Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(7):730-737. doi:10.7150/ijms.23638 This issue Cite
Research Paper
Prognostic Utility of Soluble Suppression of Tumorigenicity 2 level as a Predictor of Clinical Outcomes in Incident Hemodialysis Patients
1. Cardiovascular Center and Cardiology Division, Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea
2. Nephrology Division, Department of Internal Medicine, Incheon St. Mary's Hospital, The Catholic University of Korea, Incheon, Korea
Received 2017-11-2; Accepted 2018-4-12; Published 2018-5-14
Abstract
Background: The suppression of tumorigenicity 2 (ST2) is associated with cardiac remodeling and tissue fibrosis. It is well known as a novel biomarker on predictor of cardiovascular events in patients with heart failure. In patients needed to start dialysis treatment, most of them had congestive heart failure. However, the prognostic implications of serum ST2 level are unknown in incident hemodialysis patients.
Methods: A total 182 patients undergoing incident hemodialysis were consecutively enrolled from November 2011 to December 2014. These patients were classified into two groups according to their median ST2 levels. The two groups were subsequently compared with respect to their major adverse cerebro-cardiovascular events (MACCE) including all-cause mortality, heart failure admission, acute coronary syndrome, and nonfatal stroke.
Results: The median duration of follow up was 628 days (interquartile range 382 to 1,052 days). ST2 was significant correlated with variable echocardiographic parameters. The parameters of diastolic function, deceleration time of the early filing velocity and maximal tricuspid regurgitation velocity were independently associated with the ST2 levels. High ST2 group had significantly higher incidence of all-cause mortality, and MACCE. High ST2 was a significant independent predictor of MACCE (adjusted hazard ratio 2.33, 95% confidence interval 1.12 to 4.87, p=0.024).
Conclusion: The ST2 is associated with diastolic function and may be a predictor of clinical outcomes in incident hemodialysis patients.
Keywords: suppression of tumorigenicity 2, heat failure, incident hemodialysis
Introduction
Chronic renal failure can lead to cardiovascular changes such as atherosclerosis and cardiac structural and functional abnormalities caused by the kidney disease itself and by dialysis treatment. About 20% of dialysis patients have systolic dysfunction (1). However, diastolic dysfunction is more frequent and may be associated with poorer prognosis than systolic dysfunction (2). Even most patients who begin dialysis treatment already have heart failure (3).
Although there have been tremendous improvements in the quality and utility of dialysis in recent years, death from cardiovascular events is still the biggest problem of dialysis (4). Therefore, it is very important to predict the occurrence of cardiovascular disease in chronic dialysis patients, and many studies have been conducted on whether various biomarkers can play such roles.
The suppression of tumorigenicity 2 (ST2) is expressed as a response to myocardial stress and injury and is known as a member of the interleukin-1 receptor family (5). It can be regarded as a marker of fibrosis, remodeling, and inflammation. ST2 is well known as a new biomarker to predict cardiovascular events in patients with heart failure. (6~8). There are still few studies on the clinical usefulness of ST2 in dialysis patients, especially those who started hemodialysis for the first time, and few studies have investigated the association of ST2 levels with cardiac function and prognosis in these patients.
Our objective was to analyze the relationship between the ST2 level and echocardiographic parameter of cardiac function, and the prognostic value of ST2 in incident hemodialysis patients.
Methods
Study population
This study consisted of 182 consecutive patients who started hemodialysis treatment for the first time in Incheon St. Mary's Hospital between November 2011 and December 2014. Patients who provided informed consent to enroll the study and blood bank. No industries were involved in the design or performance of the study or the analysis of its results. The study protocol was reviewed and approved by the appropriate institutional review board.
Echocardiographic data
We could analyze the echocardiographic data of 172 patients. Transthoracic echocardiography was performed before the first hemodialysis or as early as possible after first hemodialysis and stabilization of patients. Two-dimensionally directed left ventricular (LV) M-mode dimensions were acquired from the parasternal long axis and carefully obtained perpendicular to the LV long axis and measured at the level of the mitral valve leaflet tips at end-diastole following the recommendations of the American Society of Echocardiography (9). LV end-systolic volume and LV ejection fraction (LVEF) were calculated using modified Simpson's method. Diastolic function was assessed by 2D and Doppler methods (10). Peak early diastolic flow velocity (E), its deceleration time (DT), peak late diastolic flow velocity (A), and a ratio of E wave, and A wave (E/A ratio) were assessed form the mitral valve inflow velocity curve using pulsed wave Doppler at the tips of the mitral valve leaflet. Septal mitral annular early peak velocity (e´) was obtained from tissue Doppler imaging of the mitral annulus. A ratio of peak early diastolic flow velocity to septal mitral annular velocity (E/e´ ratio), an estimate of LV filling pressure, was calculated. The maximal tricuspid regurgitation (TR) velocity (TR Vmax) was acquired from apical four-chamber view with color flow imaging to obtain highest Doppler velocity aligned with continuous wave. Left atrial (LA) volume was measured by the biplane area length method using the disk summation algorithm similar to that used to measure LV volume (11).
Measurement of biomarkers
The blood sample was stored by venipuncture prior to the first hemodialysis in EDTA-containing tubes. After centrifugation, plasma samples were stored at -80 ℃ in a refrigerator. Serum Galectin-3 levels were measured by an optimized enzyme-linked immunosorbent assay (ELISA) using a Human Gal-3 Quantikine Kit (R&D Systems, Inc., Minneapolis, Minnesota, USA). ST2 serum concentrations were measured by ELISA using Presage® ST2 (Critical Diagnostics, San Diego, CA, USA). Serum Galectin-3 and ST2 levels were measured by fiduciary institutions that professionally analyzes clinical specimens.
Study definition and clinical analysis
The primary study end point was major adverse cerebro-cardiovascular events (MACCE) including all-cause mortality, hospitalization for heart failure, acute coronary syndrome (ACS), and nonfatal stroke. All-cause mortality was considered to be cardiac death after the exclusion of non-cardiac causes. ACS was defined unstable angina or acute myocardial infarction. Stroke, which was signified by the presence of neurologic deficits, was confirmed by a neurologist who evaluated the imaging studies of affected patients. Patient follow-up data, including censored survival data, were collected through July 31, 2015 via hospital chart, telephone interviews with patients by trained reviewers who were blinded to the study result, and reviews of the database of the National Health Insurance Corporation, Korea, using a unique personal identification number.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and are compared using Student's t-test or the Mann-Whitney U-test. Discrete variables are expressed as percentages and compared using the χ2-test or Fisher's exact test. Receiver operating characteristic (ROC) curve analyses were performed to identify the optimal cut-off value of biomarkers with the highest sensitivity and specificity associated with occurrence of events. Pearson's univariate correlation analysis for continuous variables or Spearman rank correlation analysis for discrete variables were carried out to analyze the association between the ST2 and variables. To determine variables independently associated with ST2, a stepwise multiple linear regression analysis using inclusion and exclusion criteria of 0.05 and 0.10, respectively, was performed. A multivariable Cox regression analysis (after confirming the appropriateness of the proportional hazards assumption) was carried out to identify independent predictors for cardiovascular events. Univariate Cox regression analysis was carried out with conventional risk factors and variables with a statistical p value less than < 0.05 in the baseline characteristics (Table 1.) Then, variables with a significant association (p < 0.05) in the univariate analysis and conventional risk factors were evaluated in the multivariable Cox regression model. The effect of each variable in developing models was assessed using the Wald test and described as hazard ratios (HRs) with 95 % confidence intervals (CIs). The cumulative survival was estimated using the Kaplan-Meier survival curves and compared using the log-rank tests. All statistical analyses were two-tailed, with clinical significance defined as values of p less than 0.05. Statistical analysis was carried out using Statistical Analysis Software package (SAS version 9.1, SAS Institute, Cary, North Carolina).
Results
Characteristics of the study populations
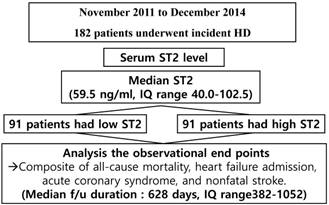
The study flow chart was briefly presented in figure 1. Serum Gal-3 levels ranged from 21 to 280 ng/ml. The mean serum ST2 level was 80.7±59.2 ng/ml, and the median serum ST2 level was 59.5 ng/ml (interquartile range (IQR) 40-102.5). All the patients enrolled herein were divided into the following two groups according to their median ST2 levels: a high ST2 group (n=91) and a low ST2 group (n=91).
Baseline characteristics between the two groups are shown in table 1. High ST2 group were older and had more reduced kidney function. These patients with high ST2 were more likely to have higher high sensitivity C-reactive protein (hs-CRP), creatine kinase-MB fraction (CK-MB), galectin-3, and B-type natriuretic peptide (BNP) and lower albumin level. Echocardiographic data was obtained in 172 patients. Patients with high ST2 had a worse diastolic function than those with low ST2 and no significant difference in systolic function compared to those with low ST2.
The study flow chart. f/u=follow up, HD=hemodialysis; IQ=interquartile; ST2=suppression of tumorigenicity 2
Baseline patient demographic, clinical, and echocardiographic data according to ST2
| Variables | Low ST2 (n=91) | High ST2 (n=91) | p value |
|---|---|---|---|
| Demographics | |||
| Age, year | 61.9±13.3 | 60.6±15.3 | 0.567 |
| Age ≥65 yrs | 41 (45.1) | 39 (42.9) | 0.881 |
| Male gender | 51 (56.0) | 55 (60.4) | 0.548 |
| Risk factors | |||
| BMI (kg/m2) | 23.8±3.8 | 23.8±4.3 | 0.984 |
| Diabetes mellitus | 46 (50.5) | 56 (61.5) | 0.179 |
| Hypertension | 77 (84.6) | 70 (76.9) | 0.259 |
| Current smoking | 21 (23.1) | 20 (22.0) | 1.000 |
| Prior history of stroke | 8 (8.8) | 13 (14.3) | 0.353 |
| Prior history of MI | 0 (0) | 2 (2.2) | 0.497 |
| Prior history of PCI | 0 (0) | 3 (3.3) | 0.246 |
| Discharge medication | |||
| Aspirin | 27 (29.7) | 35 (38.5) | 0.274 |
| Statin | 38 (41.8) | 34 (37.4) | 0.649 |
| Beta-blocker | 39 (42.9) | 38 (41.8) | 1.000 |
| ACEI or ARB | 31 (34.1) | 39 (42.9) | 0.286 |
| CCB | 42 (46.2) | 52 (57.1) | 0.182 |
| Laboratory data | |||
| Hemoglobin, g/dl | 9.29±1.60 | 9.06±1.76 | 0.359 |
| HbA1c (%) | 6.5±1.6 | 69.9±1.9 | 0.215 |
| BUN, mg/dl | 75.2±25.0 | 90.1±28.8 | <0.001 |
| Creatinine, mg/dl | 6.66±2.69 | 8.22±4.21 | 0.003 |
| eGFR, mL/min/1.73 m2 | 8.81±3.75 | 7.58±3.43 | 0.022 |
| Albumin, g/dl | 3.52±0.63 | 3.25±0.68 | 0.005 |
| Uric acid, mg/dl | 8.00±2.36 | 8.33±2.27 | 0.331 |
| Total cholesterol, mg/dl | 170.5±59.8 | 174.6±70.5 | 0.684 |
| Triglycerides, mg/dl | 157.3±92.6 | 147.3±78.3 | 0.459 |
| HDL cholesterol, mg/dl | 40.6±15.3 | 44.5±16.5 | 0.145 |
| LDL cholesterol, mg/dl | 108.3±43.9 | 112.8±55.5 | 0.584 |
| Hs-CRP, mg/l | 11.5±42.9 | 27.9±43.2 | 0.012 |
| CK-MB, ng/ml | 2.07±3.73 | 3.56±4.87 | 0.022 |
| Troponin-t, ng/ml | 43.0±104.5 | 84.5±271.0 | 0.175 |
| BNP, pg/ml | 427.5±673.1 | 1141±1670 | <0.001 |
| Galectin-3, ng/ml | 20.6 ± 9.8 | 27.3±13.3 | <0.001 |
| ST2, ng/ml | 40.44±9.89 | 120.89±60.58 | <0.001 |
| Echocardiographic data | |||
| Diastolic function parameters | |||
| E/A ratio | 0.785±0.313 | 0.875±0.366 | 0.091 |
| Median e' (m/s) | 5.62±1.90 | 5.72±1.76 | 0.711 |
| Median E/e' | 12.51±4.98 | 13.46±4.56 | 0.199 |
| Deceleration time (msec) | 228.10±68.90 | 203.31±66.57 | 0.017 |
| TR Vmax (m/s) | 2.35±0.41 | 2.54±0.58 | 0.014 |
| LAVI (ml/m2) | 48.99±13.83 | 59.44±23.19 | 0.001 |
| Systolic function parameters | |||
| LVMI (g/m2) | 124.05±29.38 | 132.17±37.10 | 0.143 |
| LVEF (%) | 59.03±7.82 | 59.07±11.57 | 0.194 |
| Median s` (m/s) | 7.08±1.65 | 6.72±1.79 | 0.176 |
| LVEDVI (ml/m2) | 61.71±16.05 | 64.92±22.04 | 0.310 |
Data are presented as the mean ± standard deviation or n (%).
ACEI/ARB=angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; BMI=body mass index; BNP=B-type natriuretic peptide; BUN=blood urea nitrogen; CCB=calcium channel blocker; CK-MB=creatine kinase-MB fraction; e'=pulsed-wave tissue Doppler imaging-derived septal mitral annular early peak velocity; E/A ratio=ratio of the peak early (E) to late (A) diastolic flow velocities; E/e' ratio=ratio of the peak early (E) diastolic flow velocities to septal mitral annular early peak velocity (e'); eGFR=estimated glomerular filtration rate; HbA1c=Glycated hemoglobin; HDL=high-density lipoprotein; Hs-CRP=high-sensitivity C-reactive protein; LAVI=left atrium volume index; LDL=low-density lipoprotein; LVEDVI=left ventricular end-diastolic volume index; LVEF=left ventricular ejection fraction; LVMI=left ventricular mass index; MI=myocardial infarction; PCI=percutaneous coronary intervention; s'= pulsed-wave tissue Doppler imaging-derived mitral annular systolic velocity; ST2=suppression of tumorigenicity 2; TR Vmax=maximal tricuspid regurgitation velocity.
Level of ST2 according to presence or absence of individual echocardiographic function parameters and diastolic dysfunction
| No, n (%) | ST2 Median (interquartile) | Yes, n (%) | ST2 Median (interquartile) | p value† | |
|---|---|---|---|---|---|
| E/e' > 14 | 117/172 (68) | 52.0(38,84) | 55/172 (32) | 72.0 (54,115) | 0.003 |
| e' (m/s) < 7 | 37/172 (21.5) | 53(38,118.5) | 135/172 (78.5) | 61 (41,95) | 0.526 |
| TR Vmax (m/s) > 2.8 | 140/172 (81.4) | 54.5(38.3,83.5) | 32/172 (18.6) | 89.5 (57.5,171.5) | <0.001 |
| LAVI (ml/m2) > 34 | 16/150 (10.7) | 42(30.5,73) | 134/150 (89.3) | 59.5 (39.8,96) | 0.069 |
| LVMI (g/m2) > 115 (men), 95 (women) | 35/150 (23.3) | 49(40,86) | 115/150 (76.7) | 59 (39,88) | 0.522 |
| LVEF (%) < 40 | 161/172 (93.6) | 58(39.5,88.5) | 11/172 (6.4) | 124 (88,221) | 0.007 |
| Diastolic dysfunction* | 35/150 (23.3) | 44(33,73) | 115/150 (76.7) | 62 (41,107) | 0.033 |
e'=pulsed-wave tissue Doppler imaging-derived septal mitral annular early peak velocity; E/e' ratio=ratio of the peak early (E) diastolic flow velocities to septal mitral annular early peak velocity (e'); LAVI=left atrium volume index; LVEF=left ventricular ejection fraction; LVMI=left ventricular mass index; ST2=suppression of tumorigenicity 2; TR Vmax=maximal tricuspid regurgitation velocity.
*normal diastolic function versus intermediate or abnormal diastolic function.
The cutoff of each parameter followed the guidelines of echocardiography (9,10).
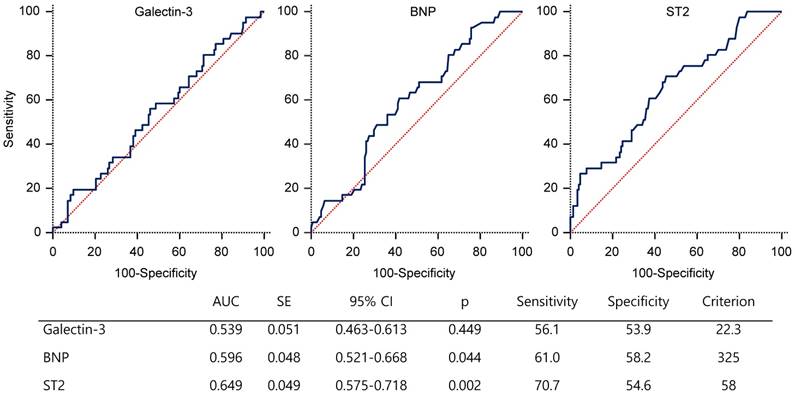
Receiver-operator characteristic curve of biomarkers for the prediction of MACCE. AUC=area under the curve; BNP=B-type natriuretic peptide; CI=confidence interval; HD=hemodialysis; IQ=interquartile; SE=standard error; ST2=suppression of tumorigenicity 2
Association of ST2 with echocardiographic functional parameters
Table 2 showed that there is a difference in median ST2 level according to presence or absence of echocardiographic functional abnormality. When the function of each echocardiography was abnormal, the median value of ST2 was higher. With the exceptions of e', LA volume index (LAVI), and LV mass index (LVMI), the presence of each abnormality of echocardiographic function was significantly associated with higher median ST2 level. A univariate analysis showed that E/A, DT, TR Vmax, LAVI, and LVEF were significantly correlated with ST2. In the stepwise multiple linear regression analysis, we included variables with p-value of < 0.05 in a univariate analysis, DT and LAVI were significantly correlated with ST2 level (table 3).
Linear regression analysis of echocardiographic predictors for sST2 level
| Echocardiographic parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| r | p | Beta coefficient | p | |
| Diastolic function parameters | ||||
| E/A | 0.159 | 0.040 | ||
| E/e' | 0.117 | 0.125 | ||
| e' | 0.036 | 0.642 | ||
| DT(msec) | -0.210 | 0.006 | -0.197 | 0.014 |
| TR Vmax (m/s) | 0.257 | 0.001 | ||
| LAVI | 0.260 | 0.001 | 0.232 | 0.004 |
| Systolic function parameters | ||||
| LVMI | 0.015 | 0.853 | ||
| LVEF | 0.220 | 0.004 | ||
| s' | -0.127 | 0.098 | ||
| LVEDVI | 0.110 | 0.174 | ||
| Overall model statistics: adjusted R2=0.083; F=7.556, p=0.001 | ||||
DT=deceleration time; e'=pulsed-wave tissue Doppler imaging-derived septal mitral annular early peak velocity; E/A ratio=ratio of the peak early (E) to late (A) diastolic flow velocities; E/e' ratio=ratio of the peak early (E) diastolic flow velocities to septal mitral annular early peak velocity (e'); LAVI=left atrium volume index; LVEDVI=left ventricular end-diastolic volume index; LVEF=left ventricular ejection fraction; LVMI=left ventricular mass index; s'= pulsed-wave tissue Doppler imaging-derived mitral annular systolic velocity; ST2=suppression of tumorigenicity 2; TR Vmax=maximal tricuspid regurgitation velocity.
Clinical outcomes for the study populations
The median duration of follow-up period was 628 days (IQR, 382-1052). Complete follow-up data for MACCE were obtained in 100% of the overall cohort for the duration of this study.
ROC curve analysis showed that the serum ST2 level with the highest sensitivity and specificity for MACCE was 58 ng/ml (area under curve (AUC), 0.649; 95% CI 0.575~0.718; p=0.002). The AUC for galectin-3 and BNP levels were lower than that for ST2 (figure 2).
Table 4 shows the univariate Cox regression for MACCE of various variables. ST2 level were all meaningful even with continuous, binary, and logarithmic transformational variables. In addition, age, creatinine, hs-CRP, CK-MB, BNP, median E/e', TR Vmax, LAVI and LVEF have significant correlations.
Predictors of the MACCE as determined by univariate Cox regression analysis
| Unadjusted HR (95% CI) | p value | |
|---|---|---|
| ST2 (binary)* | 2.378(1.231~4.593) | 0.010 |
| ST2 (continuous)† | 1.008(1.004~1.013) | <0.001 |
| ST2 (log)‡ | 2.356(1.468~3.783) | <0.001 |
| Age | 1.046(1.021~1.072) | <0.001 |
| Male gander | 0.591(0.320~1.093) | 0.094 |
| Hypertension | 0.899(0.429~1.885) | 0.778 |
| Diabetes | 1.172(0.625~2.195) | 0.621 |
| Current smoking | 0.593(0.263~1.342) | 0.210 |
| Hemoglobin | 1.161(0.975~1.384) | 0.094 |
| Creatinine | 0.871(0.773~0.982) | 0.025 |
| Albumin | 0.757(0.481~1.192) | 0.230 |
| High-sensitivity C-reactive protein | 1.005(1.001-1.010) | 0.017 |
| Creatine kinase-MB fraction | 1.078(1.001~1.161) | 0.047 |
| Troponin-T | 1.000(0.999~1.001) | 0.968 |
| B-type natriuretic peptide | 1.000(1.000~1.001) | 0.002 |
| Galectin-3 | 1.015(0.991~1.039) | 0.223 |
| Median E/e' | 1.085(1.025~1.148) | 0.005 |
| Deceleration time | 1.000(0.995-~1.005) | 0.954 |
| TR Vmax | 2.555(1.385~4.716) | 0.003 |
| LAVI | 1.022(1.006~1.039) | 0.007 |
| LVMI | 1.003(0.993~1.013) | 0.580 |
| LVEF | 0.961(0.938~0.983) | 0.001 |
| LVEDVI | 1.004(0.986~1.022) | 0.653 |
E/e' ratio=ratio of the peak early (E) diastolic flow velocities to septal mitral annular early peak velocity (e'); LAVI=left atrium volume index; LVEDVI=left ventricular end-diastolic volume index; LVEF=left ventricular ejection fraction; LVMI=left ventricular mass index; MI=myocardial infarction; PCI=percutaneous coronary intervention; s'= pulsed-wave tissue Doppler imaging-derived mitral annular systolic velocity; ST2=suppression of tumorigenicity 2; TR Vmax=maximal tricuspid regurgitation velocity.
*ST2 as a categorical variable (low galectin-3 versus high galectin-3)
†ST-2 as a continuous variable.
‡ST2 as a logarithmic transformed variable.
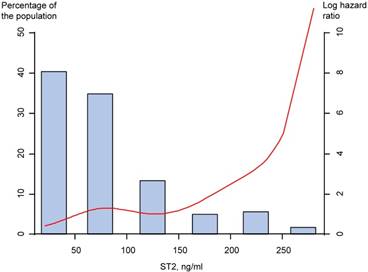
In the high ST2 group, the MACCE occurred in a total of 28 patients (30.8%), while in the low ST2 group, only 13 patients (14.3%) during long-term follow-up. The incidence of all-cause mortality and composite of all-cause mortality and heart failure admission were significantly higher in patients with high ST2 than in those with low ST2 (Table 5). Based on analysis of the study population, the high ST2 showed significant association with the MACCE (unadjusted HR 2.38, 95% CI 1.23 to 4.59, p=0.01), and multivariate analysis showed the high ST2 was associated with MACCE (adjusted HR 2.33, 95% CI 1.12 to 4.87, p=0.024) (Table 5). Restricted cubic spline regression showed the ST2 has a positive increase in hazard of the MACCE (figure 3).
Restricted cubic spline regression model of the hazard of the MACCE by serum ST2 level. MACCE=major adverse cerebro-cardiovascular events; ST2=suppression of tumorigenicity 2
Because of the small study population, multivariate Cox regression was performed in several models (table 6). The continuous variable of ST2 level had a significant association with MACCE in all 6 models. The binary variable divided by low and high group had a significant association with models 1 through 5, but not model 6 with echocardiographic parameters added.
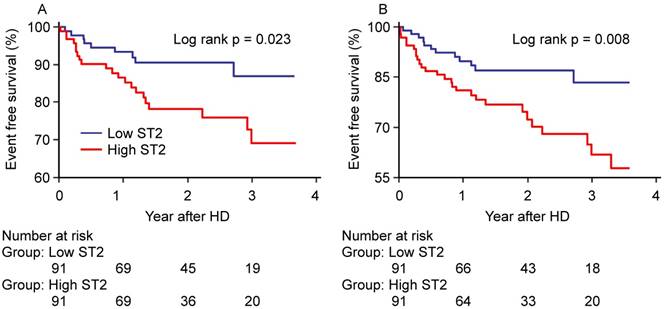
The Kaplan-Meier survival curves (figure 4) showed that high ST2 showed significantly worse hard outcomes than the low ST2 as determined by the log-rank test; all-cause mortality and MACCE (p=0.023 and p=0.008, respectively).
Discussion
This study provides evidence that initial serum ST2 levels is significantly associated with LV diastolic dysfunction and can be used to predict clinical outcomes, especially all-cause mortality, in incident hemodialysis patients. The serum ST2 levels is a significant predictor even after major risk factors, including baseline conventional risk factors, major biomarkers of heart failure, and echocardiographic parameters, have been taken into account. To our knowledge, this study is the first data which show the clinical impact of ST2 in incident hemodialysis patients.
Comparison of clinical outcome rates in patients with low and high ST2 levels
| Low ST2 (n=91) | High ST2 (n=91) | Unadjusted HR (95% CI) | p value | Adjusted* HR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| All-cause mortality | 9 (9.9) | 21 (23.1) | 2.41 (1.10-5.26) | 0.021 | 2.62 (1.11-6.24) | 0.029 |
| Cardiac mortality | 5 (5.5) | 13 (14.3) | 2.68 (0.96-7.53) | 0.061 | 1.05 (1.01-9.90) | 0.057 |
| HF admission | 5 (5.5) | 9 (9.9) | 1.98 (0.66-5.91) | 0.221 | ||
| Acute coronary syndrome | 2 (2.2) | 3 (3.3) | 1.67 (0.28-10.0) | 0.573 | ||
| Nonfatal stroke | 1 (1.1) | 3 (3.3) | 3.09 (0.32-29.7) | 0.329 | ||
| All-cause mortality + HF admission | 12 (13.2) | 26 (28.6) | 2.32(1.17-4.60) | 0.016 | 2.11(0.98~4.54) | 0.055 |
| MACCE | 13 (14.3) | 28 (30.8) | 2.38 (1.23-4.59) | 0.010 | 2.33 (1.12-4.87) | 0.024 |
CI=confidence interval; ST2=suppression of tumorigenicity 2; HR=hazard ratio; HF=heart failure; MACCE=major adverse cerebro-cardiovascular events.
*Adjusted covariates included age, sex, hypertension, diabetes mellitus, current smoker, hemoglobin, albumin, high-sensitivity C-reactive protein, galectin-3, and B type natriuretic peptide
Multivariate Cox proportional hazard models of ST2 for MACCE
| ST2 (continuous) | ST2 (low versus high) | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Model 1 - age, gender | 1.008(1.004~1.013) | <0.001 | 2.663(1.375~5.156) | 0.004 |
| Model 2 - Model 1 + DM, HTN, smoking | 1.008(1.004~1.013) | <0.001 | 2.675(1.365~5.240) | 0.004 |
| Model 3 - Model 2 + Hb, albumin, Hs-CRP | 1.008(1.003~1.013) | 0.001 | 2.595(1.314~5.127) | 0.006 |
| Model 4 - Model 3 + galectin-3, BNP | 1.008(1.002~1.013) | 0.004 | 2.334(1.119~4.867) | 0.024 |
| Model 5 - Model 1 + DT, LAVI, LVEF | 1.007(1.002~1.012) | 0.010 | 2.347(1.034~5.331) | 0.041 |
| Model 6 - Model 4 + DT, LAVI, LVEF | 1.007(1.000~1.013) | 0.038 | 1.975(0.799~4.883) | 0.141 |
BNP=B-type natriuretic peptide; CI=confidence interval; DM=diabetes; DT=deceleration time; Hb=hemoglobin; HTN=hypertension; Hs-CRP=high-sensitivity C-reactive protein; LAVI=left atrium volume index; LVEF=left ventricular ejection fraction; MACCE=major adverse cardiac and cerebral events; ST2=suppression of tumorigenicity 2
Kaplan-Meier Curves for (A) all-cause mortality and (B) MACCE. MACCE=major adverse cerebro-cardiovascular events.
Several studies have shown that ST2 level is a prognostic factor in patients with acute or chronic HF and has additional prognostic features when used with BNP (12-15). In addition, it was confirmed that ST2 level associated with new heart failure and cardiovascular mortality in patients with acute myocardial infarction (16) and cardiac reverse remodeling in patients with heart failure (17). Another study showed that ST2 was an independent prognostic factor and had a better prognostic ability than BNP in chronic hemodialysis patients (18). In other study showing that ST2 is a predictor of all-cause and cardiovascular mortality in maintenance dialysis patients, ST2 showed no greater predictive power than BNP but showed greater predictive power when used with BNP (19).
ST2 is a member of the interleukin-1 receptor family and is formally known as interleukin 1 receptor like 1. In rat model, ST2 was rapidly expressed by mechanical overload to cardiac myocytes (20). The ligand of ST2 is interleukin-33, and interleukin-33 is involved in reducing the fibrosis or hypertrophy of mechanically stressed tissues. Thus, ST2 plays a role in suppressing the effects of IL-33, so that excessive or abnormal signing of ST2 results in myocardial hypertrophy, fibrosis, and ventricular dysfunction (21).
Unlike BNP or galectin-3, ST2 is unique in that it's serum concentration has minimal effect on impaired renal function (22,23). Galectin-3 and BNP are also major prognostic factors in patients with renal impairment, but increased concentration of these marker as it is partially handled and cleared by the kidney may complicate the interpretation of the prognosis in patients with renal dysfunction (24). In fact, one study showed that the actual prognostic ability decreased by adjusted with impaired renal function (25). Thus, in patients with renal impairment, ST2 may be more helpful in predicting prognosis, and in this study, galectin-3 did not predict outcome events unlike ST2.
Left ventricular hypertrophy and systolic dysfunction, represented by LVMI and LVEF, have been established as predictors of all-cause mortality or cardiovascular mortality in end-stage renal disease patients (26). Early detection of diastolic dysfunction on echocardiography is crucial in maintenance hemodialysis patients. This is because patients with diastolic dysfunction have a poor prognosis than patients with systolic dysfunction. Also, as previously established, loss of diastolic function usually precedes systolic dysfunction (27). In the present study, LVEF was associated with ST2 in association with several diastolic parameters, but it was remarkable that LAVI and DT correlated with ST2 in multivariable analysis. LAVI is a strong indicator of LA and LV filling pressure (28). In general population and hemodialysis patients, LAVI is associated with a severity of diastolic dysfunction. LAVI is also a predictor of mortality independent of LV geometry (29,30). The elevation of LAVI is an independent predictor associated with the risk of stroke (31).
Echocardiography allows accurate assessment of cardiac function and provides prognostic information in hemodialysis patients, but it is not readily available in all dialysis units. Although this study was performed with small number of patients, ST2 is associated with echocardiographic parameters and all-cause mortality, it is likely that ST2 can be used as a tool for early risk stratification in patients who initiate hemodialysis treatment.
There are some limitations to this study. First, because this present study was nonrandomized and observational design, it may have been influenced by selection bias and confounding factors. Second, we measured the serum ST2 level only once at the initial hemodialysis time point. Therefore, it is not known whether plasma ST2 levels fluctuate during the follow-up period of maintenance hemodialysis. Third, only the medications prescribed at discharge were recorded, and any changes in medication and non-adherence or adverse drug effect of medicine during the follow-up period which may potentially influence clinical outcomes were not documented. Finally, our study is also limited as patients of single center and little sample size. More researches are needed in the large population setting.
Conclusion
The serum ST2 level is significantly associated with diastolic function and can predict all-cause mortality and clinical outcomes in incident hemodialysis patients.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pecoits-Filho R, Barberato SH. Echocardiography in chronic kidney disease: diagnostic and prognostic implications. Nephron Clin Pract. 2010;114:c242-7
2. Ahmed A, Rich MW, Sanders PW. et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol. 2007;99:393-8
3. Avorn J, Bohn RL, Levy E. et al. Nephrologist care and mortality in patients with chronic renal insufficiency. Arch Intern Med. 2002;162:2002-6
4. Collins AJ, Foley RN, Herzog C. et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 2010;55(Suppl 1):S1-420
5. Weinberg EO, Shimpo M, De Keulenaer GW. et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961-6
6. Rehman SU, Mueller T, Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458-65
7. Manzano-Fernández S, Mueller T, Pascual-Figal D. et al. Usefulness of soluble concentrations of interleukin family member ST2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107:259-67
8. Felker GM, Fiuzat M, Thompson V. et al. Soluble ST2 in ambulatory patients with heart failure: Association with functional capacity and long-term outcomes. Circ Heart Fail. 2013;6:1172-9
9. Lang RM, Badano LP, Mor-Avi V. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39
10. Nagueh SF, Smiseth OA, Appleton CP. et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277-314
11. Jiamsripong P, Honda T, Reuss CS. et al. Three methods for evaluation of left atrial volume. Eur J Echocardiogr. 2008;9:351-5
12. Rehman SU1, Mueller T, Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458-65
13. Bayes-Genis A, de Antonio M, Galán A. et al. Combined use of high-sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur J Heart Fail. 2012;14:32-8
14. Ky B1, French B, McCloskey K. et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180-7
15. Felker GM, Fiuzat M, Thompson V. et al. Soluble ST2 in ambulatory patients with heart failure: Association with functional capacity and long-term outcomes. Circ Heart Fail. 2013;6:1172-9
16. Kohli P, Bonaca MP, Kakkar R. et al. Role of ST2 in non-ST-elevation acute coronary syndrome in the MERLIN-TIMI 36 trial. Clin Chem. 2012;58:257-66
17. Lupón J, Gaggin HK, de Antonio M. et al. Biomarker-assist score for reverse remodeling prediction in heart failure: The ST2-R2 score. Int J Cardiol. 2015;184:337-43
18. Obokata M, Sunaga H, Ishida H. et al. Independent and incremental prognostic value of novel cardiac biomarkers in chronic hemodialysis patients. Am Heart J. 2016;179:29-41
19. Zhang Z, Shen B, Cao X. et al. Increased soluble suppression of tumorigenicity 2 level predicts all-cause and cardiovascular mortality in maintenance hemodialysis patients: A prospective cohort study. Blood Purif. 2017;43:37-45
20. Weinberg EO1, Shimpo M, De Keulenaer GW. et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961-6
21. Kakkar R1, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827-40
22. Lok DJA, van Der Meer P, de la Porte PWB-A. et al. Prognostic value of galectin-3, a novel biomarker of fibrosis, in patients with chronic heart failure: data from the DEAL-HF study. Clin Res Cardiol. 2010;99:323-8
23. Barnes ME, Miyasaka Y, Seward JB. et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008-14
24. Meijers WC, van der Velde AR, Ruifrok WP. et al. Renal handling of galectin-3 in the general population, chronic heart failure, and hemodialysis. J Am Heart Assoc. 2014;3:e000962
25. de Boer RA, Lok DJ, Jaarsma T. et al. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60-8
26. Foley RN, Parfrey PS, Harnett JD. et al. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024-31
27. Fathi R, Isbel N, Haluska B. et al. Correlates of subclinical left ventricular dysfunction in ESRD. Am J Kidney Dis. 2003;41:1016-25
28. Moya-Mur JL, Garcia-Martin A, Garcia-LIedo A. et al. Indexed left atrial volume is a more sensitive indicator of filling pressures and left heart function than is anteroposterior left atrial diameter. Echocardiography. 2010;27:1049-55
29. Patel DA, Lavie CJ, Milani RV. et al. Left atrial volume index predictive of mortality independent of left ventricular geometry in a large clinical cohort with preserved ejection fraction. Mayo Clin Proc. 2011;86:730-7
30. Shizuku J, Yamashita T, Ohba T. et al. Left atrial volume is an independent predictor of all-cause mortality in chronic hemodialysis patients. Intern Med. 2012;51:1479-85
31. Barnes ME1, Miyasaka Y, Seward JB. et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008-14
Author contact
![]() Corresponding author: Seok Joon Shin, MD, PhD, Nephrology Division, Department of Internal Medicine, Incheon St. Mary's Hospital, The Catholic University of Korea, 56 Dongsu-ro, Bupyeong-gu, Incheon 21431, Korea. Tel: +82.32-280-5091; Fax: +82.32-280-5987; E-mail: imkidneyac.kr
Corresponding author: Seok Joon Shin, MD, PhD, Nephrology Division, Department of Internal Medicine, Incheon St. Mary's Hospital, The Catholic University of Korea, 56 Dongsu-ro, Bupyeong-gu, Incheon 21431, Korea. Tel: +82.32-280-5091; Fax: +82.32-280-5987; E-mail: imkidneyac.kr

 Global reach, higher impact
Global reach, higher impact