Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(8):796-801. doi:10.7150/ijms.25047 This issue Cite
Research Paper
Efficacy and safety of nucleos(t)ide analogues to prevent hepatitis B virus mother-to-child transmission in pregnant women with high viremia: real life practice from China
1. Department of Infectious Disease, Shengjing Hospital, China Medical University, Shenyang 110022, China
2. The Sixth People's Hospital of Shenyang, Shenyang 110006, China
Received 2018-1-19; Accepted 2018-4-27; Published 2018-5-22
Abstract
Purpose: To evaluate the efficacy and safety of nucleos(t)ide analogues, especially telbivudine (LdT) for the prevention of mother-to-child transmission (MTCT) of hepatitis B virus (HBV) in women with high viremia.
Methods: We conducted a prospective, open-label, multicenter study of LdT for treating pregnant women having high viral loads of hepatitis B virus (HBV DNA>5 log10 IU/mL) but normal levels of alanine aminotransferase (ALT). Maternal HBV DNA, HBV serologic status and ALT were measured at baseline, 4 weeks after therapy, before delivery, 4 weeks after delivery, and 12 weeks after delivery. Infant HBV serologic status and HBV DNA levels were measured at 7 months. We calculated the MTCT rate of LdT-treated and LdT-untreated groups and analyzed the efficacy and safety of LdT.
Results: Ninety-one women (the treatment group) were treated with LdT, and twenty-one patients (the observation group) did not undergo antiviral therapy. The baseline HBV DNA levels were 8.15±0.82 log10 IU/mL in the treatment group, and 8.09±1.04 log10 IU/mL in the observation group. The MTCT rate was 0% in the treatment group, and 9.5% in the observation group (p=0.042). In the treatment group, HBV DNA levels were 5.02±0.74 log10 IU/mL at one month after therapy, and 3.95±0.94 log10 IU/mL before delivery. Both groups had significant differences from baseline levels in HBV DNA levels (p<0.001). In total, five patients had elevated ALT levels but without evidence of decompensate liver function. No severe adverse events or complications were observed in women or infants.
Conclusions: For pregnant women with HBV DNA greater than 5 log10IU/mL, LdT therapy was effective in reducing HBV MTCT. If serum HBV DNA was detectable at delivery, discontinuation of LdT immediately was found to be safe and rarely induced off-treatment hepatitis flare.
Keywords: HBV, chronic hepatitis B, pregnancy, mother-to-child-transmission, antiviral therapy, nucleos(t)ide analogues, telbivudine
Introduction
Hepatitis B virus (HBV) infection can cause many severe diseases like cirrhosis, hepatic cellular carcinoma (HCC) and liver failure. Worldwide, more than 800,000 people die every year due to complications from hepatitis B [1]. That is a heavy burden to public health.
HBV is transmitted through many routes [2, 3]. In China, mother-to-child transmission (MTCT) is the most common [1, 4]. Importantly, HBV transmitted through MTCT in the perinatal period often leads to chronic infection [5]. Without intervention, 40%-95% of infants born to hepatitis B surface antigen (HBsAg)-positive women will acquire HBV infection [6, 7]. Newborns inoculated with HBV vaccine and hepatitis B immunoglobulin (HBIG) have reduced MTCT rates ranging from 5-10% overall [8]. However, immune prophylaxis failure still occurs. The most important risk factor in MTCT is high maternal HBV DNA levels [9-13]. In infants born to women with HBV DNA levels of more than 6 log10 copies/ml, the risk of MTCT may rise to 30% despite immune prophylaxis [13-18]. Our previous studies in China demonstrated that high viral loads are common in women during the perinatal period [19, 20]. So antiviral therapy, such as nucleos(t)ide analogues (NAs) in late pregnancy to prevent MTCT is recommended now.
According to the US Food and Drug Administration (FDA) classification standard, all HBV antiviral NAs are category C (teratogenic in animals, but unknown in humans), except for telbivudine (LdT) and tenofovir disoproxil fumarate (TDF), which are category B drugs (no risk in animal studies, but unknown in humans) [21]. In our study, we chose LdT as our treatment agent.
The purposes of this study were to investigate the efficacy of LdT therapy, when to stop the drug, and the safety of drug discontinuation.
Materials and methods
Patients selected
This was a prospective, open-label, multicenter study. Patients were from Shengjing Hospital of China Medical University and the Sixth People's Hospital of Shenyang and were enrolled between Jan 2013 and Dec 2015. The trial was approved by the ethics committee of Shengjing Hospital. All patients signed informed consent forms before screening. The inclusion criteria were as follows: 1. Women between 20 to 40 years of age, confirmed pregnancy, HBsAg positive, alanine aminotransferase (ALT) below the upper limit of normal (ULN) (40 IU/mL). 2. HBV DNA>5 log10 IU/mL between 24 and 32 weeks of pregnancy. The exclusion criteria included: 1. Evidence of cirrhosis or hepatic cellular carcinoma (HCC), co-infection with hepatitis A, C, D or E or human immunodeficiency virus (HIV). 2. Use of antiviral therapy before or during pregnancy. 3. Combination use of other immune modulators, steroids and cytotoxic drugs. 4. Evidence of miscarriage or fetal deformity. Patients fulfilling the inclusion and exclusion criteria were enrolled in our study.
Treatment regime
Baseline serum HBV DNA levels were measured between 24 and 32 weeks of pregnancy. Antiviral therapy was initiated when the serum HBV DNA levels exceeded 5 log10 IU/mL. Based on the patients' choice, they were divided into treatment or observation groups. Patients in the treatment group were given oral LdT 600 mg daily. If HBV DNA levels declined less than 2 log10 IU/mL at 4 weeks after initiation of therapy (compared to baseline), LdT was changed to TDF. If hepatitis flare occurred during pregnancy, antiviral therapy was continued after delivery. If there was a hepatitis flare after delivery, patients were treated like other chronic hepatitis B (CHB) patients without pregnancy. At the time of the predelivery visit, if serum HBV DNA was detectable, the agent was immediately discontinued after delivery. Otherwise, the patients continued to take the agent after delivery until reaching the drug withdrawal criteria for CHB. Patients in the observation group received no antiviral therapy.
All infants received HBIG 100 IU and recombinant HBV vaccine 10 ug within 12 hours of birth. The second and third does of recombinant HBV vaccine were administered at 1 and 6 months of age, respectively. Women could breastfeed their babies after 1 week of agent cessation. Otherwise, breastfeeding was forbidden with agent.
Detection indexes
ALT, aspartate aminotransferase (AST), total bilirubin (TBIL), HBV DNA, HBV serologic status, and creatine kinase (CK) levels were measured at baseline for the patients in both groups, and at 4 weeks after therapy, before delivery, 4 weeks after delivery and 12 weeks after delivery for women in the treatment group. Infant HBV serologic status and HBV DNA levels were measured at 7 months of age (1 month after final vaccine). We calculated the MTCT rate between the two groups and analyzed the efficacy and safety of the antiviral agent.
Biochemical and virologic assessments
HBV serologic markers, including HBsAg, anti-HBs antibody, hepatitis B e antigen (HBeAg), anti-HBe and anti-HBc antibodies titers were assayed with a chemi-luminescent microparticle immunoassay using an automated Abbott AxSYM analyzer (Abbott, USA). HBV DNA levels were measured by real-time polymerase chain reaction (PCR) assay using a COBAS AmpliPrep/COBAS TaqMan 48 analyzer (Roche Diagnostics, Switzerland).
Definitions
MTCT was defined as detectable levels of HBV DNA or HBsAg in peripheral serum samples of infants at 7 months age. Hepatitis flare was defined as ALT ≥ 2×ULN during or after treatment.
Statistical analysis
Baseline characteristics and laboratory results were summarized by means of descriptive statistics, including percentage, and means ± standard deviation (SD). The t test was used for group comparisons of quantitative variables. The chi-square test was used to compare group differences of categorical variables. Significance levels were set at p<0.05. All data were analyzed by SPSS 16.0.
Results
General characteristics
During the three years of study, 127 chronic hepatitis B infected pregnant women with normal ALT levels were referred to the infectious disease clinic. One-hundred and sixteen (116/127, 91.3%) patients had high viral loads (>5 log10 IU/mL). Three patients failed to attend the hepatology/infectious disease clinic for treatment before 32 weeks of pregnancy, and one patient underwent an abortion for worrying about fetus safety. In total, 112 patients were enrolled in this study; all had positive HBeAg levels. Ninety-one (91/112, 80.5%) patients (treatment group) accepted antiviral therapy. The remaining 21 patients were enrolled in the observation group. In the treatment group, 2 (2/91, 2.2%) patients switched to TDF due to HBV DNA levels decline of less than 2 log10 IU/mL after 4 weeks of therapy compared to baseline. Four (4/91, 4.4%) patients continued to take the antiviral agent after delivery. Of these four patients, two had undetectable serum HBV DNA levels before delivery, and the other two experienced hepatitis flare during the pregnancy. Three patients discontinued the agent at the time of delivery but were retreated after delivery due to hepatitis flare.
Baseline characteristics
In the treatment group, the median age was 27 (range, 21-40) years, the baseline HBV DNA load was 8.15±0.82 log10 IU/mL (range, 5.54-9.53), the average ALT level was 26.53±8.32 U/L (range, 6-40), and the HBsAg and HBeAg levels were 4.34±0.33 log10 IU/mL (range, 3.22-5.05) and 1179.14±371.09 s/co (range, 5.3-1842.5), respectively. The mean duration of therapy was 13.62±2.12 weeks (range, 8-16). In the observation group, the median age was 26 (range, 20-34) years, the baseline HBV DNA load was 8.09±1.04 log10 IU/mL (range, 5.38-9.72), the average ALT was 23.62±6.51 U/L (range, 10-36), and the HBsAg and HBeAg level was 4.22±0.30 log10 IU/mL (range, 3.50-4.59) and 1294.94±329.29 s/co (range, 736.25-1867.35), respectively. There were no differences of baseline values between the treatment and observation groups (Table 1).
Maternal baseline values of the two study groups
| Parameter | Treatment group (n=91) | Observation group (n=21) | P value |
|---|---|---|---|
| Age (years) | 27.8±4.17 | 26.8±3.66 | 0.442 |
| ALT (U/L) | 26.53±8.32 | 23.62±6.51 | 0.934 |
| HBV DNA (log10 IU/ mL) | 8.15±0.82 | 8.09±1.04 | 0.410 |
| HBsAg (log10 IU/mL) | 4.34±0.33 | 4.22±0.30 | 0.690 |
| HBeAg (s/co) | 1179.14±371.09 | 1294.94±329.29 | 0.448 |
ALT, alanine aminotransferase; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen.
MTCT rate
Ninety-two babies were born to the 91 women in the treatment group. All of them were inoculated with HBIG and HBV vaccine within 12 hours of birth, and then completed the HBV vaccine series at 1 and 6 months. However, 13 infants were lost to follow up at 7 months of age. None of the other 79 infants (0/79, 0%) were HBsAg positive or had detectable serum HBV DNA levels at 7 months of age. The MTCT rate was 0 in our treatment group.
In contrast, in the observation group, there were 21 infants born to 21 women; they were followed up at 7 months. Even with standard immune prophylaxis, two infants (2/21, 9.52%) were HBV infected. The difference between the two study groups was significant (p=0.042). One infant was born via vaginal delivery, while the other was born by cesarean section. Both infants infected with HBV were breastfed.
Efficacy analysis
In the treatment group, the average HBV DNA level at 4 weeks after therapy was 5.02±0.74 log10 IU/mL (range, 3.42-6.85); it declined by 3.13 log10 IU/mL compared to that of baseline (p<0.001). Before delivery, the average HBV DNA level was 3.95±0.94 log10 IU/mL (range, 0-5.34), declining by 1.07 log10 IU/mL compared to that of 1-month therapy (p<0.001); it declined by 4.20 log10 IU/mL compared to that of baseline (P<0.001). At the 1 month visit after delivery, the serum HBV DNA levels rebounded to 7.75±1.68 log10 IU/mL (range, 0-8.95) (Fig. 1)
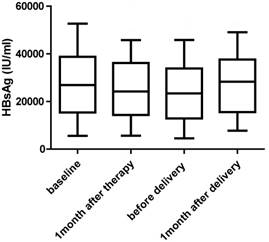
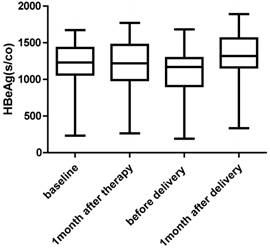
There was no significant change in HBsAg and HBeAg levels. Two patients who had low levels of HBsAg and HBeAg at baseline had undetectable serum HBV DNA levels before delivery (Fig. 2 and Fig. 3)
HBV DNA loads at baseline, 1 month after therapy, before delivery and 1 month after delivery for women undergoing antiviral therapy (n=91).

HBsAg levels at baseline, 1 month after therapy, before delivery and 1 month after delivery for women undergoing antiviral therapy (n=91).
HBeAg levels at baseline, 1 month after therapy, before delivery and 1 month after delivery for women undergoing antiviral therapy (n=91).
Safety analysis
In the treatment group, only 2 (2/91, 2.2%) patients had abnormal ALT levels during the therapy before delivery. One patient's ALT level increased to 3×ULN, and the other one increased to 2×ULN. They continued to take LdT after delivery, and the ALT returned to normal at 1 month after delivery. Both patients had normal TBIL, albumin and prothrombin time (PT), without other evidence of decompensate liver function. Three patients experienced hepatitis flare. The ALT levels of two patients rise to 4×ULN and 6×ULN at one month after delivery separately. For the third patient, ALT went up to 16×ULN at 3 months after delivery. All three patients showed no evidence of decompensate liver function; they accepted retreatment with antiviral therapy and had normal ALT levels at the next visit. The other 86 patients maintained normal ALT levels not only during the therapy but also after stopping the agent.
Among the treatment group, all but one patient had normal CK values during the LdT therapy (1/91, 1.1%). This patient had mild CK elevation (1.45×ULN) without any symptoms, levels which returned to normal by the time of the next visit. One (1/91, 1.1%) patient developed a rash during LdT therapy but remitted after several days without agent interruption. The two patients who switched from LdT to TDF did not show renal impairment during the therapy.
In the treatment group, 56 (56/91, 61.5%) women chose cesarean section; in the observation group, 12 (12/21, 57.1%) women chose cesarean section. There was no obvious difference between the two groups (p=0.71). Among the 87 patients in the treatment group who discontinued drug treatment at the time of delivery, 30 (30/87, 34.4%) breastfed their infants. No congenital malformations were identified. All neonates had normal Apgar scores at birth and developed normally.
Discussion
Antiviral therapy is recommended to avoid MTCT, as described in the guidelines of the European Association for the Study of the Liver (EASL)[22], the American Association for the Study of Liver Diseases (AASLD)[23] and the Asian Pacific Association for the Study of the Liver (APASL)[24]. Based on these guidelines, antiviral therapy is recommended for pregnant women with high viremia during the third trimester. These recommendations are based on the findings that high maternal HBV DNA levels and high HBsAg titers are closely correlated to MTCT [9, 10, 25, 26], especially the former. We previously (2012) investigated HBV-infected pregnant women. In those earlier studies, there were 249 cases enrolled, and 167 (167/249, 67.07%) were HBeAg positive. We measured their serum HBV DNA levels, and found that 37 (37/167, 22.2%) in HBeAg positive cases had high viral loads, greater than 7 log10 IU/mL. This cohort has a high risk of immune prophylaxis failure [19]. Furthermore, we still see MTCT occur in infants born to women with lower HBV DNA levels between 5-7 log10 IU/mL [11-13, 27-29]. The first trimester of pregnancy is the most critical stage for organogenesis, and long exposure to NAs in the HBV-immune tolerant phase can easily cause HBV mutations. Therefore, in our study, we set HBV DNA levels greater than 5 log10 IU/mL as the threshold for antiviral therapy, and we started antiviral treatment between 24 and 32 weeks of pregnancy; the mean duration of treatment was 13.6±2.1 weeks (range, 8-16). Before delivery, the average HBV DNA levels declined to 3.95±0.94 log10 IU/mL. None of the infants born to these women were infected with HBV. We conclude that if maternal HBV DNA levels are less than 4 log10 IU/mL and the course of treatment is suitable, nearly 100% infants will not be infected with HBV.
EASL 2017 guidelines recommended that all pregnant women with high HBV DNA levels should start antiviral prophylaxis with TDF at week 24-28 of pregnancy [22]. AASLD 2016 guidelines suggested LdT and TDF be used for prevention [23]. APASL 2016 guidelines recommended using either TDF or LdT for those women with HBV DNA levels above 6-7 log10 IU/ml [24]. In China, LdT was approved by Chinese FDA in 2007, and was included in the health care list in 2009. TDF was approved by Chinese FDA much later and was not included in the health care list. Moreover, there was a study reported that whole-body bone mineral content of TDF-exposed infants born to HIV-infected women was lower than for unexposed infants [30]. There are much more data about the safety of LdT than for TDF in China [14, 31]. There are concerns about the primary resistance to LdT. Studies from Zhuang H, et al. [32] found that younger women with a high HBV DNA levels harbor fewer NA mutations and that this population may respond more readily to NA treatment for the prevention of MTCT. We chose LdT as the antiviral agent in our initial therapy. Only 2 patients transferred from LdT to TDF due to HBV DNA decline to less than 2 log10 IU/mL compared to baseline at 4 weeks after therapy. After drug switch, they both had HBV DNA levels less than 5 log10 IU/mL before delivery, and with no evidence of MTCT. We conclude that LdT is effective for pregnant women with high viremia and that LdT may be used for HBV MTCT prophylaxis. In the rare cases where LdT therapy efficacy is insufficient, we can switch the therapy to TDF [33, 34].
Safety of antiviral therapy with LdT during the third trimester of pregnancy has been reported [14, 31, 35]. For women, mild adverse events (AEs) could be seen, such as headache, diarrhea, nausea, arthralgia, dizziness, dyspepsia, abdominal pain, insomnia, and ALT elevation. Asymptomatic mild CK elevation (<2-3×ULN) was reported in 1.5% (4/263) of cases, but without abnormal electrocardiography (EKG). Levels were all normal after drug cessation [14]. In our study, asymptomatic mild CK elevation (1.45×ULN) was observed only in one woman, and all women tolerated the agent well and rarely felt uncomfortable. Infants were all with normal Apgar scores and without congenital malformations. However, more safety data about the infants' growth and development in future are needed.
The timing for therapy discontinuation is still controversial. EASL 2017 guidelines suggest drug withdrawal at 12 weeks after delivery [22]. Antiviral therapy be discontinued at birth to 3 months postpartum according to AASLD 2016 guidelines [23]. APASL 2016 guidelines recommend that NAs be stopped at birth [24]. Early withdrawal of the antiviral therapy at birth may shorten the use of NA, which avoids resistance and allows earlier breastfeeding, in accordance with the recommendations by WHO. However, avoiding hepatitis flare is the main reason for clinicians to stop NA at a later time. In our study, we wanted patients to discontinue the antiviral drug immediately at birth if the HBV DNA levels were detectable before delivery. Otherwise, patients should continue taking the agent after delivery until reaching the CHB drug withdrawal criteria. The reasons are the following. Patients who have a good response to NA therapy with a rapid decline in HBV DNA levels usually have active CHB, and are not carriers. Therefore, if the patients have undetectable serum HBV DNA levels after such a short duration, we do not allow them to stop the agent. If not, the patient may have a high possibility for hepatitis flare when the drug is withdrawn. In our study, there were two patients in the treatment group with undetectable HBV DNA levels before delivery. We asked them to continue taking LdT after delivery without breastfeeding. Furthermore, there were only three patients (3/87, 3.5%) in our study who had off-treatment hepatitis flare, a much lower rate than in other studies [14, 15, 36]. In Zhang's study [14], 303 patients in the treatment group stopped antiviral therapy at postpartum week 4. Among them, 5.3% (16 of 303) had off-treatment ALT elevations (range, 1.38-2.57×ULN) at postpartum week 8. Pan et al. [15] observed the safety and efficacy of TDF in highly viremic pregnant women. All the patients in the TDF group received treatment from 30-32 weeks of pregnancy until postpartum week 4. They found 45% (44/97) had higher serum ALT elevations after the TDF discontinuation (p=0.03). Therefore, our drug discontinuation criteria seem much safer.
There are some limitations to this current study. First, we discontinued NA drugs early after delivery, with generally positive results; however, we still need more data to clarify the safety of early discontinuation of NA. Second, HBV DNA levels of two patients in the treatment group failed to decrease more than 2 log10 IU/mL. We regrettably did not sequence the HBV DNA to identify possible mutations. Detection of the sequence and any mutations of HBV DNA may identify the mechanisms of ineffective treatments and help to better prevent MTCT.
In conclusion, for women having HBV DNA levels greater than 5 log10 IU/mL, LdT therapy from 24 weeks of pregnancy may effectively and safely reduce HBV MTCT. If there are detectable serum HBV DNA levels at delivery, patients may safely stop the drug. Such discontinuation infrequently results in off-treatment hepatitis flare.
Acknowledgements
This work was supported by grants from the National Science and Technology Major Project (2017ZX10201201, 2017ZX10202202, 2017ZX10202203), Liaoning Provincial Science and Technology Major Project for Liver Disease Control (2013-41), and Outstanding Research Fund from Shengjing Hospital of China Medical University (2011-02).
Competing Interests
The authors have declared that no competing interest exists.
References
1. World Health Organization. Hepatitis B fact sheet, reviewed July 2017. http://who.int/mediacentre/factsheets/fs204/en
2. Custer B, Sullivan SD, Hazlet TK. et al. Global epidemiology of hepatitis B virus. Journal of clinical gastroenterology. 2004;38(Suppl 3):S158-68
3. Mohamed R, Desmond P, Suh DJ. et al. Practical difficulties in the management of hepatitis B in the Asia-Pacific region. Journal of gastroenterology and hepatology. 2004;19:958-69
4. Komatsu H, Inui A, Fujisawa T. et al. Transmission route and genotype of chronic hepatitis B virus infection in children in Japan between 1976 and 2010: A retrospective, multicenter study. Hepatology research. 2015;45:629-37
5. Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. Journal of hepatology. 2003;39(Suppl 1):S64-9
6. Okada K, Kamiyama I, Inomata M. et al. E antigen and anti-e in the serum of asymptomatic carrier mothers as indicators of positive and negative transmission of hepatitis B virus to their infants. The New England journal of medicine. 1976;294:746-9
7. Centers for Disease Control and Prevention. Postvaccination serologic testing results for infants aged </=24 months exposed to hepatitis B virus at birth: United States, 2008-2011. Morbidity and mortality weekly report. 2012;61:768-71
8. del Canho R, Grosheide PM, Mazel JA. et al. Ten-year neonatal hepatitis B vaccination program, The Netherlands, 1982-1992: protective efficacy and long-term immunogenicity. Vaccine. 1997;15:1624-30
9. Lv N, Chu XD, Sun YH. et al. Analysis on the outcomes of hepatitis B virus perinatal vertical transmission: nested case-control study. European journal of gastroenterology & hepatology. 2014;26:1286-91
10. Singh AE, Plitt SS, Osiowy C. et al. Factors associated with vaccine failure and vertical transmission of hepatitis B among a cohort of Canadian mothers and infants. Journal of viral hepatitis. 2011;18:468-73
11. Chen Y, Wang L, Xu Y. et al. Role of maternal viremia and placental infection in hepatitis B virus intrauterine transmission. Microbes and infection. 2013;15:409-15
12. Sellier P, Maylin S, Amarsy R. et al. Untreated highly viraemic pregnant women from Asia or sub-Saharan Africa often transmit hepatitis B virus despite serovaccination to newborns. Liver international. 2015;35:409-16
13. Zou H, Chen Y, Duan Z. et al. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. Journal of viral hepatitis. 2012;19:e18-25
14. Zhang H, Pan CQ, Pang Q. et al. Telbivudine or lamivudine use in late pregnancy safely reduces perinatal transmission of hepatitis B virus in real-life practice. Hepatology. 2014;60:468-76
15. Pan CQ, Duan Z, Dai E. et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. The New England journal of medicine. 2016;374:2324-34
16. Pan CQ, Han GR, Jiang HX. et al. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clinical gastroenterology and hepatology. 2012;10:520-6
17. Xu WM, Cui YT, Wang L. et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. Journal of viral hepatitis. 2009;16:94-103
18. del Canho R, Grosheide PM, Schalm SW. et al. Failure of neonatal hepatitis B vaccination: the role of HBV-DNA levels in hepatitis B carrier mothers and HLA antigens in neonates. Journal of hepatology. 1994;20:483-6
19. Ding Y, Sheng Q, Ma L. et al. Chronic HBV infection among pregnant women and their infants in Shenyang, China. Virology journal. 2013;10:17
20. Sheng QJ, Ding Y, Li BJ. et al. Telbivudine for prevention of perinatal transmission in pregnant women infected with hepatitis B virus in immune-tolerant phase: a study of efficacy and safety of drug withdrawal. Chinese journal of hepatology. 2016;24:258-64
21. Giles M, Visvanathan K, Sasadeusz J. Antiviral therapy for hepatitis B infection during pregnancy and breastfeeding. Antiviral therapy. 2011;16:621-8
22. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of hepatology. 2017;67:370-98
23. Terrault NA, Bzowej NH, Chang KM. et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-83
24. Sarin SK, Kumar M, Lau GK. et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatology international. 2016;10:1-98
25. Wen WH, Huang CW, Chie WC. et al. Quantitative maternal hepatitis B surface antigen predicts maternally transmitted hepatitis B virus infection. Hepatology. 2016;64:1451-61
26. Brown RSJ, McMahon BJ, Lok AS. et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatology. 2016;63:319-33
27. Pan CQ, Duan ZP, Bhamidimarri KR. et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clinical gastroenterology and hepatology. 2012;10:452-9
28. Pan CQ, Lee HM. Antiviral therapy for chronic hepatitis B in pregnancy. Seminars in liver disease. 2013;33:138-46
29. Sarkar M, Terrault NA. Ending vertical transmission of hepatitis B: the third trimester intervention. Hepatology. 2014;60:448-51
30. Siberry GK, Jacobson DL, Kalkwarf HJ. et al. Lower Newborn Bone Mineral Content Associated With Maternal Use of Tenofovir Disoproxil Fumarate During Pregnancy. Clinical infectious diseases. 2015;61:996-1003
31. Han GR, Cao MK, Zhao W. et al. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. Journal of hepatology. 2011;55:1215-21
32. Chen J, Yan L, Zhu FC. et al. Amino acid polymorphism in the reverse transcriptase region of hepatitis B virus and the relationship with nucleos(t)ide analogues treatment for preventing mother-to-infant transmission. Journal of medical virology. 2014;86:1288-95
33. Keeffe EB, Dieterich DT, Han SH. et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clinical gastroenterology and hepatology. 2008;6:1315-41 quiz 1286
34. Tong MJ, Pan CQ, Hann HW. et al. The management of chronic hepatitis B in Asian Americans. Digestive diseases and sciences. 2011;56:3143-62
35. Wu Q, Huang H, Sun X. et al. Telbivudine prevents vertical transmission of hepatitis B virus from women with high viral loads: a prospective long-term study. Clinical gastroenterology and hepatology. 2015;13:1170-6
36. Liu J, Wang J, Jin D. et al. Hepatic flare after telbivudine withdrawal and efficacy of postpartum antiviral therapy for pregnancies with chronic hepatitis B virus. Journal of gastroenterology and hepatology. 2017;32:177-83
Author contact
![]() Corresponding author: Dr. Xiaoguang Dou, Professor of Department of Infectious Diseases, Shengjing Hospital, China Medical University, No. 39 Huaxiang Road, Tiexi District, Shenyang 110022, China. Phone: 86-18940251121; E-mail: guang40com
Corresponding author: Dr. Xiaoguang Dou, Professor of Department of Infectious Diseases, Shengjing Hospital, China Medical University, No. 39 Huaxiang Road, Tiexi District, Shenyang 110022, China. Phone: 86-18940251121; E-mail: guang40com

 Global reach, higher impact
Global reach, higher impact