3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(11):1171-1178. doi:10.7150/ijms.26685 This issue Cite
Research Paper
High chloride channel accessory 1 expression predicts poor prognoses in patients with rectal cancer receiving chemoradiotherapy
1. Department of Pathology, Chi Mei Medical Center, Tainan, Taiwan
2. Department of Optometry, Chung Hwa University of Medical Technology, Tainan, Taiwan
3. Institute of Biomedical Science, National Sun Yat-sen University, Kaohsiung, Taiwan
4. Department of Radiation Oncology, Chi Mei Medical Center, Liouying, Tainan, Taiwan
5. Institute of Biomedical Sciences, National Sun Yat-Sen University, Kaohsiung, Taiwan
6. Department of Pharmacy, Chia-Nan University of Pharmacy and Science, Tainan, Taiwan
7. Division of General Surgery, Department of Surgery, Chi Mei Medical Center, Tainan, Taiwan.
8. Department of Health & Nutrition, Chia Nan University of Pharmacy and Science, Tainan, Taiwan.
9. National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan
10. Division of Hematology and Oncology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Received 2018-4-15; Accepted 2018-6-30; Published 2018-7-30
Abstract
Background: Concurrent chemoradiotherapy (CCRT) has now become the standard of treatments for advanced rectal cancer before surgery. To search the biological molecules with prognostic and therapeutic potential of CCRT could be beneficial for these patients. Recently, aberrant expression of chloride channels has been linked to radio-resistance in glioblastoma; however, its clinical implication has not been well-studied in rectal cancers. Therefore, we examined the clinical significance of targetable drivers associated with chloride channel activity in patients with rectal cancer receiving CCRT.
Methods: After datamining from a published transcriptome of rectal cancers, upregulation of CLCA1 gene was recognized to be significantly correlated with non-responders of CCRT. In validation cohort of rectal cancers, the expression levels of CLCA1 were accessed by using immunohistochemistry assays in 172 tumor specimens that were obtained before any treatment. Expression levels of CLCA1 were statistically analyzed with principal clinicopathological features and survival outcomes in this substantial cohort.
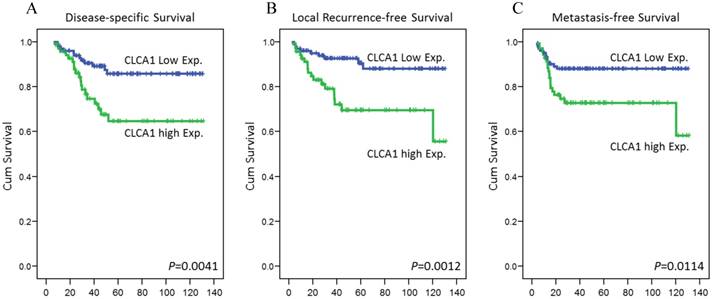
Results: In validation cohort, high expression of CLCA1 was significantly associated with higher pre-treatment tumor nodal stages (P=0.032), vascular invasion (P=0.028), and inferior tumor regression grade (P=0.042). In survival evaluations, high expression of CLCA1 was significantly correlated with worse local recurrence-free survival (LRFS; P=0.0012), metastasis-free survival (MeFS; P =0.0114), and disease-specific survival (DSS; P=0.0041). Furthermore, high expression of CLCA1 remained an independent prognosticator of shorter LRFS (P=0.029, hazard ratio=2.555), MeFS (P=0.044, hazard ratio=2.125) and DSS (P=0.044, hazard ratio=2.172).
Conclusions: High expression of CLCA1 is significantly associated with poor therapeutic response and survival outcomes in rectal cancer patients with CCRT treatment before surgery. With the development of specific inhibitors, our findings indicate not only prognostic but also therapeutic potential of CLCA1 in rectal cancers.
Keywords: CLCA1, rectal cancer, concurrent chemoradiotherapy
Introduction
The incidence of rectal cancer, a malignant disease located in the colon distal to rectosigmoid junction, has been steadily increasing in Taiwan in a decade [1]. Colorectal cancer (CRC) is always considered a prevalent disease in developed countries [2]; the increasing incidence of rectal cancer in Taiwan might be attributed to the habit alterations to Western-style diet [3]. Due to the anatomic characteristic, the major difference in treatments between these epithelium malignancies originating from rectum and other colonic sites is the introduction of radiotherapy. Especially in locally advanced rectal cancers (LARC; T3, T4 and node positive diseases), concurrent chemoradiotherapy (CCRT) followed by tumor resection is considered the standard of treatment nowadays. In addition to improved local control rates and reduced toxicity profiles, neoadjuvant CCRT could offer some patients the opportunities to undergo sphincter-preserving surgery [4-7]. In spite of these advantages, the 5-year disease recurrence and overall survival rates of these patients receiving neoadjuvant CCRT are 36% and 65%, respectively [4-6]. These unsatisfactory clinical outcomes suggest that more efforts would be made to advance the efficacy of CCRT on rectal cancers.
The key features of cancer cells include the capacity to continuous proliferation, apoptosis escape, metabolic re-programming, invasive migration as well as neo-angiogenesis stimulation [8]. In addition to established onco-proteins, majorly focusing on receptor kinases, metabolic enzymes and signaling transducers to maintain malignant behaviors of cancers cells, several trans-membrane ion channels have been identified to regulate the development and progression of cancer cells [9-10]. Genetic or functional aberrations in these trans-membrane proteins which control transportation of specific ions between extracellular and intracellular environments have always been recognized as a key player in various diseases involved in neurological, cardiovascular, endocrine and immune systems [11-13]. Recently, dysregulated expression of ion channels has also been reported in a variety of human cancers, including CRC [14]. Increasing evidence from different laboratory work has also suggested that aberrant expression of ion channels could regulate cellular functions in proliferation, invasion, migration and angiogenesis [15-17]. In light of a broad clinical development of pharmacological modulators targeting ion channels [18], it deserves to search significant ion transporters with potential of clinical impact on rectal cancers.
Recently, several studies have demonstrated importance of ion transporters in regulation of cancer cell responses after irradiation exposure, especially chloride channels in glioblastoma (GBM) cells [19-21]. Through the modifications of these channels, alterations of chloride ion concentrations between cancer cells and their surrounding environments can confer cellular resistance to irradiation. Accordingly, we aim to decipher the potential prognostic role of chloride channels in radiotherapy for rectal cancers in this study. After initial data mining, focusing on chloride channels, from a previously published transcriptome of patients with rectal cancer receiving CCRT (GSE35452), the upregulation of chloride channel accessory 1 (CLCA1) was identified to be substantially associated with poor response to CCRT. The transmembrane protein CLCA1 belongs to a family of ion channels which function in regulating chloride conductance dependent on calcium activation [22]. In intestinal epithelium, chloride channels are crucial to control epithelial volume via electrolyte transportation [23]. Increased expression of CLCA1 has been demonstrated to affect spheroid aggregate of ovarian cancer cells [24]; however, its clinical implications on rectal cancers remain to be elucidated. Therefore, in this study, the clinical significance of CLCA1 expression was further analyzed by examining a clearly-defined cohort of rectal cancers receiving CCRT before surgery.
Materials and Methods
Data mining of the published transcriptomic dataset
To determine the significant chloride channel associated with the response of CCRT, the transcriptome dataset which was derived from tissues of rectal cancers (n=46) and deposited in Gene Expression Omnibus (GSE35452) was assessed. According to the response to neoadjuvant CCRT, the tumors were categorized into “responder” and “non-responder”. Nexus Expression 3 software (BioDiscovery) was utilized to analyze all probe sets from raw files of GeneChip® Human Genome U133 Plus 2.0 array platform (Affymetrix) without filtering or pre-selection. By comparing responder and non-responder, with special attention to chloride channel activity (GO:0005254), statistically significant genes were examined. Those transcripts with P-value <0.01 and fold change of log2-transformed expression >0.1 were designated for further analyses.
Study cohort of patients
The analyses of clinical and pathological information in this study have been approved by the institutional review board of Chi-Mei Medical Center (IRB 10302014). Totally 172 LARC patients who were histologically confirmed rectum adenocarcinoma were enrolled from Chi-Mei Medical Center between 1998 and 2004. The pre-operative clinical staging was decided by using chest X-radiography and abdominopelvic CT and/or pelvic magnetic resonance imaging (MRI). All 172 LARC patients received neoadjuvant CCRT followed by surgery as previously described [25]. Briefly, a total dose of 45 Gy in 25 fractions was delivered to all patients over a period of 5 weeks concurrently with infusion of 5-fluorouracil before surgery (225 mg/m2/day). The administration of adjuvant systemic chemotherapy was based on the multidiscipline guideline at Chi-Mei Medical Center (if initial clinical tumor stage was beyond T3 or N1). These patients were routinely and completely followed up at Chi-Mei Medical Center as previously described [25].
Histopathologic assessments of tumor specimens
Tumor specimens derived from these LARC patients were evaluated by two independent pathologists who were blinded in any clinical information of this study. Post-operative tumor stages of all patients were judged based on the 8th American Joint Committee on Cancer (AJCC) TNM staging system [26]. Tumor regression grade (TRG) according to the study reported by Dworak et al. was investigated in all patients for tumor response after neoadjuvant CCRT as previously described [25,27].
CLCA1 immunohistochemical staining and scoring
In immunohistochemical staining, tumor tissues derived from patients before any treatment were cut, deparaffinized, rehydrated, heated, quenched and washed as previously described [25]. The primary antibody targeting CLCA1 (1:100, Thermo Fisher Scientific, PA5-21288) was subsequently incubated with tumor tissue sections for 1 h. After secondary antibody incubation and hematoxylin counterstaining, the immunoexpression of CLCA1 in all tumor tissues were interpreted by two independent pathologists. Normal bowel tissues stained with or without CLCA1 primary antibody were employed in parallel as the positive or negative control. The expression levels of CLCA1 were determined by using H-score as previously described [25]. The equation of this scoring system is defined as follows: H-score = ΣPi (i + 1), in which i stands for the intensity of the tumor staining (0 to 3+), and Pi stands for the percentage of tumor staining with a variety of intensities (0 to 100%). The CLCA1 scoring no less or below the median of all analyzed subjects was categorized as high or low expression, respectively.
Statistical analysis
All statistical analyses were completed using SPSS 14 software package (SPSS Inc., Chicago, IL, USA) in this study. The relationship between CLCA1 expression levels and various principal clinical and pathological features were compared by using Chi-square test. The interval of clinical outcomes, including local recurrence-free survival (LRFS), metastasis-free survival (MeFS), and disease-specific survival (DSS) were calculated from the date of operation to the date of event. Survival curves of each subgroup with different CLCA1 expression were depicted by using the Kaplan-Meier method. The prognostic significance of miscellaneous clinical or pathological features was evaluated by using log-rank tests. Multivariate analysis used to determine the independence of identified prognostic factor was carried out by using the Cox proportional hazards model. For all analyses, P value < 0.05 under two-sided tests was decided statistically significant.
Results
High CLCA1 transcription correlates with non-responder with CCRT treatment
Through datamining from the public transcriptome GSE35452 comprising 46 rectal cancer cases, probes covering genes associated with chloride channel activity (GO:0005254) were focused. In non-responder with CCRT treatment, CLCA1 demonstrated the top-ranking significance among all identified genes with upregulated transcription (comparison log2 ratio=2.1851, P=0.0001, Figure 1, Table 1). These results suggest that a potential prognostic role of CLCA1 playing in patients with rectal cancer. Accordingly, clinical relevance of CLCA1 expression in patients with rectal cancers receiving CCRT was further investigated in our validation cohort.
The association between immunohistochemical expression of CLCA1 and clinicopathological features
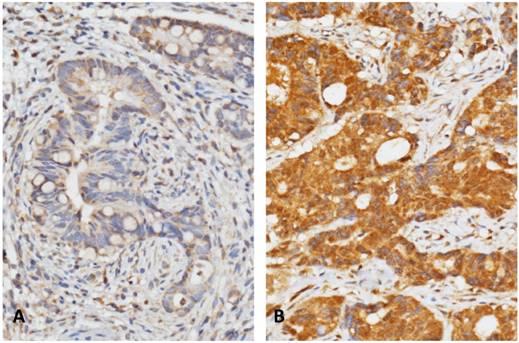
In order to further investigate the association between the expression of CLCA1 and clinicopatholigical features in our cohort of rectal cancers, immunohistochemical staining was employed to determine the expression level of CLCA1 in tumor specimens. In all 172 rectal tumors, CLCA1 immunoexpression detected on cellular membrane was completely examined with a broad range of H-score, spanning from 105 to 365 (Figure-2). After analyzing the association with clinicopathological parameters, high immunoexpression of CLCA1 was correlated with an advanced Pre-Tx nodal stages (P=0.032), and vascular invasion (P=0.028), respectively (Table 2). Moreover, high expression of CLCA1 was significantly correlated with the lower TRG degree, meaning inferior tumor response to CCRT in our cohort of rectal patients (P=0.042, Table 2). These results also imply that CLCA1 expression levels in rectal cancers would be linked to tumor response of CCRT. All principal clinicopathological characteristics of all patients are summarized in Table-2.
Analysis of CLCA1 expression between responders and non-responder of CCRT in a published transcriptome database composed of rectal cancers. In the clustering analysis of upregulated genes associated with chloride channel activity (GO:0005254), CLCA1 was significantly correlated with non-responders of CCRT. Tumor specimens derived from non-responder (blue lines) and responder (yellow lines) tissue specimens were marked on top of the heatmap, and expression levels of associated genes were illustrated as a series of brightness in red and green colors, respectively. Those with unaltered mRNA expression were coded as black color in the heatmap.
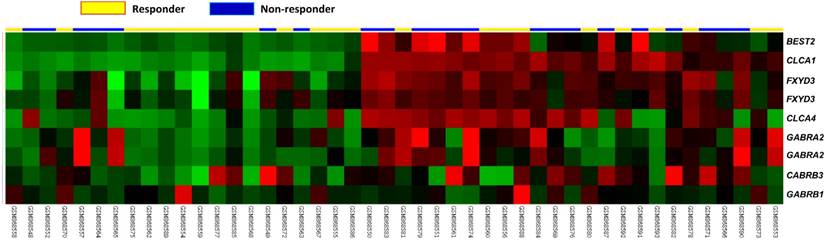
Summary of differentially expressed genes associated with chloride channel activity (GO:0005254) in relation to response to CCRT in rectal carcinoma
| Probe | Comparison log ratio | Comparison p-value | Gene Symbol | Gene Name | Biological Process | Molecular Function |
|---|---|---|---|---|---|---|
| 210107_at | 2.1851 | 0.0001 | CLCA1 | chloride channel; calcium activated; family member 1 | transport | chloride channel activity |
| 220026_at | 1.4829 | 0.0043 | CLCA4 | chloride channel; calcium activated; family member 4 | transport | chloride channel activity |
| 207432_at | 0.8953 | 0.0004 | BEST2 | bestrophin 2 | ion transport, transport | calcium ion binding, chloride channel activity, chloride ion binding, ion channel activity |
| 207014_at | 0.8246 | 0.0001 | GABRA2 | gamma-aminobutyric acid (GABA) A receptor; alpha 2 | chloride transport, gamma-aminobutyric acid signaling pathway, ion transport, regulation of neurotransmitter levels, transport | GABA-A receptor activity, benzodiazepine receptor activity, chloride channel activity, chloride ion binding, extracellular ligand-gated ion channel activity, ion channel activity, neurotransmitter receptor activity |
| 202488_s_at | 0.8124 | 0.0005 | FXYD3 | FXYD domain containing ion transport regulator 3 | chloride transport, ion transport, transport | chloride channel activity, chloride ion binding, ion channel activity |
| 202489_s_at | 0.5951 | 0.0018 | FXYD3 | FXYD domain containing ion transport regulator 3 | chloride transport, ion transport, transport | chloride channel activity, chloride ion binding, ion channel activity |
| 1554308_s_at | 0.2243 | 0.0004 | GABRA2 | gamma-aminobutyric acid (GABA) A receptor; alpha 2 | chloride transport, gamma-aminobutyric acid signaling pathway, ion transport, regulation of neurotransmitter levels, transport | GABA-A receptor activity, benzodiazepine receptor activity, chloride channel activity, chloride ion binding, extracellular ligand-gated ion channel activity, ion channel activity, neurotransmitter receptor activity |
| 1561316_at | 0.1145 | 0.0089 | GABRB3 | Gamma-aminobutyric acid (GABA) A receptor; beta 3 | ion transport, signal transduction | GABA-A receptor activity, chloride channel activity, chloride ion binding, extracellular ligand-gated ion channel activity, ion channel activity, neurotransmitter receptor activity |
| 1552296_at | 0.0412 | 0.4682 | BEST4 | bestrophin 4 | ion transport, transport | calcium ion binding, chloride channel activity, chloride ion binding, ion channel activity |
High immunohistochemical expression of CLCA1 predicts shorter survivals in rectal cancer receiving CCRT
To determine the prognostic role of CLCA1 expression in rectal cancer patients receiving CCRT, its correlation with various survival outcomes were further analyzed. In univariate analysis, Pre-Tx tumor and nodal stages, Post-Tx tumor stages, vascular invasion, perineurial invasion and TRG were significantly correlated with at least one of the three survival outcomes (Table 3). Remarkably, high expression of CLCA1 also was comparable to a more tragic disease course in rectal cancers, with significantly decreased DSS (P=0.0041), LRFS (P=0.0012), and MeFS (P=0.0114), as shown in Figure 3. In multivariate analyses, high expression of CLCA1 remained an independent prognosticator for shorter DSS (P =0.044, hazard ratio [HR] = 2.172), LRFS (P =0.029, HR = 2.555) and MeFS (P =0.044, HR = 2.125), as well as the other important clinical prognostic predictor, TRG. These findings indicate the prognostic value of CLCA1 expression in patients with rectal cancer receiving neoadjuvant CCRT.
Representative immunohistochemical staining of CLCA1 expression in our validation cohort of rectal cancers. Cases with low expression (A) and high expression (B) of CLCA1 from tumor specimens before treatment were demonstrated respectively.
Kaplan-Meier survival curves plotted to represent survivals in rectal cancers. By using log-rank test, high expression of CLCA1 was significantly correlated with shorter disease-specific survival (A), local recurrence-free survival (B), and metastases-free survival (C).
Associations and comparisons between CLCA1 expression and clinicopathological factors in 172 rectal cancer patients receiving neoadjuvant CCRT.
| Parameter | No. | CLCA1 Expression | p-value | ||
|---|---|---|---|---|---|
| Low Exp. | High Exp. | ||||
| Gender | Male | 108 (62.8%) | 66 (64.1%) | 42 (60.9%) | 0.670 |
| Female | 64 (37.2%) | 37 (35.9%) | 27 (39.1%) | ||
| Age | <70 | 106 (61.6%) | 62 (60.2%) | 44 (63.8%) | 0.637 |
| ≧70 | 66 (38.4%) | 41 (39.8%) | 25 (36.2%) | ||
| Pre-Tx tumor status (Pre-T) | T1-T2 | 81 (47.1%) | 52 (50.5%) | 29 (42.0%) | 0.276 |
| T3-T4 | 91 (52.9%) | 51 (49.5%) | 40 (58.0%) | ||
| Pre-Tx nodal status (Pre-N) | N0 | 125 (72.7%) | 81 (78.6%) | 44 (63.8%) | 0.032* |
| N1-N2 | 47 (27.3%) | 22 (21.4%) | 25 (36.2%) | ||
| Post-Tx tumor status (Post-T) | T1-T2 | 86 (50%) | 56 (54.4%) | 30 (43.5%) | 0.161 |
| T3-T4 | 86 (50%) | 47 (45.6%) | 39 (56.5%) | ||
| Post-Tx nodal status (Post-N) | N0 | 123 (71.5%) | 78 (75.7%) | 45 (65.2%) | 0.134 |
| N1-N2 | 49 (28.5%) | 25 (24.3%) | 24 (34.8%) | ||
| Vascular invasion | Absent | 157 (91.3%) | 98 (95.1%) | 59 (85.5%) | 0.028* |
| Present | 15 (8.7%) | 5 (4.9%) | 10 (14.5%) | ||
| Perineurial invasion | Absent | 167 (97.1%) | 101 (98.1%) | 66 (95.7%) | 0.357 |
| Present | 5 (2.9%) | 2 (1.9%) | 3 (4.3%) | ||
| Tumor regression grade | Grade 0-1 | 37 (21.5%) | 18 (17.5%) | 19 (27.5%) | 0.042* |
| Grade 2~3 | 118 (68.6%) | 78 (75.7%) | 40 (58.0%) | ||
| Grade 4 | 17 (9.9%) | 7 (6.8%) | 10 (14.5%) | ||
*, statistically significant
Univariate log-rank analysis for important clinicopathological variables and CLCA1 expression
| Parameter | No. of case | DSS | LRFS | MeFS | ||||
|---|---|---|---|---|---|---|---|---|
| No. of event | p-value | No. of event | p-value | No. of event | p-value | |||
| Gender | Male | 108 (62.8%) | 20 (18.5%) | 0.6027 | 15 (13.9%) | 0.3096 | 14 (13.0%) | 0.1047 |
| Female | 64 (37.2%) | 11 (17.2%) | 7 (10.9%) | 15 (23.4%) | ||||
| Age | <70 | 106 (61.6%) | 19 (17.9%) | 0.7158 | 14 (13.2%) | 0.9630 | 18 (17.0%) | 0.9520 |
| ≧70 | 66 (38.4%) | 12 (18.2%) | 8 (12.1%) | 11 (16.7%) | ||||
| Pre-Tx tumor status (Pre-T) | T1-T2 | 81 (47.1%) | 10 (12.3%) | 0.0484* | 7 (8.6%) | 0.0836 | 10 (12.3%) | 0.1288 |
| T3-T4 | 91 (52.9%) | 21 (23%) | 15 (16.5%) | 19 (20.9%) | ||||
| Pre-Tx nodal status (Pre-N) | N0 | 125 (72.7%) | 19 (15.2%) | 0.0059* | 12 (9.6%) | 0.0025* | 18 (14.4%) | 0.0866 |
| N1-N2 | 47 (27.3%) | 21 (44.7%) | 10 (21.3%) | 11 (23.4%) | ||||
| Post-Tx tumor status (Post-T) | T1-T2 | 86 (50%) | 7 (8.1%) | 0.0014* | 5 (5.8%) | 0.0056* | 8 (9.3%) | 0.0123* |
| T3-T4 | 86 (50%) | 24 (27.9%) | 17 (19.8%) | 21 (24.4%) | ||||
| Post-Tx nodal status (Post-N) | N0 | 123 (71.5%) | 21 (17%) | 0.4654 | 15 (12.2%) | 0.6267 | 20 (16.3%) | 0.8403 |
| N1-N2 | 49 (28.5%) | 10 (20.4%) | 7 (14.3%) | 9 (18.4%) | ||||
| Vascular invasion | Absent | 157 (91.3%) | 25 (15.9%) | 0.0123* | 17 (10.8%) | 0.0023* | 26 (16.6%) | 0.7236 |
| Present | 15 (8.7%) | 6 (40%) | 5 (33.3%) | 3 (20%) | ||||
| Perineurial invasion | Absent | 167 (97.1%) | 29 (17.4%) | 0.0994 | 20 (12.0%) | 0.0083* | 28 (16.8%) | 0.8157 |
| Present | 5 (2.9%) | 2 (40%) | 2 (40%) | 1 (20%) | ||||
| Tumor regression grade | Grade 0-1 | 37 (21.5%) | 13 (35.1%) | 0.0037* | 10 (27.0%) | 0.0021* | 14 (37.8%) | 0.0008* |
| Grade 2~3 | 118 (68.6%) | 17 (14.4%) | 12 (10.2%) | 14 (11.9%) | ||||
| Grade 4 | 17 (9.9%) | 1 (5.9%) | 0 (0%) | 1 (5.9%) | ||||
| CLCA1 expression | Low Exp. | 103 (59.9%) | 12 (11.7%) | 0.0041* | 9 (8.7%) | 0.0012* | 12 (11.7%) | 0.0114* |
| High Exp. | 69 (40.1%) | 19 (27.5%) | 18 (26.1%) | 19 (27.5%) | ||||
DSS, disease-specific survival; LRFS, local recurrence-free survival; MeFS, metastasis-free survival; *, statistically significant
Multivariate analysis
| Parameter | DSS | LRFS | MeFS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H.R | 95% CI | p-Value | H.R | 95% CI | p-Value | H.R | 95% CI | p-Value | |
| Tumor regression grade | 2.105 | 1.055-4.202 | 0.035* | 2.283 | 1.077-4.853 | 0.031* | 2.427 | 1.221-4.831 | 0.011* |
| CLCA1 expression | 2.172 | 1.022-4.620 | 0.044* | 2.555 | 1.102-5.921 | 0.029* | 2.125 | 1.021-4.421 | 0.044* |
| Vascular invasion | 1.774 | 0.647-4.867 | 0.266 | 1.987 | 0.707-5.587 | 0.193 | - | - | - |
| Post-Tx tumor status (Post-T) | 2.404 | 0.964-5.995 | 0.060 | 1.886 | 0.755-4.711 | 0.175 | 1.988 | 0.843-4.688 | 0.117 |
| Pre-Tx nodal status (Pre-N) | 1.228 | 0.507-2.975 | 0.649 | 1.762 | 0.728-4.263 | 0.209 | - | - | - |
| Pre-Tx tumor status (Pre-T) | 1.286 | 0.543-3.043 | 0.567 | - | - | - | - | - | - |
| Perineurial invasion | - | - | - | 1.317 | 0.273-6.335 | 0.731 | - | - | - |
DSS, disease-specific survival; LRFS, local recurrence-free survival; MeFS, metastasis-free survival; *, statistically significant
Discussion
In current anti-cancer treatments, radiotherapy alone or combined with chemotherapy acts a leading character indispensably. The necrotic or apoptotic cancer cell death followed by radiotherapy mainly comes from DNA damage induced by ionizing radiation itself or free radical produced by the ionization of cellular molecules, such as H2O [28]. Therefore, the exploration of therapeutic approach to impair DNA repair activity or increased DNA damage in cancer cells with radiation exposure would be an ideal way to enhance the efficacy of radiotherapy in human malignancies, including rectal cancers. Inspiringly, by a lot of in vitro or in vivo laboratory work, a large number of ion channels have been shown to be able to modulate the cytotoxicity of ionizing radiation in cancer cells [29,30]. The impact of these channels working on radio-sensitivity in cancer cells might be contributed by increased DNA damage, attenuated DNA repair energy or limited cellular migration. Through the control of electrolyte fluxes, ion channels could be responsible for alterations of main cellular responses to external stress stimulation. Among all these critical ion-conducting proteins, chloride channels have been reported to regulate cellular volume in intestine epithelium and differentiation in colon cancer cells [31-33]. Consequently, in this study, chloride channels were focused in initial datamining from a published transcriptome and CLCA1 expression was further distinguished to estimate tumor responses of CCRT in rectal cancers. Moreover, in our validation cohort, high expression of CLCA1 was verified to be significantly correlated with poor response and inferior survivals in patients with rectal cancer receiving CCRT. These novel findings indicate clinical significance of CLCA1 in rectal cancers with CCRT treatment.
CLCA1 belongs to the CLCA family which consists of four genes in humans [34,35]. In addition to chloride transportation activated by calcium stimulation, functions of these CLCA proteins include the regulation of mucus production in respiratory systems [36]. Correspondingly, high expression of CLCA1 has also been linked to various pulmonary inflammatory diseases, as well as a tumor suppressor in colorectal carcinogenesis. These diverse biological functions of CLCA1 indicate the complex role of this protein acting in clinical implications of rectal cancers. In previous studies, CLCA1 expression is reported to be adversely correlated with tumorigenicity of human CRC [31]. The inhibition of CLCA1 expression is also demonstrated to increase proliferation and reduce differentiation in CRC cell lines [33]. In a recent study, inactivation of CLCA1 expression, by using the CRISPR/Cas9 technique, is shown to promote invasion and migration activities in CRC cells [37]. The expression levels of acting molecules participated in the epithelial-mesenchymal transition and the Wnt/beta-catenin signaling pathway were also increased in CLCA1-knockout cells. In the current study, increased percentage of low CLCA1 expression present in our validation cohort supports the role of this protein acting as a tumor suppressor in rectal cancers (59.9%, Table 1). Contradictorily, these patients with low CLCA1 expression levels were correlated with better prognoses of CCRT treatment. These clinical outcomes of our validation cohort conflict with those reported by Yang et al. In this previous study analyzing patients with CRC, poorer survival outcomes were revealed in those with low CLCA1 expression levels [38]. However, patients with a variety of originating sites and tumor stages were recruited and examined in Yang's study. Moreover, only a few patients received radiotherapy for tumor control in this study. The heterogeneity of subjects enrolled and treatment modalities employed can lead to the discrepancy of survival analyses between Yang's and our study. More importantly, several recent researches indicate that the phenotype of radiogenic hypermigration can be induced by irradiation exposure in cancer cells, especially in GBM cells [19-21]. After irradiation treatment, these malignant brain cells gain the capacity to invade the surrounding parenchyma primarily by the decrease and re-increase of cellular volume. This reshaping of cellular volume during the process of migration needs effective control of intra-cellular water transportation mediated by chloride ion efflux [39]. For instance, the regulatory role of one chloride channel, voltage-gated chloride channel (ClC)-3, has been demonstrated in hypermigration phenotype of glioblastoma cells [40]. After down-regulation of this specific chloride channel expression, the competence of invasion in glioma cells would be impeded. Since chloride conductance is crucial for cancer cells to escape from irradiation stress, further studies to investigate the correlation between CLCA1 expression and radiogenic hypermigration in cancer cells are warranted.
The other important mechanism for cancer cells to acquire radiotherapy resistance is “stemness” transformation. The selection of stem-like cells by ionizing irradiation has also been reported in glioblastoma cells [41]. Compared with differentiated cancer cells, stem-like cells are believed to own higher talent of radio-resistance [42]. Interestingly, in ovarian cancer cells, the ability of sphenoid formation conferred by CLCA1 expression has been reported recently [24]. Through a proteomics survey, CLCA1 upregulation is identified to be correlated with sphenoid aggregations in ovarian cancer cells. In functional assay of this study, aggregate formation of cancer cells could be reduced by suppression of CLCA1 expression. Furthermore, Pauli et al. has reported the potential of CLCA1 expression to modulate adhesion ability of various lung-metastatic cancer cells in lung microvascular endothelium [43]. These findings also indicate that complicated biological roles of CLCA1 can act in different stages of tumor progression and cellular response to radiotherapy, other than a pure tumor suppressor.
Due to the function in mediating mucus or fluid secretion, clinical utility of several potential CLCA inhibitors haven been studied in various kinds of diseases, such as secretory diarrhea, asthma and cystic fibrosis [44,45]. Among these inhibitors, niflumic acid (NFA), a drug clinically indicated for the relief of muscular pain, has revealed its talent as an anti-cancer agent. After NFA treatment, reduced cellular proliferation, adhesion and invasion has been shown in ovarian cancer cell lines [24,46]. Furthermore, through a large-scale drug screening platform, two potent CLCA inhibitors, CaCCinh-A01 and CaCCinh-B01, have been identified with satisfactory IC50 values [47]. Vigorous inhibition of chloride flux induced by these inhibitors has also been manifested in CRC cells [47]. Accordingly, the studies to investigate if these potent chloride channel inhibitors would enhance the efficacy of radiotherapy in rectal cancers are highly anticipated.
In conclusion, this is the first study to show that high expression of CLCA1 is positively correlated with inferior response to CCRT in patients with rectal cancers. Additionally, high expression of CLCA1 displays its predictive value in worse clinical outcomes of rectal cancers, including more tumor recurrences and shorter patients' survival. Recently, more specific and potent CLCA1 inhibitors have been developed through the advance of drug screening platforms. Our findings would offer novel therapeutic insight in the combination of these inhibitors and radiotherapy in rectal cancers.
Acknowledgements
The authors are grateful to Dr. Chien-Feng Li and the Translational Research Laboratory of Human Cancers of Chi-Mei Medical Center for providing critical technical assistance. This work was supported by Health and Welfare Surcharge of Tobacco Products grant MOHW107-TDU-B-212-114020.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Health Promotion Administration, Ministry of Health and Welfare. Accessed 1 Jan 2018. https://www.hpa.gov.tw/Pages/List.aspx?nodeid=119
2. Ferlay J, Soerjomataram I, Dikshit R. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-86
3. Wu CY, Lin JT. The changing epidemiology of Asian digestive cancers: From etiologies and incidences to preventive strategies. Best Pract Res Clin Gastroenterol. 2015;29:843-53
4. Sauer R, Becker H, Hohenberger W. et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-40
5. Gérard JP, Conroy T, Bonnetain F. et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-5
6. Bosset JF, Collette L, Calais G. et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-23
7. Bosset JF. Distal rectal cancer: sphincter-sparing is also a challenge for the radiation oncologist. Radiother Oncol. 2006;80:1-3
8. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011Mar4;144(5):646-74
9. Leanza L, Biasutto L, Managò A. et al. Intracellular ion channels and cancer. Front Physiol. 2013Sep3;4:227
10. Leanza L, Zoratti M, Gulbins E. et al. Mitochondrial ion channels as oncological targets. Oncogene. 2014Dec4;33(49):5569-81
11. Newsom-Davis J. Neuromuscular junction channelopathies: a brief overview. Acta Neurol Belg. 2005Dec;105(4):181-6
12. Campuzano O, Beltrán-Alvarez P, Iglesias A. et al. Genetics and cardiac channelopathies. Genet Med. 2010May;12(5):260-7
13. Vincent A. Autoimmune channelopathies: well-established and emerging immunotherapy-responsive diseases of the peripheral and central nervous systems. J Clin Immunol. 2010May;30(Suppl 1):S97-102
14. Lastraioli E, Iorio J, Arcangeli A. Ion channel expression as promising cancer biomarker. Biochim Biophys Acta. 2015Oct;1848(10 Pt B):2685-702
15. Lang F, Ritter M, Gamper N. et al. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol Biochem. 2000;10(5-6):417-28
16. Schwab A, Fabian A, Hanley PJ. et al. Role of ion channels and transporters in cell migration. Physiol Rev. 2012Oct;92(4):1865-913
17. Munaron L. Systems biology of ion channels and transporters in tumor angiogenesis: An omics view. Biochim Biophys Acta. 2015Oct;1848(10 Pt B):2647-56
18. Djamgoz MB, Onkal R. Persistent current blockers of voltage-gated sodium channels: a clinical opportunity for controlling metastatic disease. Recent Pat Anticancer Drug Discov. 2013Jan1;8(1):66-84
19. Wild-Bode C, Weller M, Rimner A. et al. Sublethal irradiation promotes migration and invasiveness of glioma cells: implications for radiotherapy of human glioblastoma. Cancer Res. 2001Mar15;61(6):2744-50
20. Canazza A, Calatozzolo C, Fumagalli L. et al. Increased migration of a human glioma cell line after in vitro CyberKnife irradiation. Cancer Biol Ther. 2011Oct1;12(7):629-33
21. Zhou W, Xu Y, Gao G. et al. Irradiated normal brain promotes invasion of glioblastoma through vascular endothelial growth and stromal cell-derived factor 1α. Neuroreport. 2013Sep11;24(13):730-4
22. Gruber AD, Elble RC, Ji HL. et al. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl- channel proteins. Genomics. 1998Dec1;54(2):200-14
23. Yang B, Cao L, Liu B. et al. The transition from proliferation to differentiation in colorectal cancer is regulated by the calcium activated chloride channel A1. PLoS One. 2013Apr12;8(4):e60861
24. Musrap N, Tuccitto A, Karagiannis GS. et al. Comparative Proteomics of Ovarian Cancer Aggregate Formation Reveals an Increased Expression of Calcium-activated Chloride Channel Regulator 1 (CLCA1). J Biol Chem. 2015Jul10;290(28):17218-27
25. Li CF, He HL, Wang JY. et al. Fibroblast growth factor receptor 2 overexpression is predictive of poor prognosis in rectal cancer patients receiving neoadjuvant chemoradiotherapy. J Clin Pathol. 2014Dec;67(12):1056-61
26. Edge SB, Byrd DR, Compton CC. et al. AJCC cancer staging manual. 7th edn. New York: Springer. 2010
27. Dworak OKL, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23
28. Harada H. How can we overcome tumor hypoxia in radiation therapy? J Radiat Res. 2011;52(5):545-56
29. Litan A, Langhans SA. Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Front Cell Neurosci. 2015Mar17;9:86
30. Huber SM, Butz L, Stegen B. et al. Role of ion channels in ionizing radiation-induced cell death. Biochim Biophys Acta. 2015Oct;1848(10 Pt B):2657-64
31. Bustin SA, Li SR, Dorudi S. Expression of the Ca2+-activated chloride channel genes CLCA1 and CLCA2 is downregulated in human colorectal cancer. DNA Cell Biol. 2001Jun;20(6):331-8
32. Okada Y, Shimizu T, Maeno E. et al. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J Membr Biol. 2006Jan;209(1):21-9
33. Yang B, Cao L, Liu B. et al. The transition from proliferation to differentiation in colorectal cancer is regulated by the calcium activated chloride channel A1. PLoS One. 2013Apr12;8(4):e60861
34. Elble RC, Widom J, Gruber AD. et al. Cloning and characterization of lung-endothelial cell adhesion molecule-1 suggest it is an endothelial chloride channel. J Biol Chem. 1997Oct31;272(44):27853-61
35. Fuller CM, Ji HL, Tousson A. et al. Ca(2+)-activated Cl(-) channels: a newly emerging anion transport family. Pflugers Arch. 2001;443(Suppl 1):S107-10
36. Patel AC, Brett TJ, Holtzman MJ. The role of CLCA proteins in inflammatory airway disease. Annu Rev Physiol. 2009;71:425-49
37. Li X, Hu W, Zhou J. et al. CLCA1 suppresses colorectal cancer aggressiveness via inhibition of the Wnt/beta-catenin signaling pathway. Cell Commun Signal. 2017Oct3;15(1):38
38. Yang B, Cao L, Liu J. et al. Low expression of chloride channel accessory 1 predicts a poor prognosis in colorectal cancer. Cancer. 2015May15;121(10):1570-80
39. Habela CW, Ernest NJ, Swindall AF. et al. Chloride accumulation drives volume dynamics underlying cell proliferation and migration. J Neurophysiol. 2009Feb;101(2):750-7
40. Cuddapah VA, Sontheimer H. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J Biol Chem. 2010Apr9;285(15):11188-96
41. Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R's of radiobiology revisited. Stem Cells. 2010Apr;28(4):639-48
42. Jamal M, Rath BH, Tsang PS. et al. The brain microenvironment preferentially enhances the radioresistance of CD133(+) glioblastoma stem-like cells. Neoplasia. 2012Feb;14(2):150-8
43. Pauli BU, Abdel-Ghany M, Cheng HC. et al. Molecular characteristics and functional diversity of CLCA family members. Clin Exp Pharmacol Physiol. 2000Nov;27(11):901-5
44. Schultheiss G, Siefjediers A, Diener M. Muscarinic receptor stimulation activates a Ca(2+)-dependent Cl(-) conductance in rat distal colon. J Membr Biol. 2005Apr;204(3):117-27
45. Hegab AE, Sakamoto T, Nomura A. et al. Niflumic acid and AG-1478 reduce cigarette smoke-induced mucin synthesis: the role of hCLCA1. Chest. 2007Apr;131(4):1149-56
46. Li M, Wang Q, Lin W. et al. Regulation of ovarian cancer cell adhesion and invasion by chloride channels. Int J Gynecol Cancer. 2009May;19(4):526-30
47. De La Fuente R, Namkung W, Mills A. et al. Small-molecule screen identifies inhibitors of a human intestinal calcium-activated chloride channel. Mol Pharmacol. 2008Mar;73(3):758-68
Author contact
![]() Corresponding author: Shang Hung Chen, MD, PhD. National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan. E-mail: bryanchenorg.tw
Corresponding author: Shang Hung Chen, MD, PhD. National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan. E-mail: bryanchenorg.tw

 Global reach, higher impact
Global reach, higher impact