 treatment expressed in term of ratio between the percentage of blue cells in treated sample and the relative percentage of blue cells in untreated sample after 1 h (Panel A) and 2 h (Panel B) of treatment. In each panel the effect of every H2O2 concentration was presented after 24, 48 and 72 h from the end of the stimulus. Data were obtained in triplicate from one subject and expressed as ratio (n=3) ± SD. Control value is 1 (not shown) and treatment was arbitrarily considered effective when ratio was >3. In Panels (C) and (D) the effects of different H2O2 concentrations on hWJ-MSC SA β-Gal activity tested at 48 h from the end of the treatment are shown. In Panel (C), a graph representative of three (obtained from three different subjects) showing the percentage of blue cells in untreated (CTR) and treated cells after 1 and 2 h of H2O2 treatment. Data were expressed as percentage of blue cells ± SD. Panel (D): representative SA β-Gal staining images of hWJ-MSCs untreated (CTR) and treated with H2O2 at final concentrations of 100 μM, 150 μM and 200 μM after 2-h treatment. Cells were analyzed under a light microscope (at 200× magnification) and cell images were detected under bright field illumination with the Leica MC170 HD Imaging System. Blue staining indicates senescent cells and scale bars correspond to 100 μm.
Morphological changes of hASCs after reseeding post H2O2 treatments: control (CTR) cells and cells treated with H2O2 100, 150 and 200 μM. Cells were analyzed under a light microscope (at 200× magnification) and cell images were detected under bright field illumination with the Leica MC170 HD Imaging System. Scale bars: 100 μm.

Morphological changes of hWJ-MSCs after reseeding post H2O2 treatments: control (CTR) cells and cells treated with H2O2 100, 150 and 200 μM. Cells were analyzed under a light microscope (at 200× magnification) and cell images were detected under bright field illumination with the Leica MC170 HD Imaging System. Scale bars: 100 μm.
Effect of H2O2 on hMSCs gene expression
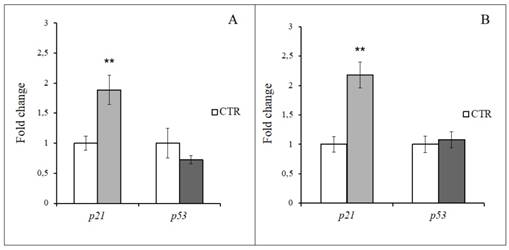
p21 and p53 expression was investigated at 48 h from the end of the H2O2 specific treatment both in hASCs (i.e. 150 µM for 2 h) and hWJ-MSCs (i.e. 200 µM for 2 h). As depicted in Figure 12, hydrogen peroxide strongly acted on p21 overexpression in hMSCs independently of their source (p<0.01). At the same time, H2O2 treatment in hASCs resulted in a down regulation of p53 (Figure 12A), while in hWJ-MSCs it shows a stable expression in comparison with the control (Figure 12B).
Discussion
Both hASCs and hWJ-MSCs are frequently used in stem cell-based protocols [2]. These cells undergo significant decline in their rescuing potential as they age in vivo during the lifespan, or when they become senescent during their expansion in vitro, with significant impairment in the outcome of cell therapy efforts [22-24].
The availability of reliable accelerated methods to induce stem cell senescence in vitro will avoid cumbersome long-lasting (2-3 months) culture protocols and facilitate the investigation of senescence mechanisms and source-related senescence susceptibility among different hMSC populations [13].
To this end, we observed that hASCs and hWJ-MSCs exhibited different proliferation profiles over time, indicating that basal metabolism may be higher in hWJ-MSCs than in hASCs. Akin to this observation is the present finding that hWJ-MSCs required a greater number of culture passages than hASCs for undergoing senescence, as it can also be inferred from the ability of hWJ-MSCs to exhibit long-lasting stemness properties and preserve a typical fibroblast-like morphology while undergoing multiple culture passages in vitro [43,74].
Gene expression analysis in hASCs and hWJ-MSCs after 48 h from 2h-H2O2 treatment. A: p21 and p53 relative expression in hASCs treated with 150 μM H2O2. B: p21 and p53 relative expression in hWJ-MSCs treated with 200 μM H2O2. Data were expressed as mean fold change (n=3) ± SD, **p<0.01.
Here, we found that in both cell populations, H2O2 not only inhibited cell proliferation in a dose-dependent fashion, but it also affected the persistence of the anti-proliferative effect after the end of exposure (up to 48 or 72 h) in a manner that was dependent upon the duration (1 or 2 h) of the treatment itself. These results are coherent with the reduction of cell vitality observed in hWJ-MSCs [43-45] and hASCs [39,42] treated with higher concentrations of H2O2 (until 2000 μM) but at low subcultured passages. We also observed a growth slowdown of cells treated with H2O2 400 μM, and a cell inability to re-plate. On the other hand, cells treated with 100, 150 and 200 μM H2O2 were still able to undergo at least 2 passages, although an increase in H2O2 concentration and exposure time were associated to morphology changes. These results were consistent with those obtained in the study of Choo and coll. (2014), where hWJ-MSCs exposed to H2O2 resulted enlarged, with cytoplasm enriched of granules and able to undergo until 3-4 passages after H2O2 treatment [43].
The possibility that the observed difference in basal proliferative activity among hASCs and hWJ-MSCs may entail different senescence susceptibilities [13,75] was confirmed by the finding that the same H2O2 concentrations that remarkably inhibited proliferation in hASCs, only weakly affected hWJ-MSC proliferation, and that a significant decrease in the percentage of reduced Resazurin could be achieved in these cells solely after the exposure to the H2O2 concentration that proved the most effective one in hASCs. Our results are reinforced by the previous evidence that low H2O2 concentrations (50 and 100 μM) did not affect hWJ-MSCs proliferation treated for 24 h [45].
The analysis of the effect of H2O2 treatment on proliferation in cells that had been subjected to different, even long-term subculture passages (until 14-16th), provided the chance of further compounding the multifaceted ways different hMSC populations may progress in senescence. For the first time we demonstrated that H2O2 given for 2 h, reduced hASC proliferation both in early and late subculture passages, while it decreased hWJ-MSC proliferation in a passage-dependent fashion, in particular only at higher subculture passages.
These observations were all corroborated by results obtained from our analysis of SA β-Gal activity after 24, 48 and 72 h from the end of the H2O2 treatment, where both hASCs and hWJ-MSCs showed an increase of the SA β-Gal activity. In particular, the higher senescent effect was evident at 48 h. A similar trend was seen in other researches, although it was obtained at different experimental times [42-44].
Comparing SA β-Gal assay results, it is evident that hWJ-MSCs and hASCs have a different way to respond to oxidative stress. To this end, the SA β-Gal activity increase observed in hWJ-MSCs was dependent on both H2O2 concentration and exposure time, reaching a maximum with 200 μM H2O2 for 2 h, while hASCs showed the greatest increase with 150 μM H2O2. In fact, if hASCs were exposed to H2O2 at low concentrations (i.e. 100 μM) they were able to counteract pro-senescence stress, while if cells were treated with 200 μM H2O2 they showed a SA β-Gal activity increase, although weaker than that induced by 150 μM H2O2. This observation could be explained by the well documented ability of H2O2 to promote not only senescence but also apoptosis depending upon the experimental conditions [43,76].
The persistence of the hydrogen peroxide effects on cell senescence was shown also by the gene expression analysis of two of the major cell cycle regulators, p21 and p53. At 48 h from the end of the most effective and specific H2O2 treatment, in both hMSC types the inhibition of cell cycle progression was associated with a strong overexpression of p21, a transient growth arrest mediator, able to slowdown cell proliferation [22]. At the same time, p53 transcription was quite stable in hWJ-MSCs, while being down-regulated in hASCs, suggesting that after 2 days from the selected H2O2 administration, hMSCs did not start an apoptotic program.
Overall the present data not only provide evidence for the effectiveness of the H2O2 exposure method in recapitulating over time senescence features in hMSCs, but also indicate its usefulness in detecting the proneness of different hMSC populations to undergo senescence on the basis of their tissue source [13,73].
In particular, hWJ-MSCs present several advantages comparing with hASCs: not only their easy availability and isolation, their independence from donor age, but also their natural proneness to undergo senescence at late culture passages and their ability to efficiently counteract oxidative stress stimuli.
Moreover, within cell therapy contexts, the present study may also set the basis to investigate and establish the limit(s) for in vitro expansion of targeted hMSC populations in order to achieve an optimal balance between the cell number available for transplantation and the risk for cell expansion-related senescence. These considerations may also apply to the development of patient-specific protocols for the expansion of defined hMSC populations in the future perspective of Precision Medicine approaches.
Abbreviations
hASCs: human Adipose tissue-derived Stem Cells; hWJ-MSCs: human Wharton's Jelly-derived Mesenchymal Stem Cells; H2O2: hydrogen peroxide; hMSCs: human Mesenchymal Stem Cells; SA β-Gal: Senescence-Associated β-Galactosidase; ROS: Reactive Species of Oxygen; OSIPS: Oxidative Stress-Induced Premature Senescence; SOD: Superoxide Dismutase; GSH: Glutathione Synthetase; MAPK14: mitogen-activated protein kinase 14 (alias p38); SIRT1: sirtuin 1; α-MEM: alfa-Minimal Essential Medium; DMEM: Dulbecco's Modified Eagle's Medium; FBS: Fetal Bovine Serum; HPRT1: hypoxanthine phosphoribosyl transferase 1; TBP: TATA box binding protein; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; CDKN1A: cyclin dependent kinase inhibitor 1A (alias p21); TP53: tumor protein p53 (alias p53); p: passage; h: hour; SD: standard deviation; SEM: standard error of the mean.
Acknowledgements
This study was funded by Eldor Lab, via Vittor Pisani 16, 20124 Milan, Italy.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen C, Fingerhut JM, Yamashita YM. The ins(ide) and outs(ide) of asymmetric stem cell division. Curr Opin Cell Biol. 2016;43:1-6
2. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-317
3. Smiler D, Soltan M, Albitar M. Toward the identification of mesenchymal stem cells in bone marrow and peripheral blood for bone regeneration. Implant Dent. 2008;17(3):236-247
4. Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7-14
5. In't Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338-1345
6. Corrao S, La Rocca G, Lo Iacono M, Corsello T, Farina F, Anzalone R. Umbilical cord revisited: From Wharton's jelly myofibroblasts to mesenchymal stem cells. Histol Histopathol. 2013;28(10):1235-1244
7. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235-242
8. Kwon A, Kim Y, Kim M, Kim J, Choi H, Jekarl DW, Lee S, Kim JM, Shin JC, Park IY. Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci Rep. 2016;6:23544
9. Chen JY, Mou XZ, Du XC, Xiang C. Comparative analysis of biological characteristics of adult mesenchymal stem cells with different tissue origins. Asian Pac J Trop Med. 2015;8(9):739-746
10. Billing AM, Ben Hamidane H, Dib SS, Cotton RJ, Bhagwat AM, Kumar P, Hayat S, Yousri NA, Goswami N, Suhre K, Rafii A, Graumann J. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep. 2016;6:21507
11. Dabrowski FA. Comparison of the paracrine activity of mesenchymal stem cells derived from human umbilical cord, amniotic membrane and adipose tissue. J Obstet Gynaecol Res. 2017;43(11):1758-1768
12. Maleki M, Ghanbarvand F, Reza Behvarz M, Ejtemaei M, Ghadirkhomi E. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells. 2014;7(2):118-126
13. Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl Med. 2017;6(12):2173-2185
14. Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:496218
15. Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol Sin. 2013;34(6):747-754
16. Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283
17. Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15(6):862-870
18. Chang C, Wang X, Niu D, Zhang Z, Zhao H, Gong F. Mesenchymal stem cells adopt beta-cell fate upon diabetic pancreatic microenvironment. Pancreas. 2009;38(3):275-281
19. Kuo YR, Goto S, Shih HS, Wang FS, Lin CC, Wang CT, Huang EY, Chen CL, Wei FC, Zheng XX, Lee WP. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation. 2009;87(12):1769-1777
20. Souza BS, Nogueira RC, de Oliveira SA, de Freitas LA, Lyra LG, Ribeiro dos Santos R, Lyra AC, Soares MB. Current status of stem cell therapy for liver diseases. Cell Transplant. 2009;18(12):1261-1279
21. Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci USA. 2009;106(33):14022-14027
22. Turinetto V, Vitale E, Giachino C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int J Mol Sci. 2016;17(7):E1164
23. Ho AD, Wagner W, Mahlknecht U. Stem cells and ageing. The potential of stem cells to overcome age-related deteriorations of the body in regenerative medicine. EMBO Rep. 2005;6:S35-S38
24. Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE. 2009;4(6):e5846
25. Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614-636
26. Benameur L, Charif N, Li Y, Stoltz JF, de Isla N. Toward an understanding of mechanism of aging induced oxidative stress in human mesenchymal stem cells. Biomed Mater Eng. 2015;25(1 Suppl):41-46
27. Reuter S, Gupta SC, Chaturvedi MM, Aggarwa BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49(11):1603-1616
28. Haines DD, Juhasz B, Tosaki A. Management of multicellular senescence and oxidative stress. J Cell Mol Med. 2013;17(8):936-957
29. Chen JH, Stoeber K, Kingsbury S, Ozanne SE, Williams GH, Hales CN. Loss of proliferative capacity and induction of senescence in oxidatively stressed human fibroblasts. J Biol Chem. 2004;279(47):49439-49446
30. Passos JF, von Zglinicki T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res. 2006;40(12):1277-1283
31. Harmand D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298-300
32. Valle-Prieto A, Conget PA. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010;19(12):1885-1893
33. Denu RA, Hematti P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid Med Cell Longev. 2016;2016:2989076
34. Brandl A, Meyer M, Bechmann V, Nerlich M, Angele P. Oxidative stress induces senescence in human mesenchymal stem cells. Exp Cell Res. 2011;317(11):1541-1547
35. Kim JS, Kim EJ, Kim HJ, Yang JY, Hwang GS, Kim CW. Proteomic and metabolomic analysis of H2O2-induced premature senescent human mesenchymal stem cells. Exp Gerontol. 2011;46(6):500-510
36. Park SY, Jeong AJ, Kim GY, Jo A, Lee JE, Leem SH, Yoon JH, Ye SK, Chung JW. Lactoferrin Protects Human Mesenchymal Stem Cells from Oxidative Stress-Induced Senescence and Apoptosis. J Microbiol Biotechnol. 2017;27(10):1877-1884
37. Zhou L, Chen X, Liu T, Gong Y, Chen S, Pan G, Cui W, Luo ZP, Pei M, Yang H, He F. Melatonin reverses H2O2-induced premature senescence in mesenchymal stem cells via the SIRT1-dependent pathway. J Pineal Res. 2015;59(2):190-205
38. Lee JH, Jung HK, Han YS, Yoon YM, Yun CW, Sun HY, Cho HW, Lee SH. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol Med Rep. 2016;14(4):3777-3784
39. Wang N, Wang F, Gao Y, Yin P, Pan C, Liu W, Zhou Z, Wang J. Curcumin protects human adipose-derived mesenchymal stem cells against oxidative stress-induced inhibition of osteogenesis. J Pharmacol Sci. 2016;132(3):192-200
40. Baldari S, Di Rocco G, Trivisonno A, Samengo D, Pani G, Toietta G. Promotion of survival and engraftment of transplanted adipose tissue-derived stromal and vascular cells by overexpression of manganese superoxide dismutase. Int J Mol Sci. 2016;17(7):1082
41. Altanerova V, Horvathova E, Matuskova M, Kucerova L, Altaner C. Genotoxic damage of human adipose-tissue derived mesenchymal stem cells triggers their terminal differentiation. Neoplasma. 2009;56(6):542-547
42. Rajamani K, Lin YC, Wen TC, Hsieh J, Subeq YM, Liu JW, Lin PC, Harn HJ, Lin SZ, Chiou TW. The antisenescence effect of trans-cinnamaldehyde on adipose-derived stem cells. Cell Transplant. 2015;24(3):493-507
43. Choo KB, Tai L, Hymavathee KS, Wong CY, Nguyen PN, Huang CJ, Cheong SK, Kamarul T. Oxidative stress-induced premature senescence in Wharton's jelly-derived mesenchymal stem cells. Int J Med Sci. 2014;11(11):1201-1207
44. Wajid N, Mehmood A, Bhatti FU, Khan SN, Riazuddin S. Lovastatin protects chondrocytes derived from Wharton's jelly of human cord against hydrogen-peroxide-induced in vitro injury. Cell Tissue Res. 2013;351(3):433-443
45. Nimsanor N, Phetfong J, Plabplueng C, Jangpatarapongsa K, Prachayasittikul V, Supokawej A. Inhibitory effect of oxidative damage on cardiomyocyte differentiation from Wharton's jelly-derived mesenchymal stem cells. Exp Ther Med. 2017;14(6):5329-5338
46. Nouri F, Salehinejad P, Nematollahi-Mahani SN, Kamarul T, Zarrindast MR, Sharifi AM. Deferoxamine Preconditioning of Neural-Like Cells Derived from Human Wharton's Jelly Mesenchymal Stem Cells as a Strategy to Promote Their Tolerance and Therapeutic Potential: An In Vitro Study. Cell Mol Neurobiol. 2016;36(5):689-700
47. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163-173
48. Huang K, Zhou DH, Huang SL, Liang SH. Age related biological characteristics of human bone marrow mesenchymal stem cells from different age donors. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2005;13(6):1049-1053
49. Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919-926
50. Madonna R, Renna FV, Cellini C, Cotellese R, Picardi N, Francomano F, Innocenti P, De Caterina R. Age- dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. Eur J Clin Invest. 2011;41(2):126-133
51. Alt EU, Senst C, Murthy SN, Slakey DP, Dupin CL, Chaffin AE, Kadowitz PJ, Izadpanah R. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8(2):215-225
52. Davies JE, Walker JT, Keating A. Concise review: Wharton's Jelly: The rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl Med. 2017;6(7):1620-1630
53. Kalaszczynska I, Ferdyn K. Wharton's jelly derived mesenchymal stem cells: future of regenerative medicine? Recent findings and clinical significance. Biomed Res Int. 2015;2015:430847
54. Conconi MT, Liddo RD, Tommasini M, Calore CC, Parnigotto PP. Phenotype and differentiation potential of stromal populations obtained from various zones of human umbilical cord: an overview. Open Tissue Eng Regen Med J. 2011;4:6-20
55. Anzalone R, Iacono ML, Corrao S, Magno F, Loria T, Cappello F, Zummo G, Farina F, La Rocca G. New emerging potentials for human Wharton's jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010;19(4):423-438
56. Nekanti U, Mohanty L, Venugopal P, Balasubramanian S, Totey S, Ta M. Optimization and scale-up of Wharton's jelly derived mesenchymal stem cells for clinical applications. Stem Cell Res. 2010;5(3):244-254
57. Tong CK, Vellasamy S, Tan BC, Abdullah M, Vidyadaran S, Seow HF, Ramasamy R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35(3):221-226
58. Fong CY, Chak LL, Biswas A, Tan JH, Gauthaman K, Chan WK, Bongso A. Human Wharton's jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2011;7(1):1-16
59. Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14(10):828-835
60. Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14(6):11692-11712
61. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150-154
62. Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24(3):781-792
63. Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22(11):2063-2077
64. Canaider S, Maioli M, Facchin F, Bianconi E, Santaniello S, Pigliaru G, Ljungberg L, Burigana F, Bianchi F, Olivi E, Tremolada C, Biava PM, Ventura C. Human stem cell exposure to developmental stage zebrafish extracts: a novel strategy for tuning stemness and senescence patterning. CellR4. 2014;2:e1226
65. Paradisi M, Alviano F, Pirondi S, Lanzoni G, Fernandez M, Lizzo G, Giardino L, Giuliani A, Costa R, Marchionni C, Bonsi L, Calzà L. Human mesenchymal stem cells produce bioactive neurotrophic factors: source, individual variability and differentiation issues. Int J Immunopathol Pharmacol. 2014;27(3):391-402
66. La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M, Di Stefano A, Giannuzzi P, Marasà L, Cappello F, Zummo G, Farina F. Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol. 2009;131(2):267-282
67. Präbst K, Engelhardt H, Ringgeler S, Hübner H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. Methods Mol Biol. 2017;1601:1-17
68. Rinaldi S, Maioli M, Santaniello S, Castagna A, Pigliaru G, Gualini S, Margotti ML, Carta A, Fontani V, Ventura C. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: an in vitro beta-galactosidase study. Clin Interv Aging. 2012;7:191-194
69. Casadei R, Piovesan A, Vitale L, Facchin F, Pelleri MC, Canaider S, Bianconi E, Frabetti F, Strippoli P. Genome-scale analysis of human mRNA 5' coding sequences based on expressed sequence tag (EST) database. Genomics. 2012;100(2):125-130
70. Facchin F, Vitale L, Bianconi E, Piva F, Frabetti F, Strippoli P, Casadei R, Pelleri MC, Piovesan A, Canaider S. Complexity of bidirectional transcription and alternative splicing at human RCAN3 locus. PLoS One. 2011;6:e24508
71. Beraudi A, Bianconi E, Catalani S, Canaider S, De Pasquale D, Apostoli P, Bordini B, Stea S, Toni A, Facchin F. In vivo response of heme-oxygenase-1 to metal ions released from metal-on-metal hip prostheses. Mol Med Rep. 2016;14(1):474-480
72. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611-622
73. Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, Lotti Margotti M, Bagella L, Fontani V, Ventura C. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Dordr). 2014;36(1):9-20
74. Carvalho MM, Teixeira FG, Reis RL, Sousa N, Salgado AJ. Mesenchymal stem cells in the umbilical cord: phenotypic characterization, secretome and applications in central nervous system regenerative medicine. Curr Stem Cell Res Ther. 2011;6(3):221-228
75. Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Comm Signal. 2011;9:12
76. Chen QM. Replicative senescence and oxidant-induced premature senescence. Beyond the control of cell cycle checkpoints. Ann NY Acad Sci. 2000;908:111-125
Author contact
![]() Corresponding author: Silvia Canaider (Department of Experimental, Diagnostic and Specialty Medicine -DIMES, University of Bologna, Via Massarenti 9, 40138 Bologna, Italy, Tel: +39-051-2094104, Fax: +39-051-2094110 or E-mail address: silvia.canaiderit)
Corresponding author: Silvia Canaider (Department of Experimental, Diagnostic and Specialty Medicine -DIMES, University of Bologna, Via Massarenti 9, 40138 Bologna, Italy, Tel: +39-051-2094104, Fax: +39-051-2094110 or E-mail address: silvia.canaiderit)

 Global reach, higher impact
Global reach, higher impact