3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(6):628-637. doi:10.7150/ijms.22723 This issue Cite
Research Paper
Resveratrol attenuates denervation-induced muscle atrophy due to the blockade of atrogin-1 and p62 accumulation
1. Research Center for Physical Fitness, Sports and Health, Toyohashi University of Technology, 1-1 Hibarigaoka, Tenpaku-cho, Toyohashi 441-8580, Japan
2. Institute for Liberal Arts, Environment and Society, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan
3. FANCL Research Institute, 12-13 Kamishinano, Totsuka-ku, Yokohama, 244-0806, Japan
Received 2017-9-6; Accepted 2018-3-2; Published 2018-4-3
Abstract
Decrease in activity stress induces skeletal muscle atrophy. A previous study showed that treatment with resveratrol inhibits muscular atrophy in mdx mice, a model of DMD. However, almost all studies using resveratrol supplementation have only looked at adaptive changes in the muscle weight. The present study was designed to elucidate whether the resveratrol-inducing attenuation of skeletal muscle actually reflects the adaptation of muscle fibers themselves, based on the modulation of atrogin-1- or p62-dependent signaling. Mice were fed either a normal diet or 0.5% resveratrol diet. One week later, the right sciatic nerve was cut. The wet weight, mean fiber area, and amount of atrogin-1 and p62 proteins were examined in the gastrocnemius muscle at 14 days after denervation. The 0.5% resveratrol diet significantly prevented denervation-induced decreases in both the muscle weight and fiber atrophy. In addition, dietary resveratrol suppressed the denervation-induced atrogin-1 and p62 immunoreactivity. In contrast, 0.5% resveratrol supplementation did not significantly modulate the total protein amount of atrogin-1 or p62 in the denervated muscle of mice. Resveratrol supplementation significantly prevents muscle atrophy after denervation in mice, possibly due to the decrease in atrogin-1 and p62-dependent signaling.
Keywords: resveratrol, muscle atrophy, supplementation, atrogin-1, p62, denervation
1. Introduction
Skeletal muscle possesses a highly plastic potential to cope with the demands of various environmental conditions. Skeletal muscle is composed of a variety of proteins and is regulated by the balance between protein synthesis and degradation. However, marked increase in protein degradation and decreases in protein synthesis will result in muscle atrophy. Muscle atrophy occurs in the presence of inactivity, sarcopenia, and various neuromuscular diseases, leading to a marked decrease in physical activity and the inevitable progression of muscle wasting. For example, sarcopenia is the common denominator of the aging process, responsible for a general and substantial decline in physical performance, which ultimately leads to physical disability.
Various approaches such as resistance training [1], hormonal [2] and pharmacological [3] treatment, nutritional [4] supplementation, and caloric restriction [5] has been attempted to inhibit muscle atrophy, particularly in sarcopenia. For example, treatment with androgen and nandrolone enhance protein synthesis, markedly increasing the muscle volume and strength [2]. Examining frail elderly subjects in different coutries, Becker et al. [3] showed that a phase two trial of treatment with myostatin antibody (LY2495655: LY) improved the lean body mass and several indicators of muscle power at 24 weeks. In addition, many researchers have tried various forms of supplementation to prevent muscle atrophy in vivo in humans and rodents.
Resveratrol, which was discovered in veratrum grandiflorum, was reported to upregulate the expression of sirtuin and lead to increased longevity in various species [6-8]. Resveratrol supplementation has been reported to enhance the activities of AMPK and PGC-1, leading to the upregulation of the insulin sensitivity and mitochondrial biogenesis in mice [9] and obese humans [10], but not nonobese women [11]. In addition, treatment with resveratrol improves the negative influence of a high-calorie diet in mice [12]. Furthermore, Chen et al. [13] demonstrated that resveratrol administration attenuates the abnormal accumulation of adipose tissue in mice continuously fed a high-fat diet. All of these findings suggest that resveratrol supplementation exhibits anti-aging effect and attenuates metabolic disorders.
Several researchers investigated the effect of resveratrol on morphometric paramerters such as the mass of skeletal muscle. Hori et al. [14] demonstrated that treatment with resveratrol inhibited muscle atrophy in mdx mice, a model of DMD. Shadfar et al. [15] also found that resveratrol administration inhibited muscle atrophy in a mouse model of cancer cachexia, possibly due to a decrease in immune cytokines. However, almost all studies using resveratrol supplementation have only looked at adaptive changes in the muscle weight [16, 17]. Since the muscle weight is determined by the water content, connective tissues such as tendons, and intramuscular lipids as well as muscle fibers, the increase of the muscle weight may not reflect the actual adaptation of muscle fibers. In addition, many studies involved a descriptive investigation of the adaptation of SIRT [16-20], AMPK [16, 19], PGC-1 [16-18, 20], apoptosis- [18-20], and anti-oxidant- regulated molecules [18, 20] in skeletal muscle after resveratrol supplementation. It is unknown whether resveratrol modulates the main pathway of protein degradation [ubiquitin -proteasome system (UPS) and autophagy]. A single result of atrogin-1 mRNA in muscle fed resveratrol [21] cannot reflect the adaptive role of these proteins at all. All of these findings indicate insufficient investigation of the protein degradation-linked molecules (UPS and autophagy system) at the protein level in particular.
In the present study, we investigated whether the resveratrol-inducing attenuation of skeletal muscle actually reflects the adaptation of muscle fibers themselves, and is attributable to the adaptation of representative protein degradation signaling such as UPS and autophagy-dependent signaling.
2. Materials and Methods
2.1 Experimental animals
Experiments were conducted in male ICR mice (12-weeks of age, Japan SLC, Shizuoka, Japan). The animals were housed in a temperature (22±2ºC) and humidity (60±5%)-controlled room regulated to provide alternating 12-h periods of light and darkness and were allowed to feed (commercial chow, Japan SLC) and drink ad libitum. After acclimatization for 1 week, mice were randomized and divided into two groups: the mice fed either normal (n=12) or resveratrol [0.5% content (w/w) n=12] diets. Mice continued to receive the normal or resveratrol diets until the termination of the experiments 3 weeks later. These special diets were provided by Oriental Yeast Co., Ltd. (Tokyo, Japan). At 1 weeks of diet exchange, the sciatic nerve of the left leg of mice was cut and a 10 mm piece was excised under anaesthetization. At 2 weeks of denervation, mice were killed by excess pentobarbital, and the medial gastrocnemius muscles dissected. The muscles were quickly weighed, frozen in liquid nitrogen, and then stored at -80ºC. This experimental procedure was approved by the Committee for Animal Research in Toyohashi University of Technology.
2.2 Primary antibodies
The antibodies employed in the present study were as follows: affinity-purified mouse monoclonal antibody to dystrophin (1:50, Cat. No. #25013, Sigma Aldrich); rabbit polyclonal antibody to atrogin-1/MAFbx (1:300, Cat. No. SC-33782, Santa Cruz Biotechnology, Santa Cruz, CA), and to p62 (1:200, Cat. No. M-115, MBL, Nagoya); to affinity-purified goat polyclonal antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:400, Cat. No. SC-20357, Santa Cruz Biotechnology, Santa Cruz, CA).
2.3 Tissue preparation, gel electrophoresis, and immunoblotting
The gastrocnemius muscle of both mice was homogenized in 10-20 vols of 50 mM Tris-HCl pH 7.4, 5 mM EDTA, 10 µg/ml phenylmethylsulfonylfluoride, 0.5 µg/ml leupeptin, 0.2 µg/ml aprotinin, 0.2% NP-40, 0.1% Triton X-100, and 1mM Na3VO4 in a polytron (DIAX 900, Heidolph-Instruments, Schwabach, Germany) for 30 s. The homogenized tissues were then centrifuged at 15,000×g for 25 min at 4 ºC, and the protein concentration of the supernatant was colorimetrically determined (Bio-Rad protein determination kit, Bio-Rad Laboratories, Richmond, CA). Sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10-15% acrylamide for detection of atrogin-1, cleaved caspase-3 and GAPDH) and Western blotting were performed next as described previously [22]. Proteins separated by SDS-PAGE were electrophoretically transferred onto nitrocellulose membranes (Hybond-ECL Western, Amersham, Arlington Heights, IL). The blots were then incubated with blocking buffer composed of 0.1% Tween-20 and 1% gelatin in 10 mM Tris-buffered saline (TBS, 10 mM Tris, 135 mM NaCl, 1 mM KCl, and 0.02% NaN3, pH 7.4) for 5 min. The blots were next incubated with primary antibodies (each concentration Atrogin-1 1:400, p62 1:2000, and GAPDH 1:400) overnight and with goat anti-rabbit or rabbit anti-goat IgG -conjugated AP (1:10000, Cat. No. 611-105-122 and 16376, Rockland Immunochemicals, Inc., USA) for lh and were visualized with Western blue, a stabilized substrate for alkaline phosphatase (Promega). By using GAPDH bands as loading control, a densitometric analysis of each blot was performed with a computerized image processing system (NIH Image 1.63).
2.4 Immunofluorescence
Mouse gastrocnemius muscle was isolated and frozen in isopentane. Serial 7-µm transverse sections made with a cryostat (Microm Coldtome, HM-520, Germany) were mounted on silanized slides (Dako Japan, Tokyo). Cryosections were fixed with 4% paraformaldehyde (5 min) and incubated in blocking solution (10 % normal horse serum in PBS) for 30 min at room temperature. Sections were incubated with polyclonal anti-atrogin-1 and anti-dystrophin antibodies for 90 min at room temperature. After being washed in PBS, sections were incubated with anti-rabbit FITC-conjugated (1:100 final dilution; Rockland Immunochemicals, Inc., USA) and anti-goat Rhodamine- conjugated (1:100 final dilution; Chemicon International Inc., USA) secondary antibodies. The muscle sections were mounted in a slowfade antifade kit with 4'-6-Diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA, USA). Images were acquired on an Olympus BX50 inverted microscope with a fluorescent attachment (Olympus) and Photonic Science CCD camera (Olympus DP70). As described previously [23], we calculated in percentages of nuclei possessing atrogin-1 immunoreactivity by analyzing DAPI-positive nuclei from 10-15 different parts of cross-sections using 10 animals. We conducted the analysis of cross-sectional area (CSA) in gastrocnemius muscles of mice by analyzing 200 muscle fibers.
2.5 Statistical analysis
All values were expressed as means ± S.D. A one-way analysis of variance (ANOVA) was used to evaluate the significance of differences in morphometric parameter. Individual differences between groups were assessed with Scheffés multiple range test. P < 0.05 was considered to be statistically significant.
3. Results
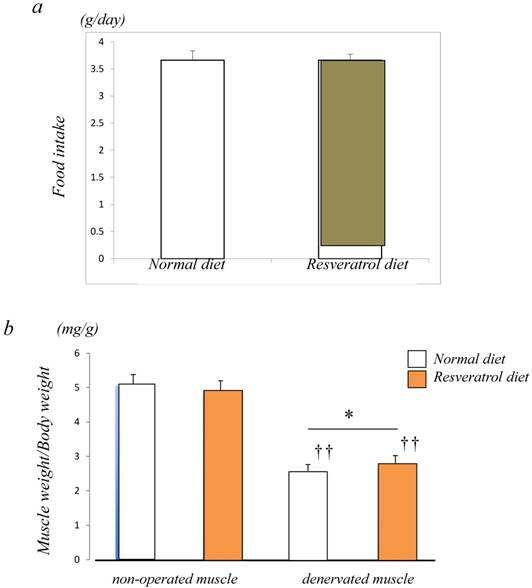
Figure 1a shows a similar level of food intake between the control (3.7±0.2 g/day) and resveratrol (3.7±0.1 g/day)-fed groups. To examine the inhibitory effects of resveratrol on muscle atrophy after denervation, the left leg muscles of ICR mice were denervated by sectioning the sciatic nerve while the right leg muscles of mice were sham-operated. The denervated mice were divided into two groups. There were no significant differences in body weight (data not shown) between the two groups of mice. In the mice fed a normal diet, the muscle weight of denervated gastrocnemius muscle decreased by 50% 14 days after denervation. Similar but significantly lower extent of denervation-induced atrophy was observed in the muscle of mice fed the resveratrol diet (b) (p < 0.05) [Fig. 1b].
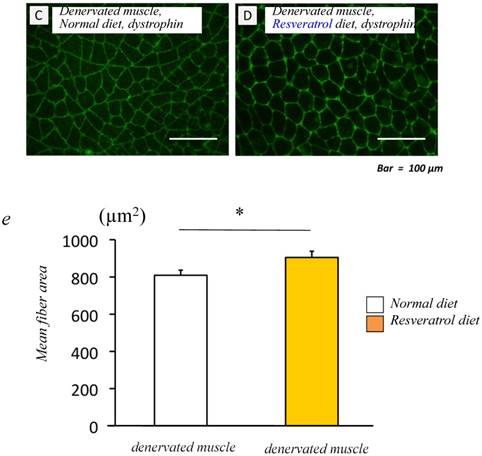
We examined the effects of dietary resveratrol on the denervation-mediated decrease in the fiber area of the gastrocnemius muscle. The denervated muscle of mice fed the resveratrol diet exhibited a significantly larger fiber area (905.0±33.5 μm2) than those fed a normal diet (809.3±27.7 μm2) (p < 0.05) [Fig. 1c-e].
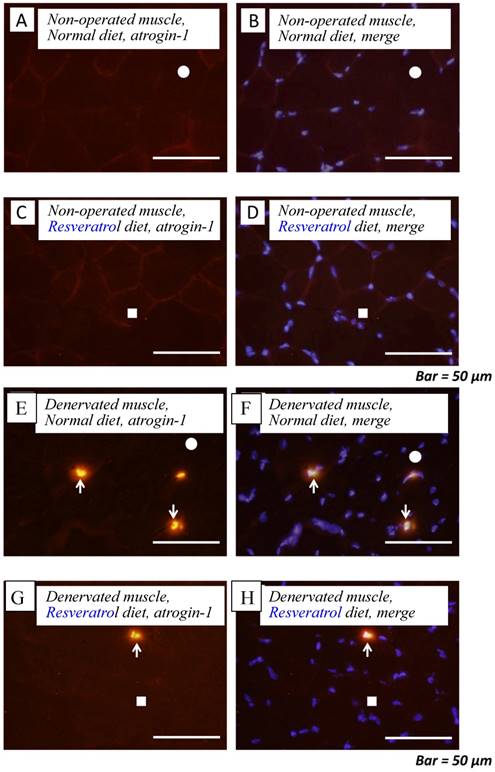
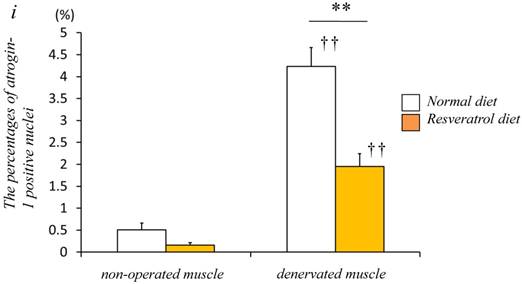
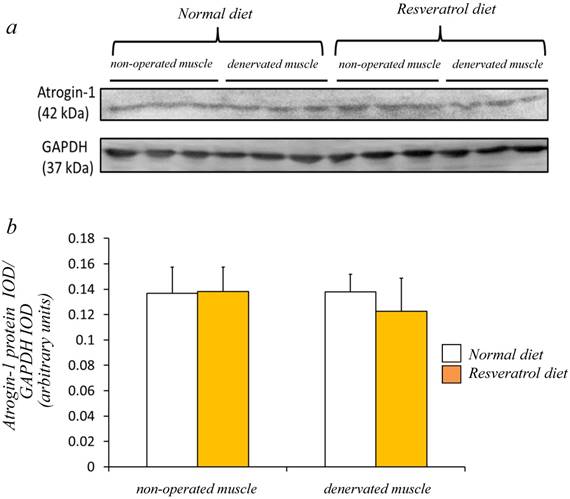
Next, we investigated whether the level of atrogin-1 protein expression differs in the denervated gastrocnemius muscle between those fed normal and resveratrol diets. We performed immunofluorescence staining of a single cryosection, which can be used to detect atrogin-1 immunoreactivity by visualizing using Rhodamine-conjugated secondary antibody. Irrespective of the differences in the diet, normal unoperated muscle did not exhibit the expression of atrogin-1 immunoreactivity in the nuclei [Fig. 2a-d]. Denervation significantly enhanced the atrogin-1-positive nuclei in muscle (p < 0.01) [Fig. 2e-h], but these expression levels were not significantly reduced with the resveratrol diet (p < 0.01) (2.0±0.3%) compared with those of normal diet (4.2±0.4%) [Fig. 2i]. Western blot analysis using crude homogenates of the total muscle indicated prominent bands of atrogin-1 and GAPDH proteins at 42 and 37 kDa, respectively [Fig. 3a]. Densitometric analysis did not detect a significant increase in the amount of atrogin-1 protein in the denervated muscle of mice receiving both diets [Fig. 3b].
Food intake and morphological characteristics of mice and the gastrocnemius muscle fed both the normal and resveratrol diets. There was no significant difference in the amount of fook intake (a). In the mice fed the normal diet, the weight of the devnervated gastrocnemius muscle had decreased by 50% at 14 days after denervation (b). Similar but significant lower extent of denervation-induced atrophy was observed in the muscle of mice fed the resveratrol diet (b). The effects of dietary resveratrol on the denervation-mediated decrease in the fiber area of the gastrocnemius muscle. The immunostaining using anti-dystrophin antibody is performed for the cryosections of the gastrocnemius muscle of mice fed normal (c) and resveratrol (d) diets. The denervated muscle of mice fed the resveratrol diet exhibited a significantly larger fiber area than those fed the normal diet (c-e). Comparing the muscule fiber size distribution maps, the mice fed the resveratrol diet exhibited more larger-sized and lower numbers of small sized muscle fibers after denervation (f). Values are means ± SD (n=10~12/group). †† : p < 0.01 compared with the non-operated muscle. *: p < 0.05 compared with normal diet.
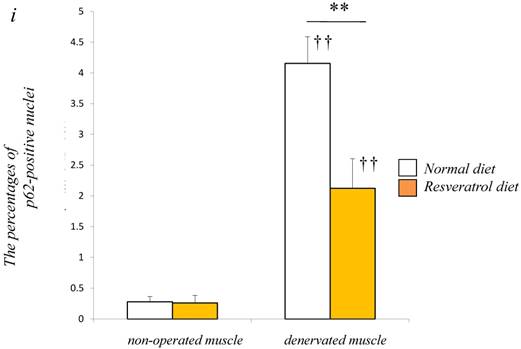
Serial cryosections of the gastrocnemius muscle of mice fed normal and resveratrol diets. Atrogin-1 immunoreactivity was visualized using Rhodamine-conjugated antibody. In non-operated gastrocnemius muscle of both diets, immunofluorescence labeling showed that atrogin-1 was not present in the any nuclei of muscle fibers (a-d). Marked increases of atrogin-1 immunoreactivity were observed in several nuclei of denervated muscle of both mice (e-h). Densitometric analysis showed significant increasee in atrogin-1 immunoreactivity in the denervated muscle of mice fed between normal and resveratrol diets (i). However, the percentage of atrogin-1 positive nuclei were significantly lower in the mice fed the resveratrol diet than those of normal one (i). White circles and squares indicate the same fibers on different immunoimages. White arrows denote the atrogin-1-positive nuclei. Bar = 50 μm. ††: p < 0.01 compared with the non-operated muscle. **: p < 0.01 compared with normal diet.
Western blot analysis showed that the denervated muscles of both mice were not significantly increased in the atrogin-1 protein compared with those non-operated muscles (a). No significant difference in the amount of atrogin-1 protein was observed in the gastrocnemius muscles between normal and resveratrol fed mice (b). The integrated optical density (IOD) of atrogin-1 protein was normalized to the IOD of each internal control (GAPDH) (arbitrary units). Values are means ± SD (n=8/group).
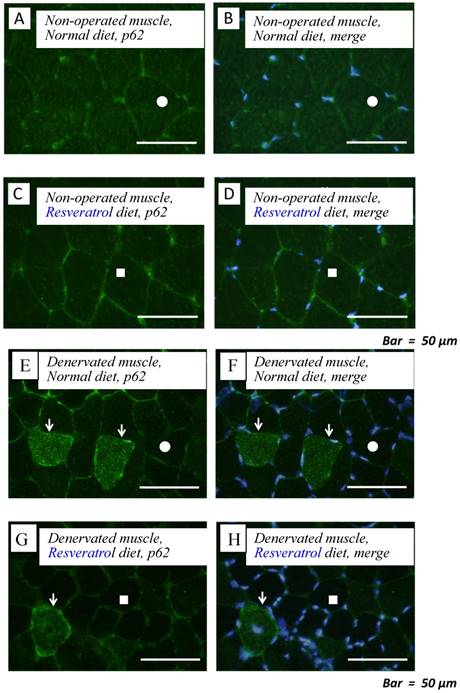
To obtain a clear understanding of whether the level of autophagic defect (p62/SQSTM1 accumulation in cytosol) differs in the denervated muscle between control and resveratrol supplementation, we performend immunofluorescence staining. p62/SQSTM1-positive fibers were detected weakly in non-operated muscle (control: 0.3±0.1% vs. resveratrol: 0.3±0.1%) [Fig. 4a-d]. Denervation significantly increased p62/SQSTM1 immunoreactivity in the cytosol of several muscle fibers with both diets (p < 0.01) [Fig. 4e-i]. Densitometric analysis clearly showed a significantly lower amount of p62-positive muscle fibers in the denervated muscle of mice with the resveratrol diet (4.2±0.4%) than normal diet (2.1±0.5%) (p < 0.01) [Fig. 4i].
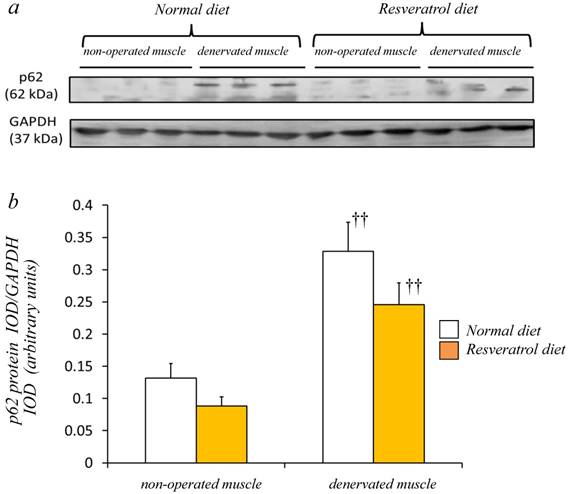
Western blot analysis using crude homogenates of the total muscle indicated prominent bands of p62/SQSTM1 and GAPDH proteins at 62 and 37 kDa, respectively [Fig. 5a]. In the denervated muscle of mice fed the normal diet, p62/SQSTM1 protein increased markedly, although such a change was smaller in those fed the resveratrol diet. Densitometric analysis indicated a significant increase in the amount of p62/SQSTM1 protein in the denervated muscle of mice with both diets (p < 0.01) [Fig. 5b]. However, we failed to observe a significantly lower expression of p62/SQSTM1 with resveratrol supplementation than with the normal diet.
4. Discussion
A previous study demonstrated that resveratrol supplementation attenuates muscular atrophy in mdx mice, a model of DMD. However, it is unknown whether such attenuation by resveratrol is attributable to the modulation of the main protein degrading system (UPS and autophagy). The present study investigated whether resveratrol supplementation attenuates denervation-induced atrophy of muscle fibers by the inhibition of UPS and autophagy. Two main conclusions can be derived from the findings of the present study. First, supplementation with resveratrol at a 0.5% of diet significantly attenuates the atrophy of muscle fibers after denervation. Second, this protective effect can be attributed to the decrease in atrogin-1 and p62 accumulation.
Serial cryosections of the gastrocnemius muscle of mice fed normal and resveratrol diets. Atrogin-1 immunoreactivity was visualized using FITC-conjugated antibody. In non-operated gastrocnemius muscle of both diets, immunofluorescence labeling showed that p62/SQSTM1 was not present in the any cytosol of muscle fibers (a-d). Marked increases of p62/SQSTM1 immunoreactivity were observed in the cytosol of several muscle fibers of denervated gastrocnemius of both mice (e-h). Densitometric analysis showed significant increasee in p62/SQSTM1 immunoreactivity in the denervated muscle of mice fed between normal and resveratrol diets (i). However, the percentage of p62/SQSTM1-potitive muscle fibers was significantly lower in the mice fed the resveratrol diet than those of normal one (i). White circles and squares indicate the same fibers on different immunoimages. White arrows denote the p62/SQSTM1-positive muscle fiber. Bar = 50 μm. †† : p < 0.01 compared with the non-operated muscle. **: p < 0.01 compared with normal diet.
In mice fed the both diets, a prominent bands of p62 protein and GAPDH were detected at 62 and 37 kDa, respectively (a). Western blot analysis showed that the denervated muscles of both mice were more abundantly expressing p62 protein (a). Although denervation increased the p62 protein in the gastrocnemius muscle, the expression levels in mice receiving the two diets were similar (b). The integrated optical density (IOD) of p62 protein was normalized to the IOD of each internal control (GAPDH) (arbitrary units). Values are means ± SD (n=8/group). ††: p < 0.01 compared with the non-operated muscle.
Since the amount of food intake in the mice fed resveratrol diet was similar to that in the mice fed normal diet, the food intake may not affect the findings (all of our data). In the present study, the ratio of the denervated/contralateral muscle weight in the resveratrol-fed mice was significantly higher than in the normal-fed mice. Our atrophy-attenuating data using resveratrol (0.5%) are similar to the data of Hori et al. [14], which demonstrated that C57BL/10ScSn-Dmdmdx/J mice (mdx mice) exhibit less muscle atrophy of the biceps brachii on receiving 0.4% resveratrol for 32 weeks. In addition, we investigated the attenuation of the atrophy of muscle fibers in the resveratrol-fed mice compared with normal-fed mice. All data indicated the attenuating effect of resveratrol supplementation on the denervated muscle of mice.
It is widely accepted that UPS cannot modulate slower muscle atrophy in sarcopenia [24-26]. In contrast, the protein degradation in the denervated muscle is highly regulated by UPS [27, 28]. Indeed, the protein content of E3 ubiquitin ligase, MuRF1, and atrogin-1 is upregulated in the denervated muscle [29], similar to the results of our study [Fig. 3]. In addition, our study indicated the lower expression of atrogin-1 immunoreactivity in the denervated muscle after resveratrol supplementation. This result supports the report of Sin et al. [20], which demonstrated decreased atrogin-1 expression by resveratrol in degenerated muscle after compression. It is widely accepted that SIRT is activated by resveratrol. SIRT1 attenuates muscle atrophy by inhibiting the movement of Foxo1 and Foxo3 transcription factor the upstream modulator of atrogin-1 [30]. Thus, resveratrol may inhibit the expression of atrogin-1 protein via the activation of SIRT1.
Our Western blot using atrogin-1 antibody could not detect a significant decrease after resveratrol supplementation. Since atrogin-1 exhibits movable components between the nucleus and cytosol [31], atrogin-1 only changes the localization and does not cause volumetric changes. The protein analysis in this study was conducted to utilize crude homogenate using whole muscle but not fractionated homogenate with the division of components such as the nucleus, cytosol, and membrane. Unfortunately, our protein analysis using crude homogenate can only determine the total amount of atrogin-1 protein. Therefore, further studies should be conducted to examine the localization of atrogin-1 protein using fractionated homogenate with the nucleus and cytosol at least to elucidate the attenuating effect on muscle atrophy of treatment with resveratrol.
The cytosol accumulation of p62/SQSTM1 is a marker of the functional defect in autophagy-lysosome signaling. Indeed, the marked accumulation of p62/SQSTM1 occurs in the atrophic muscle of mdx mice [32], sarcopenic muscle of mice [33], and sporadic inclusion-body myositis of muscle fibers [34]. The present study demonstrated the marked accumulation of p62/SQSTM1 in the cytosol of muscle fibers in the denervated muscle [Fig. 4]. Thus, denervated muscle exhibits a defect of autophagy-dependent signaling. Intriguingly, resveratrol supplementation in our study significantly decreased the percentage of p62/SQSTM1-positive muscle fibers after denervation. Western blot analysis using whole muscle homogenate also indicated a small but non-significant decrease in the amount of p62/SQSTM1 proteins after denervation than those of the control diet [Fig. 5]. These results suggest that resveratrol supplementation ameliorates the autophagic defect in denervated muscle. Resveratrol is known to activate AMPK, an upstream mediator of autophagy-lysosome signaling [10, 12]. AMPK activates autophagic signaling probably by inactivating mTORC1, which inhibits this autophagic system [35, 36]. In addition, AMPK directly activates ULK1 and Beclin1 [37, 38], which are necessary molecules for the initial step of the autophagic system. Thus, resveratrol alleviates the autophagic defect by activating AMPK. Since the autophagy-lysosome system is a degradation system, the improvement in the defect of the autophagic system may induce further muscle atrophy. However, the accumulation of unnecessary degraded protein also leads to the deterioration of muscle protein synthesis and promotes further degradation. Our results suggest that the alleviation of the autophagic defect in the denervated muscle may block muscle atrophy by degrading unnecessary accumulated proteins, and recovering the protein balance.
Our study demonstrated that treatment with resveratrol at a 0.5% of food intake attenuates denervation-induced muscle atrophy of mice. This attenuation may be ascribed to the decrease in the atrogin-1-dependent system and by improvement of the autophagic defect. It remains necessary to elucidate a more descriptive mechanism (AMPK-, SIRT1-, or PGC-1α dependent?) linked to the decrease of atrogin-1 and p62 protein in the denervated muscle during resveratrol supplementation, and assess whether treatment with resveratrol is effective for morphological parameters in other muscular atrophy models such as sarcopenia and muscular dystrophy.
Acknowledgements
This work was supported by a research Grant-in- Aid for Scientic Research C (No. 17K01755) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038-44
2. Camerino GM, Desaphy JF, De Bellis M, Capogrosso RF, Cozzoli A, Dinardo MM, Caloiero R, Musaraj K, Fonzino A, Conte E, Jagerschmidt C, Namour F, Liantonio A, De Luca A, Conte Camerino D, Pierno S. Effects of nandrolone in the counteraction of skeletal muscle atrophy in a mouse model of muscle disuse: molecular biology and functional evaluation. PLoS One. 2015;10:e0129686
3. Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med. 2013;19:101-6
4. Le NH, Kim CS, Park T, Park JH, Sung MK, Lee DG, Hong SM, Choe SY, Goto T, Kawada T, Yu R. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediators Inflamm. 2014;2014:834294
5. McKiernan SH, Colman RJ, Aiken E, Evans TD, Beasley TM, Aiken JM, Weindruch R, Anderson RM. Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys. Exp Gerontol. 2011;47:229-36
6. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191-6
7. Bauer JH, Goupil S, Garber GB, Helfand SL. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2004;101:12980-5
8. Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605-15
9. Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11:e1001603
10. Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MK, Kunz I, Schrauwen-Hinderling VB, Blaak EE, Auwerx J, Schrauwen P. Caloric restriction-like effects of 30 days resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612-22
11. Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BW, Klein S. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16:658-64
12. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337-42
13. Chen Q, Wang E, Ma L, Zhai P. Dietary resveratrol increases the expression of hepatic 7α-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis. 2012;11:56
14. Hori YS, Kuno A, Hosoda R, Tanno M, Miura T, Shimamoto K, Horio Y. Resveratrol ameliorates muscular pathology in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. J Pharmacol Exp Ther. 2011;338:784-94
15. Shadfar S, Couch ME, McKinney KA, Weinstein LJ, Yin X, Rodríguez JE, Guttridge DC, Willis M. Oral resveratrol therapy inhibits cancer-induced skeletal muscle and cardiac atrophy in vivo. Nutr Cancer. 2011;63:749-62
16. Bennet BT, Mohamed JS, Always SE. Effects of resveratrol on the recovery of muscle mass following disuse in the plantaris muscle of aged rats. PLoS One. 2013;8:e83518
17. Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, Zahariev A, Zahn S, Stein TP, Sebedio JL, Pujos-Guillot E, Falempin M, Simon C, Coxam V, Andrianjafiniony T, Gauquelin-Koch G, Picquet F, Blanc S. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25:3646-60
18. Jackson JR, Ryan MJ, Always SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66A:751-64
19. Ringholm S, Olesen J, Pedersen JT, Brandt CT, Halling JF, Hellsten Y, Prats C, Pilegaard H. Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice: impact of PGC-1a. Exp Gerontol. 2013;48:1311-8
20. Sin TK, Yu AP, Yung BY, Yip SP, Chan LW, Wong CS, Rudd JA, Siu PM. Effects of long-term resveratrol-induced SIRT1 activation on insulin and apoptotic signaling in aged skeletal muscle. Acta Diabetol. 2015;52:1063-75
21. Alamdari N, Aversa Z, Castillero E, Gurav A, Petkova V, Tizio S, Hasselgren PO. Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochim Biophys Res Comm. 2012;417:528-33
22. Sakuma K, Akiho M, Nakashima H, Nakao R, Hirata M, Inashima S, Yamaguchi A, Yasuhara M. Cyclosporin A modulates cellular localization of MEF2C protein and blocks fiber hypertrophy in the overloaded soleus muscle of mice. Acta Neuropathol (Berl). 2008;115:663-74
23. Akiho M, Nakashima H, Sakata M, Yamasa Y, Yamaguchi A, Sakuma K. Expression profile of Notch-1 in mechanically overloaded plantaris muscle of Mice. Life Sci. 2010;86:59-65
24. Sakuma K, Yamaguchi A. Sarcopenia and cachexia: the adaptation of negative regulators of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2012;3:77-94
25. Sakuma K, Aoi W, Yamaguchi A. Current understanding of sarcopenia: possible candidates modulating muscle mass. Pflügers Arch. 2014;467:213-229
26. Sandri M, Barberi L, Bijlsma AY, Blaauw B, Dyar KA, Milan G, Mammucari C, Meskers CG, Pallafacchina G, Paoli A, Pion D, Roceri M, Romanello V, Serrano AL, Toniolo L, Larsson L, Maier AB, Muñoz-Cánoves P, Musarò A, Pende M, Reggiani C, Rizzuto R, Schiaffino S. Signaling pathways regulating muscle mass in ageing skeletal muscle. The role of IGF-1-Akt-mTOR-FoxO pathway. Biogerontology. 2013;14:303-23
27. Bodine SC, Latres E, Baumhueter S, Lai VK-M, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan Z-Q, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704-8
28. Bodine SC, Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469-84
29. Sun H, Qiu J, Chen Y, Yu M, Ding F, Gu X. Proteomic and bioinformatic analysis of differentially expressed proteins in denervated skeletal muscle. Int J Mol Med. 2014;33:1586-96
30. Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J Biol Chem. 2013;288(42):30515-30526
31. Julie LC, Sabrina BP, Marie-Pierre L, Leibovitch SA. Identification of essential sequences for cellular localization in the muscle-specific ubiquitin E3 ligase MAFbx/Atrogin 1. FEBS Lett. 2012;586:362-7
32. De Palma C, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, Rovere-Querini P, Moggio M, Ripolone M, Francolini M, Sandri M, Clementi E. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis. 2012;3:e418
33. Sakuma K, Kinoshita M, Ito Y, Aizawa M, Aoi W, Yamaguchi A. p62/SQSTM1 but not LC3 is accumulated in sarcopenic muscle of mice. J Cachexia Sarcopenia Muscle. 2016;7:204-12
34. Nogalska A, Terracciano C, D'Agostino C, King Engel W, Askanas V. p62/SQSTM1 is overexpressed and prominently accumulated in inclusions of sporadic inclusion-body myositis muscle fibers, and can help differentiating it from polymyositis and dermatomyositis. Acta Neuropathol. 2009;118:407-13
35. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214-26
36. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577-90
37. Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, Guan KL. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell. 2013;152:290-303
38. Sanchez AM, Csibi A, Raibon A, Cornille K, Gay S, Bernardi H, Candau R. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J Cell Biochem. 2012;113:695-710
Author contact
![]() Corresponding author: Kunihiro Sakuma, Ph.D., Institute for Liberal Arts, Environment and Society, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan. E-mail: sakumatitech.ac.jp; TEL: 81-3-5734-3620
Corresponding author: Kunihiro Sakuma, Ph.D., Institute for Liberal Arts, Environment and Society, Tokyo Institute of Technology, 2-12-1 Ookayama, Meguro-ku, Tokyo 152-8550, Japan. E-mail: sakumatitech.ac.jp; TEL: 81-3-5734-3620

 Global reach, higher impact
Global reach, higher impact