3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(11):1210-1216. doi:10.7150/ijms.26580 This issue Cite
Research Paper
Influence Of Angiogenic Mediators And Bone Remodelling In Paget´s Disease Of Bone
1. Translational Research on Renal and Cardiovascular Diseases (TRECARD), Department of Physiology and Pharmacology, University of Salamanca, Salamanca, Spain.
2. Institute of Biomedical Research of Salamanca (IBSAL), Salamanca, Spain.
3. Molecular Medicine Unit, Department of Medicine, University of Salamanca and Institute of Molecular and Cellular Biology of Cancer (IBMCC), University of Salamanca-CSIC, Salamanca, Spain.
4. Internal Medicine Service, Virgen de la Luz Hospital, Cuenca, Spain.
5. Research Unit, Primary Care Centre of La Alamedilla, Salamanca, Spain.
6. Metabolic Bone Unit, University Hospital of Salamanca, Spain.
7. Institute of Health Sciences Studies of Castilla y Leon (IECSCYL), Research Unit, University Hospital of Salamanca, Salamanca, Spain
*These authors contributed equally to this work
Received 2018-4-9; Accepted 2018-7-2; Published 2018-7-30
Abstract
Paget´s disease of bone (PDB) is characterized by increased bone resorption followed by an excessive compensatory bone formation, with an abnormal bone structure with altered mechanical properties. Pagetic bone also has a higher vascularization and marrow fibrosis. Despite of pagetic bone being a highly vascularized tissue, there are no studies on the plasma levels of angiogenic mediators in the different states of the disease; moreover, the effect of PDB treatment on plasma levels of these angiogenic mediators is not very well known. The aim of this study was to analyse plasma levels of cytokines implicated in the increased bone turnover (OPG, RANKL, sclerostin) and hypervascularization (VEGF, PGF, ENG) observed in PDB and their evolution and response to zoledronic acid treatment in 70 PDB patients, 29 with an active disease measured by plasma alkaline phosphatase (ALP). Plasma ALP concentration was higher in active PDB than in inactive PDB patients, whereas there were no differences in OPG, RANKL, sclerostin, VEGF, PGF and ENG plasma levels between active and inactive PDB patients. ALP decreased at 3 and 12 months after zoledronic acid treatment. RANKL levels were reduced and sclerostin levels were increased after 12 months of treatment. PGF levels were lower 12 months after zoledronic acid treatment, whereas there were no differences in plasma VEGF and ENG after zoledronic acid treatment. Summarizing, zoledronic acid treatment is associated to decreases in plasma levels of ALP, RANKL, sclerostin and P1GF in active PDB patients. This treatment may reduce bone turnover and might reduce the pathological vascularisation typical of pagetic bone.
Keywords: Paget´s disease of bone, zoledronic acid, RANKL, sclerostin, PGF
Introduction
Paget´s disease of bone (PDB) is a metabolic focal disorder of bone remodelling characterized by an increase in bone resorption followed by an excessive compensatory bone formation. The main PDB alteration resides in osteoclasts that increase in size, number and activity. As a result, the bone structure is abnormal and variegated and causes alterations of its mechanical properties [1]. Over time, the hypercellularity, bone turnover and vascularization decrease, and predominates a sclerotic bone (inactive PDB) [1,2]. One of the most common and effective treatments for the symptoms in PDB patients is zoledronic acid, an aminobisphosphonate that inhibits osteoclast activity thereby reducing the rate of bone turnover and therefore reduces pain and improves quality of life of the patients [3,4] by inhibiting osteoclast proliferation [5] and inducing osteoclast apoptosis [6]. PDB is the second most frequent metabolic bone disorder after osteoporosis and affects up to 3% of caucasians over 55 years of age [7]. In Spain, prevalence is 0.7% to 1.3% with an irregular geographic distribution [8] and areas of high prevalence as Vitigudino-Salamanca (5,7%)[9].
RANK-RANKL-OPG pathway regulates bone remodelling. The first step of bone turnover is the resorption of bone by osteoclasts which activation and function is regulated by the binding of receptor activator of nuclear factor kappa B ligand (RANKL) to RANK receptor [10]. Osteoprotegerin (OPG), a decoy receptor produced by osteoblasts, neutralizes RANKL and has an inhibitory effect on osteoclast differentiation and bone resorption [11,12]. This step is followed by osteoblasts-mediated bone formation. Some osteoblasts are trapped within bone matrix and differentiate into osteocytes that act as mechanosensors releasing RANKL and sclerostin [13]. Sclerostin inhibits bone formation by modulation of OPG and RANKL levels [14]. Higher plasma levels of OPG and RANKL have been described in PDB patients [15], but there are no studies concerning changes in these proteins in the presence or absence of metabolically active PDB.
Pagetic bone is also characterized by a higher vascularization and marrow fibrosis. Angiogenesis is a tightly regulated process in which the actions of proangiogenic and antiangiogenic factors are counterbalanced. A fundamental mediator of angiogenesis is vascular endothelial growth factor (VEGF), which supports the growth of new blood vessels and promotes differentiation of hematopoietic cells and subsequently the presence of a greater number of bone-resorptive osteoclasts [16]. The placental growth factor (PGF) also stimulates angiogenesis, being also relevant in circumstances as ischemia, inflammation, wound healing and cancer [17,18]. Despite of PDB bone being a highly vascularized tissue, there are no studies on the plasma levels of angiogenic mediators in the different states of the disease; moreover, the effect of PDB treatment on plasma levels of these angiogenic mediators is unknown. On the other hand, recent studies show that endoglin (ENG), a non-signalling receptor of transforming growth factor-β1, is a better marker of vascularization [19-21] than VEGF and an indicator of vascular pathologies associated to diabetes and hypertension [22].
Thus, the purpose of this study was to analyse plasma levels of mediators (RANKL, OPG, sclerostin, ENG, VEGF, PGF, ENG) implicated in the increased bone turnover and hypervascularization observed in PDB and their evolution and response to zoledronic acid treatment in these patients.
Methods
Patients
The cohort study comprised 70 PDB naive to bisphosphonate treatment patients recruited in the Metabolic Bone Unit at the University Hospital of Salamanca (Spain) between January 2014 and February 2016. The experimental protocol was in accordance with the Declaration of Helsinki (2008) of the World Medical Association, approved by the University Hospital of Salamanca Ethics Committee and complied with Spanish data protection law (LO 15/1999) and specifications (RD 1720/2007). All who accepted to participate in the study signed a written consent. Clinical and analytical variables such as gender, age at diagnosis, family history of PDB, number of affected bones, Coutris ´s index, presence of complications (fractures and cranial nerve involvement) and alkaline phosphatase (ALP) levels were collected from each patient. Coutris' index is used to calculate the extent of the disease, expressed as the percentage of affected skeleton according to the coefficient that each bone represents in the skeleton set, and is calculated as the percentage of the skeleton affected and it responds to the following function: Patients ALP= (pagetic bone ALP × Coutris´s index/100) + (normal bone ALP × (100-Coutris's index)/100 [23,24]. ALP was adjusted according to the upper limit of ALP standard range following the function: ALP/upper ALP (adjusted ALP). PDB patients with elevated plasma ALP and normal levels of liver derived enzymes (gamma-glutamyltransferase, bilirubin, alanine transaminase and aspartate transaminase), were classified as having active PDB. Other causes of raised ALP, as intra- or extrahepatic cholestasis, were ruled out. None of the patients took medication that could affect calcium metabolism.
Study design
The study design included two subgroups of patients: normal ALP levels untreated patients (PDB patients in the inactive phase of the disease) and active treated patients (when increased levels of plasma ALP from bone origin were present, which corresponded to the active phase of the disease) who received one dose of 5 mg of intravenous zoledronic acid. Plasma samples from each PDB patient who did not receive zoledronic acid treatment were collected and stored at -80ºC at the time of consultation. In PDB patients treated with zoledronic acid, we obtained and stored three plasma samples: baseline, three months post-treatment and twelve months post-treatment.
ALP measurement
ALP levels were measured in the Clinical Biochemistry Service at the University Hospital of Salamanca (Spain) using an enzyme-linked immunosorbent assay (ELISA; MyBioSource, San Diego CA, USA).
Determination of OPG, RANKL, sclerostin, VEGF, PGF and ENG plasma levels
Protein plasma levels were measured using an enzyme-linked immunosorbent assay (ELISA) method, following the instructions of the manufacturer. Human OPG and human RANKL were from Biomedic (Vienna, Austria); human sclerostin were from RayBio (Norcross, Georgia, USA); human ENG, human PGF and human VEGF were from R&D (Abingdon, United Kingdom). Samples were measured in duplicate. Absorbance was determined using a spectrophotometer ELx800 Universal Microplates Reader (Bio-Tek Instruments Inc., Winooski, Vermont, USA) at 450 nm with a wavelength correction of 620 nm.
Clinical characteristics of PDB patients
| Clinical characteristics | Active PDB n =29 | Inactive PDB n =41 |
|---|---|---|
| Male Sex, n (%) | 19 (65,5%) | 23 (56,1%) |
| Age at diagnosis, mean ± SD | 71,85 ± 8,98 | 75,02 ± 9,00 |
| Polyostotic involvement, n (%) | 20 (69,0%) | 16 (39,0%) |
| Number of affected bones, mean ± SD | 3,27 ± 2,38 | 2,02 ± 1,89 |
| Coutris's index, mean ± SD | 17,37 ± 13,40 | 10,78 ± 8,80 |
| Adjusted ALP, mean ± SD | 1,99 ± 1,05 | 0,63 ± 0,16 |
| Familial history of PDB, n (%) | 2 (6,9%) | 3 (7,3%) |
| Fracture or fissures, n (%) | 1 (3,4%) | 3 (7,3%) |
| Cranial nerve involvement, n (%) | 3 (10,3%) | 3 (7,3%) |
| Zoledronic acid treatment, n (%) | 25 (86,2%) | 0 (0%) |
ALP: alkaline phosphatase, PDB: Paget´s disease of bone, SD: standard deviation.
Adjusted ALP (ratio ALP/upper ALP), RANKL (pmol/L), sclerostin (pg/ml), OPG (pmol/L), PGF (pg/mL), VEGF (pg/mL) and ENG (ng/mL) plasma levels in active and inactive Paget disease of bone (PDB) patients
| Active PDB | Inactive PDB | p-value | |
|---|---|---|---|
| Adjusted ALP, mean ± SD | 1.99 ± 1.05 | 0.63 ± 0.16 | <0.001 |
| RANKL, mean ± SD | 0.07 ± 0.03 | 0.06 ± 0.04 | 0.159 |
| Sclerostin, median (min; max) | 131.26 (86.59; 194.01) | 120.84 (32.34; 423.32) | 0.397 |
| OPG, median (min; max) | 4.53 (2.48; 8.36) | 7.46 (2.27; 14.31) | 0.277 |
| PGF, mean ± SD | 9.26 ± 5.68 | 10.21 ± 6.37 | 0.728 |
| VEGF, mean ± SD | 98.86 ± 107.02 | 57.11 ± 43.07 | 0.254 |
| ENG, mean ± SD | 3.80 ± 0.95 | 4.28 ± 0.69 | 0.178 |
P-values refer to differences between active and inactive PDB patients. ALP: alkaline phosphatase, ENG: endoglin, OPG: osteoprotegerin, PGF: placental growth factor, RANKL: receptor activator of nuclear factor kappa B ligand, SD: standard deviation, VEGF: vascular endothelial growth factor.
Statistical analysis
The statistical analysis was performed using SPSS v.21 software. Data following a normal distribution was analysed by analysis of variance (ANOVA); data that did not follow a normal distribution was analysed by Mann-Whitney U test. Differences with a p-value < 0.05 were considered statistically significant.
Results
Clinical characteristics of the recruited PBD patients are summarized in Table 1. 29 of the 70 PDB patients analysed (41.42%) had an active disease. After the analysis of plasma levels of ENG, OPG, VEGF, PGF, RANKL, sclerostin and ALP, we found that, as expected, circulating ALP concentration was significantly higher in active PDB than in inactive PDB patients (Table 2). However, we did not find significant differences in the plasma levels of the other proteins analysed.
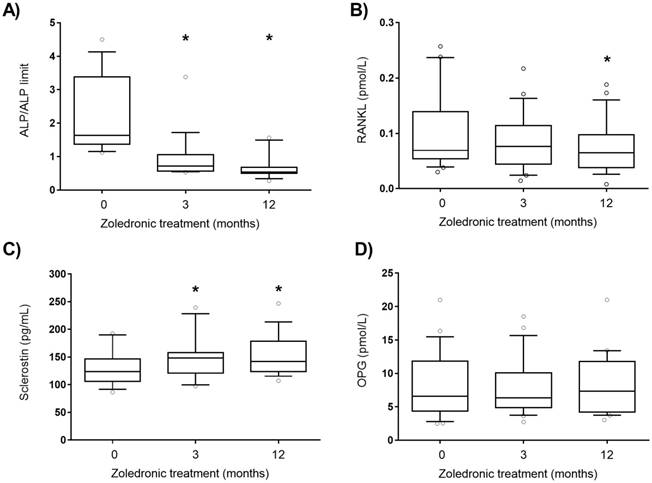
ALP from bone origin and angiogenic plasma factors levels were analysed in active PDB patients treated with zoledronic acid and followed during 12 months. Zoledronic acid is a fast and long-acting inhibitor of osteoclast and bone metabolism. As expected, ALP decreased at 3 and 12 months after treatment (Figure 1A). RANKL levels were significantly reduced and sclerostin plasma levels were increased after 12 months of treatment (Figure 1B and C). No differences were found in OPG levels throughout the treatment (Figure 1D). PGF levels were significantly lower 12 months after zoledronic acid treatment (Figure. 2A), whereas no differences were found in plasma levels of other angiogenic proteins as VEGF and ENG after zoledronic acid treatment (Figure. 2B and C).
Discussion
Few studies have been addressed to evaluate angiogenic biomarkers after zoledronic acid treatment in PDB patients. Moreover, although normal ALP can be found in some monostotic active patients, most guidelines recommend the increased level of ALP with other liver derived enzymes in the normal range as a possible situation of enough active disease to order treatment [25]. To our knowledge, this is the first study in which plasma levels of these angiogenic factors were compared in treated active PDB subjects. Plasma levels of RANKL, sclerostin, ALP and PGF decreased 12 months after the treatment, but there were no differences in the levels of OPG, RANKL, sclerostin, ENG, PGF and VEGF between active and inactive PDB. We have considered active PDB patients when increased levels of plasma ALP from bone origin were present [26]. Although some monostotic active patients show normal ALP levels, we used ALP from bone origin as a threshold for treatment. Changes in plasma levels of other bone formation markers as PINP (amino-terminal propeptide of procollagen type 1) are not the rule to start treatment. Taking into account this consideration, we have assumed that an elevation in ALP from bone origin (when other liver derived enzymes are in normal range) means an active disease with increased bone formation. It has been previously reported that PDB patients have higher levels of RANKL and OPG than healthy subjects [15,27,28] but, in these studies, the active or inactive state of PDB was not considered. In fact, Martini et al. showed that PDB patients had higher levels of OPG and RANKL than healthy individuals[15], but the data shown in that study have a high variability that could be the result of mixing patients in both active and inactive states. Our results showed that patients with active PDB have slightly higher levels of RANKL and lower levels of OPG than patients with inactive PDB, and these results are in agreement with the higher bone turnover observed in the active PDB. However, these differences in OPG and RANKL were not statistically significant, due probably to the number of patients and the considerable standard deviation.
Adjusted ALP (A), RANKL (B), sclerostin (C) and OPG (D) plasma levels after zoledronic acid treatment (5 mg, intravenous). ALP: alkaline phosphatase, OPG: osteoprotegerin, RANKL: receptor activator of nuclear factor kappa B ligand. Statistically significant differences: *p < 0.01 vs subjects before treatment (0).
Sclerostin is another protein involved in the regulation of bone resorption and in the inhibition of bone formation. In a study with 88 PDB patients, sclerostin plasma levels were higher in PDB patients than in healthy subjects [14]. However, a recent study with 40 PDB patients showed no differences in sclerostin serum levels between PDB patients and healthy subjects of the same age [29]. In neither of these studies, these levels were compared between patients with active or inactive PDB. In our study, there were no differences in sclerostin plasma levels between active and inactive PDB patients, which seems to suggest that this protein is not involved in the active phase of the disease.
Zoledronic acid is a potent and easily administered intravenous bisphosphonate that inhibits osteoclast recruitment, function and survival, resulting in an inhibition of bone resorption and improving quality of life of PDB patients [30,31]. Zoledronic acid normalizes the plasma values of markers of bone turnover (ALP), markers of bone formation (amino-terminal propeptide of procollagen type 1, P1NP), and of markers of bone resorption (carboxy-terminal telopeptide of collagen type 1) [32]. Our data show that ALP levels remain decreased 3 months after zoledronic acid treatment. It has been previously shown that zoledronic acid inhibits in vitro the osteoclast maturation indirectly by increasing OPG and decreasing RANKL expression in human osteoblasts[33,34]. Moreover, zoledronic acid causes a decrease in RANKL levels and an increase in OPG levels in patients with bone metastasis [35]. Our results show that zoledronic acid is associated to a decrease in RANKL plasma levels, but OPG levels were unaltered. This can be explained by the fact that the prescribed doses of zoledronic acid in PDB patients are lower than that used in patients with bone metastasis. Our data are in agreement with the findings of Makie et al., who described that bisphosphonates caused a reduction in RANKL expression without any modification in OPG levels in an osteosarcoma cell line [36]. We also analysed the effect of zoledronic acid treatment on plasma sclerostin levels. Our results showed an increase of sclerostin levels in patients with PDB 3 months after zoledronic acid treatment. In our knowledge, this is the first report that describes an increase of sclerostin plasma levels after zoledronic acid treatment. This treatment could reduce bone resorption that would be associated to the decrease in RANKL levels observed 12 months after treatment. On the other hand, excessive bone formation that happens in PDB patients may be reduced by the increase in the levels of sclerostin observed after zoledronic treatment in active patients.
VEGF (A), PGF (B) and ENG (C) plasma levels after zoledronic acid treatment (5 mg, intravenous). P1GF: placental growth factor, VEGF: vascular endothelial growth factor. Statistically significant differences: *p < 0.01 vs subjects without treatment (0); #p < 0.01 vs subjects after 3 months treatment (3).
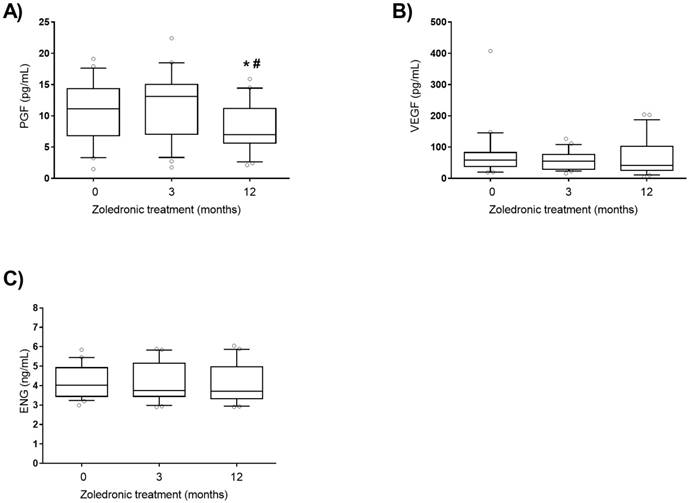
Another characteristic of the pagetic bone is its higher vascularization [1,2]. We analysed plasma levels of several angiogenesis mediators, VEGF, PGF and ENG [19-21]. We found no differences in the plasma levels of ENG, PGF and VEGF between active and inactive PDB. However, we observed that plasma levels of PGF decreased 12 months after zoledronic acid treatment in active PDB patients. PGF is a member of the vascular endothelial growth factor subfamily which binds with vascular endothelial growth factor receptor 1 (VEGFR1) and this interaction is involved in the pathological angiogenesis observed in various diseases such as ischemic cardiovascular disease, tumours, inflammatory diseases and diabetic retinopathy [18,37-39]. This is the first time that a decrease of PGF plasma concentration after zoledronic acid treatment in PDB is described, which suggests that zoledronic acid might reduce the pathological vascularisation characteristic of pagetic bone.
The main limitation of the study is that although the study is carried out in a Spanish area with a high prevalence of the disease, the sample size is not very high. Even so, the statistically significant differences found in our study are even more robust considering the number of patients recruited. On the other hand, although the age of patients is similar, activity and extension of disease is not homogeneous. These results are preliminary, and need to be replicated in another cohort of PDF patients.
Summarizing, our data show that zoledronic acid is associated to a decrease in ALP, RANKL, sclerostin and P1GF levels in PDB patients in active phase. Our data confirm that zoledronic acid treatment in PDB patients induces a decrease in bone turnover and suggest that it might reduce the pathological vascularisation typical of pagetic bone.
Abbreviations
ALP: plasma alkaline phosphatase; ANOVA: analysis of variance; ELISA: enzyme-linked immunosorbent assay; ENG: endoglin; OPG: Osteoprotegerin; PDB: Paget's disease of bone; PINP: amino-terminal propeptide of procollagen type 1; PGF: placental growth factor; RANK: receptor activator of nuclear factor kappa B; RANKL: receptor activator of nuclear factor kappa B ligand; VEGF: vascular endothelial growth factor; VEGFR1: vascular endothelial growth factor receptor 1.
Acknowledgements
This work was supported by grants from Instituto de Salud Carlos III (Ministry of Economy and Competitiveness, PI12/00959, PI13/01741, PI15/01055, Kidney Research Network REDINREN RD012/0021/0032 and RD016/0009/0025, co-funded by FEDER) and Junta de Castilla y León (Ministry of Health, GRS 969/A/14).
Author contributions
Study design: CMS, JPM, RGS; patients recruitment: ICP, JPM, LGO; experimental work: IFC, RUM, CMP; data analysis: IFC, RUM; data interpretation: IFC, RUM, JPM, RGS, CMS; drafting manuscript: IFC, RUM, CMS; revising manuscript: JPM, RGS, LGO, CMS; approving final version of the manuscript: RGS, JPM, CMS; IFC, RUM take responsibility for the integrity of the data analysis.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ralston SH, Layfield R. Pathogenesis of Paget disease of bone. Calcif Tissue Int. 2012Aug;91(2):97-113
2. Roodman GD. Insights into the pathogenesis of Paget's disease. Ann N Y Acad Sci. 2010Mar;1192:176-80
3. Ralston SH, Langston AL, Reid IR. Pathogenesis and management of Paget's disease of bone. Lancet. 2008Jul12;372(9633):155-63
4. Singer FR, Bone HG, Hosking DJ, Lyles KW, Murad MH, Reid IR. et al. Paget's disease of bone: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014Dec;99(12):4408-22
5. Coxon FP, Helfrich MH, Van't Hof R, Sebti S, Ralston SH, Hamilton A. et al. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res. 2000Aug;15(8):1467-76
6. Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001May;28(5):465-73
7. Michou L, Brown JP. Emerging strategies and therapies for treatment of Paget's disease of bone. Drug Des Devel Ther. 2011;5:225-39
8. Guañabens N, Garrido J, Gobbo M, Piga AM, del Pino J, Torrijos A. et al. Prevalence of Paget's disease of bone in Spain. Bone. 2008Dec;43(6):1006-9
9. Mirón-Canelo JA, Del Pino-Montes J, Vicente-Arroyo M, Sáenz-González MC. Epidemiological study of Paget's disease of bone in a zone of the Province of Salamanca (Spain). The Paget's disease of the bone study group of Salamanca. Eur J Epidemiol. 1997Oct;13(7):801-5
10. Boyce BF, Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl 1):S1
11. Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998Apr17;93(2):165-76
12. Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998Mar31;95(7):3597-602
13. Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011Feb;26(2):229-38
14. Yavropoulou MP, van Lierop AH, Hamdy NAT, Rizzoli R, Papapoulos SE. Serum sclerostin levels in Paget's disease and prostate cancer with bone metastases with a wide range of bone turnover. Bone. 2012Jul;51(1):153-7
15. Martini G, Gennari L, Merlotti D, Salvadori S, Franci MB, Campagna S. et al. Serum OPG and RANKL levels before and after intravenous bisphosphonate treatment in Paget's disease of bone. Bone. 2007Feb;40(2):457-63
16. Street J, Bao M, deGuzman L, Bunting S, Peale FV, Ferrara N. et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002Jul23;99(15):9656-61
17. Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008Dec;8(12):942-56
18. Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001May;7(5):575-83
19. Düwel A, Eleno N, Jerkic M, Arevalo M, Bolaños JP, Bernabeu C. et al. Reduced tumor growth and angiogenesis in endoglin-haploinsufficient mice. Tumour Biol. 2007;28(1):1-8
20. Eleno N, Düwel A, Muñoz A, Paz-Bouza J, López-Novoa J-M, Lozano F. Endoglin as a marker in cervical paragangliomas. Head Neck. 2010Jun;32(6):737-43
21. Pérez-Gómez E, Eleno N, López-Novoa JM, Ramirez JR, Velasco B, Letarte M. et al. Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene. 2005Jun23;24(27):4450-61
22. Blázquez-Medela AM, García-Ortiz L, Gómez-Marcos MA, Recio-Rodríguez JI, Sánchez-Rodríguez A, López-Novoa JM. et al. Increased plasma soluble endoglin levels as an indicator of cardiovascular alterations in hypertensive and diabetic patients. BMC Med. 2010Dec20;8:86
23. Reiner JC, Bontoux-Carre E, Seret P, Villayleck S. [How to evaluate the activity of Paget's disease in clinical practice and which patients should be treated?]. Rev Rhum Mal Osteoartic. 1984Oct;51(9):463-8
24. Coutris G, Cayla J, Rondier J, Talbot JN, Bonvarlet JP, Milhaud G. [Analysis of disorders of the principal pathways of calcium metabolism in Paget's disease. Effects of calcitonin administration. 26 cases]. Rev Rhum Mal Osteoartic. 1975Dec;42(12):759-67
25. Cundy T. Treating Paget's Disease-Why and How Much? J Bone Miner Res. 2017Jun;32(6):1163-4
26. Vallet M, Ralston SH. Biology and Treatment of Paget's Disease of Bone. J Cell Biochem. 2016Feb;117(2):289-99
27. Alvarez L, Peris P, Guañabens N, Vidal S, Ros I, Pons F. et al. Serum osteoprotegerin and its ligand in Paget's disease of bone: relationship to disease activity and effect of treatment with bisphosphonates. Arthritis Rheum. 2003Mar;48(3):824-8
28. Rendina D, Mossetti G, Viceconti R, Sorrentino M, Nunziata V. Risedronate and pamidronate treatment in the clinical management of patients with severe Paget's disease of bone and acquired resistance to bisphosphonates. Calcif Tissue Int. 2004Sep;75(3):189-96
29. Idolazzi L, Fassio A, Tripi G, Braga V, Viapiana O, Adami G. et al. Circulating Dickkopf-1 and sclerostin in patients with Paget's disease of bone. Clin Rheumatol. 2017Apr;36(4):925-8
30. Reid IR, Miller P, Lyles K, Fraser W, Brown JP, Saidi Y. et al. Comparison of a single infusion of zoledronic acid with risedronate for Paget's disease. N Engl J Med. 2005Sep1;353(9):898-908
31. Maricic M. The use of zoledronic acid for Paget's disease of bone. Curr Osteoporos Rep. 2006Mar;4(1):40-4
32. Díaz Curiel M, Serrano Morales R, De la Piedra Gordo C, Moro Alvarez MJ, Andrade Poveda M. Effect of zoledronic acid on the markers for bone remodelling in Paget's disease. Rev Osteoporos Metab Miner. 2010;2(2):21-5
33. Viereck V, Emons G, Lauck V, Frosch K-H, Blaschke S, Gründker C. et al. Bisphosphonates Pamidronate and Zoledronic Acid Stimulate Osteoprotegerin Production by Primary Human Osteoblasts. Biochemical and Biophysical Research Communications. 2002Mar1;291(3):680-6
34. Pan B, Farrugia AN, To LB, Findlay DM, Green J, Lynch K. et al. The nitrogen-containing bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblast-like cells by activating TNF-alpha converting enzyme (TACE). J Bone Miner Res. 2004Jan;19(1):147-54
35. Mercatali L, Ricci M, Scarpi E, Serra P, Fabbri F, Ricci R. et al. RANK/RANK-L/OPG in Patients with Bone Metastases Treated with Anticancer Agents and Zoledronic Acid: A Prospective Study. Int J Mol Sci. 2013May23;14(6):10683-93
36. Mackie PS, Fisher JL, Zhou H, Choong PF. Bisphosphonates regulate cell growth and gene expression in the UMR 106-01 clonal rat osteosarcoma cell line. Br J Cancer. 2001Apr6;84(7):951-8
37. Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F. et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002Aug;8(8):831-40
38. De Falco S. The discovery of placenta growth factor and its biological activity. Exp Mol Med. 2012Jan31;44(1):1-9
39. Zhou Y, Tu C, Zhao Y, Liu H, Zhang S. Placental growth factor enhances angiogenesis in human intestinal microvascular endothelial cells via PI3K/Akt pathway: Potential implications of inflammation bowel disease. Biochem Biophys Res Commun. 2016Feb19;470(4):967-74
Author contact
![]() Corresponding author: Carlos Martínez-Salgado. Phone: +34923294500 ext. 1945; Fax: +34923294669; Email: carlosmses
Corresponding author: Carlos Martínez-Salgado. Phone: +34923294500 ext. 1945; Fax: +34923294669; Email: carlosmses

 Global reach, higher impact
Global reach, higher impact