3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2018; 15(4):385-394. doi:10.7150/ijms.23054 This issue Cite
Research Paper
Transgenic Expression of A Venous Malformation Related Mutation, TIE2-R849W, Significantly Induces Multiple Malformations of Zebrafish
Department of Oral and Maxillofacial-Head and Neck Oncology, Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine, Shanghai Key Laboratory of Stomatology, Shanghai, 200011, PR China.
Received 2017-9-27; Accepted 2018-1-5; Published 2018-2-12
Abstract
A TIE2 mutation causing arginine-to-tryptophan substitution at residue 849 (TIE2-R849W) is commonly identified in heredofamilial venous malformation. However, there is no in vivo model to confirm the pathogenic role of TIE2-R849W. Humanized TIE2-R849W plasmid was constructed via PCR-mediated site-directed mutagenesis. After transcription and micro-injection, TIE2-R849W significantly induces multiple malformations in zebrafish: caudal vein plexus (CVP) defect, eye abnormalities, forebrain formation perturbations, and mandibular malformation. Histologically, these phenotypes accompany aphakia, confused retina plexiform layer, abnormal mandibular cartilage, ectopic myelencephalon proliferation and aberrant location of neurogliocytes. According to qRT-PCR, except for high expression of egfl7, the other CVP-related genes cd146, nr2f1a, and s1pr1 are not significantly different from control. TIE2-R849W also induced upregulation of the wnt signaling pathway. Gene array in vitro shows that under the effect of TIE2-R849W, consistent with high expression of pik3 and foxo1, high levels of egfl7, wnt9a, lrp5 and dkk1 were partly confirmed. This in vivo model directly identifies the venous-related pathogenic role of TIE2-R849W. Under up-regulation of TIE2-R849W, egfl7 could be considered a potential reason for venous defects. Moreover, the wnt pathway may perform an important role as a key trigger for head multi-malformations.
Keywords: TIE2-R849W, Venous malformation, Zebrafish, Developmental malformation, Egfl7, Wnt
Introduction
Venous malformation (VM), a type of vascular morphogenesis defect, is clinically characterized by soft, blue, compressive, and localized lesions. Histologically, lesions display uneven endothelial cell lining, messy extracellular matrix (ECM) structure, enlarged endothelial lumens, and disorganized, sparse vascular smooth muscle cells (VSMCs)[1-3]. While investigating genetic changes among typical heredofamilial VM (multiple cutaneous and mucosal venous malformations, VMCM), a specific genetic TIE2 mutation, a cytosine-to-thymine (C-to-T) nucleotide transition in TIE2 (2545C>T) causing an arginine-to-tryptophan substitution at residue 849 (TIE2-R849W) in the intracellular domain of TIE2[1], was commonly identified and has been accepted as a crucial pathogenic factor[4, 5]. TIE2 or TEK, a endothelial-specific receptor tyrosine kinase (RTK), is generally considered an important component involved in the regulation of angiogenesis and cardiovascular development[6, 7]. Due to its unique performance in early angiogenesis, the relationship between TIE2 and VM has been paid close attention. According to the newest ISSVA (International Society for the Study of Vascular Anomalies) classification for vascular anomalies (2014), diverse TIE2 mutations have been further regarded as causal genes of VM. Among these mutations, TIE2-R849W is the most common and unique mutation in VMCM.
Based on in vitro studies, TIE2-R849W is thought to lead to constitutive ligand-independent hyperphosphorylation of mutant TIE2, thereby activating downstream signaling pathways and enhancing corresponding cell functions[5, 8]. Compared with other VM-related TIE2 mutations, TIE2-R849W is often recognized as a natural inducer or analog of overactive natural TIE2[9]. Among activated downstream molecules, PI3K, ShcA protein and STAT1 are all confirmed to be involved in the TIE2-R849W related network[10, 11]. Aberrant STAT1 phosphorylation is unique in the TIE2-R849W state, which could inhibit fibroblast growth factor 2 (bFGF) expression[11]. It is still unknown whether other pathways could play important roles in R849W-related processes. Besides the cellular results mentioned, there is still no established TIE2-R849W-related animal model; thus, systematic in vivo data are insufficient. There is no direct evidence to prove the VM-related pathogenic role of the TIE2 mutation. Tie2‑/- mice suffer from severe embryonic lethality, dramatic reductions in endothelial cells due to malformations of the vascular network, and heart defects[12]. The feasibility of a transgenic mouse model remains controversial. Thus, more in vitro and in vivo studies are needed to illuminate role and functions of TIE2-R849W.
Fortunately, zebrafish (Danio rerio), a novel model system for studying developmental or hematopoietic disease, could express a highly conservative homologous gene and protein. Considering that zebrafish presents characteristics of a relatively diaphanous embryo, fast growth, easy manipulation of genes, and a simplified vascular developmental process, this model could provide convenient observations of comparatively significant changes in phenotype[13-15]. For confirming the mechanism and role of TIE2-R849W, we established a humanized TIE2-R849W transgenic zebrafish model via experienced transgenic manipulation to investigate the mechanism and vital signal network. According to our current work, TIE2-R849W expression impairs formation of caudal vein plexus (CVP) in zebrafish. This provides the first direct in vivo evidence to identify the venous-related pathogenic role of TIE2-R849W. EGFL7 could be considered as another potential reason for venous defect caused by TIE2-R849W. Interestingly, in addition to CVP defects, TIE2-R849W significantly induces multiple other malformations in zebrafish, including eye defects, malformed forebrain and abnormal jaw developmental extension. Corresponding to typical WNT pathway-related phenotypes, under the activation of TIE2-R849W, gene expression changes of wnt are evident and consistent. Rechecking published gene arrays in vitro is also an important supplement to our work.
Methods
Materials
Conventional chemical reagents were purchased from Sigma (Sigma-Aldrich Chemie GmbH, Munich, Germany) and Sinopharm (Sinopharm Chemie, Shanghai, China). All primers and transcription kits were purchased from Invitrogen (Invitrogen, Eugene, USA). Restriction endonucleases, Taq DNA polymerase, and other reagents used for polymerase chain reaction (PCR) were purchased from Takara (Takara Bio, Otsu, Japan). Reagents related to agarose gel electrophoresis (AGE) were purchased from Thermo Scientific (Thermo Scientific, Eugene, USA). Gel extraction kit was purchased from OMEGA (OMEGA, E.Z.N.A.® Gel Extraction). In-fusion reaction was performed with In-Fusion® HD Column kit (Clontech® Laboratories).
Zebrafish and maintenance
Zebrafish and embryos were raised and maintained under standard conditions in Shanghai Research Center for Model Organisms[13, 14]. Establishment and characterization of the fli1a: EGFP transgenic lines have been described elsewhere[16]. The Animal Care and Use Committee at Roswell Park Cancer Institute approved all animal procedures described herein. Animal procedures were approved by the Institutional Animal Care and Use Committees at Shanghai Research Center for Model Organisms. These procedures comply with the American Veterinary Medical Association's (AVMA) Panel on Euthanasia.
Construction of TIE2-R849W plasmid vector
Basic schematic diagram of mutant plasmid construction is displayed in Figure 1A. Artificial cloning plasmid pcDNA3 (Invitrogen) and humanized TIE2 cDNA (IMAGE, ID: 100004752, Product Code: OCAA49 D02, Source BioScience) were used as basic templates. After designing and synthesizing primers (shown in Table S1), linearized pcDNA3, BGH polyA and TIE2 CDS (Coding DNA Sequence) were amplified via PCR (Polymerase Chain Reaction) and were identified via electrophoresis and gel extraction. Then, the humanized normal TIE2 plasmid system was constructed via In-Fusion® HD Column reaction, electrophoresis and gel extraction. The cytosine-to-thymine (R849W) is in line with nucleotide transition in TIE2 (2545C>T). According to typical and traditional PCR-mediated site-directed mutagenesis method[17], with the help of ingeniously designed primers and missense mutation (shown in Table S1), the R849W mutation fragment in TIE2 CDS and other fragments were generated. Finally, based on diverse fragments, the humanized TIE2-R849W plasmid system was constructed via In-Fusion® HD Column reaction, electrophoresis and gel extraction. Through In-Fusion cloning, conventional transfection, shaking, and plasmid extraction, adequate mutant plasmid T7-kozak-R849W-BGHpolyA-BGHpolyA was obtained. Finally, the specific mutation site (C>T) was confirmed by traditional gene sequencing (Figure 1B).
Acquisition of mutant human TIE2 mRNA
For linearizing plasmid, the extracted final vector was treated with the restriction endonuclease KpnI. According to standard procedures, mutant human TIE2 mRNA was obtained and purified from plasmids harboring TIE2-R849W using a T7-derived transcription kit (mMESSAGE mMACHINE® T7 Ultra Kit) and mRNA purifying kit (MEGA clear® Kit).
Collection and microinjection of zebrafish embryos
Healthy mature zebrafish, with an unbalanced male:female ratio (2:1), were mated the day before microinjection, and embryos were collected after natural spawning. For the mutant group, fertilized one-celled zebrafish embryos were injected with 0.5 nl mutant human TIE2 mRNA (200 pg) into each embryo. For the control group, embryos were injected with 0.5 nl pure water. All embryos were raised and maintained under standard conditions.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from 30 to 50 embryos per group in TRIzol (Roche) according to the manufacturer's instructions. RNA was reverse transcribed using the PrimeScript RT reagent kit with gDNA Eraser (Takara). Quantification of gene expression was performed in triplicate using Bio-Rad iQ SYBR Green Supermix (Bio-Rad) with detection on the Realplex system (Eppendorf). Relative gene expression quantification was based on the comparative threshold cycle method (2 -ΔΔCt) using ef1 as endogenous control gene. Primer sequences are given in Table S2. Three independent experiments were evaluated.
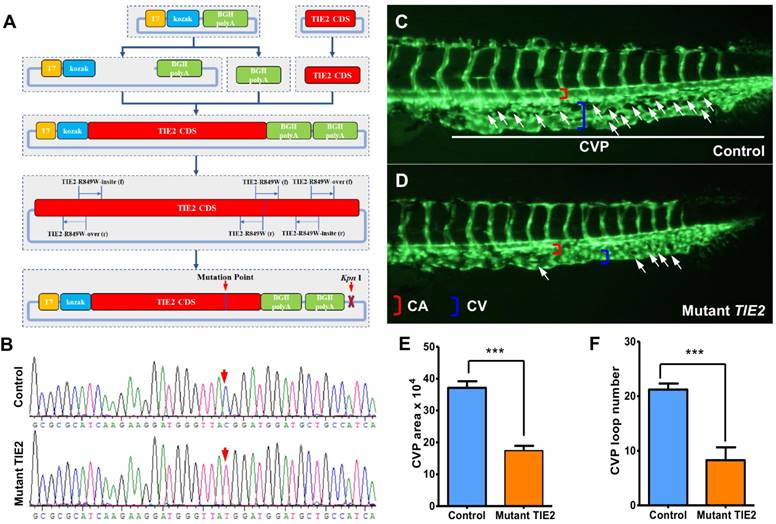
Construction of plasmid encoding humanized TIE2-R849W and CVP defect in zebrafish caused by TIE2-R849W expression. (A) Construction of plasmid encoding mutant TIE2, based on normal humanized TIE2 sequence, via PCR-mediated site-directed mutagenesis. Location of mutation-related primers and plasmid elements are indicated. (B) Traditional gene sequencing identification after construction. As shown by red arrow, nucleotide T in control group had successfully transformed to C in mutant TIE2 group. (C) At approximately 2 days after fertilization (dpf) in control embryos, CVP formed honeycomb-like structures at the tail (white arrowheads). In contrast, mutant human TIE2 mRNA (200 pg) injection resulted in specific defects in caudal vein plexus (CVP) formation (D, white arrowheads). (E, F) Quantification of area and loop formation at CVP (Scale bars are 100 μm for A-C. Error bars, s.e.m.; *** refers to P < 0.0001 by ANOVA. CVP, caudal vein plexus; CA, caudal artery; CV, caudal vein).
Histological examination
For histological analysis, all embryos were fixed in 4% paraformaldehyde/PBS (pH 7.4) at 4 degrees Celsius for at least 24 h. The tissues were embedded in paraffin, and 3 um sections were produced as previously described[18] and stained with hematoxylin and eosin (H&E, Biotech Well). Each section was then photographed under a magnification of 40×, 100× and 200×.
Gene array analysis
The microarray data were acquired in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus and are available through GEO series accession number GSE46684. In this work, statistical analysis and visualization of the microarray data were carried out using a software package of HemI (Heatmap Illustrator, version 1.0, Copyright © 2007-2014, The CUCKOO Workgroup, http://hemi.biocuckoo.org), and prepared publication-quality figures of heatmap[19].
Image acquisition and statistical analysis
Embryos and larvae were analyzed with Nikon SMZ 1500 fluorescence microscope and subsequently photographed with digital cameras. Quantitative image analyses were performed using image-based morphometric analysis (NIS-Elements D3.1, Japan). A subset of images was adjusted for levels, brightness, contrast, hue and saturation with Adobe Photoshop 7.0 software (Adobe, San Jose, California) to optimally visualize the expression patterns. All data are presented as means ± SEM. Statistical analysis and graphical representation of the data were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Statistical significance was performed using a Student's t test, ANOVA, or χ2 test as appropriate. Statistical significance is indicated by *, where P < 0.05, and ***, where P < 0.0001.
Results
Via site-directed mutagenesis, humanized TIE2-R849W expression impairs the formation of caudal vein plexus (CVP) in zebrafish
To investigate the vascular formation process affected by TIE2-R849W, a plasmid encoding mutant TIE2 was constructed based on the normal humanized TIE2 sequence via ingenious PCR-mediated site-directed mutagenesis (shown in Figure 1A, primers are shown in Table S1) and identified by traditional gene sequencing (nucleotide C had successfully transformed to T, shown in Figure 1B). Through experienced micro-injection operation, transcriptional mRNA (200 pg) encoding humanized TIE2-R849W (mutant TIE2) was injected into 1-cell stage fli1a: EGFP transgenic zebrafish embryos. The fli1a: EGFP transgenic zebrafish exhibited fluorescent protein-positive vasculature[16], providing more convenience for dynamic and real-time observation of the vascular formation process in vivo. As the control group, equal doses of pure water were also injected into embryos to exclude the potential influence of injection. As an important window of observing angiogenesis, in the control group, normal CVP were formed with honeycomb-like structures at the tail approximately 2 days after fertilization (dpf) (Figure 1C), while the TIE2-R849W group exhibited obvious formation obstacles and defects in CVP (Figure 1D). All zebrafish developed normal segmental vasculature and caudal arteries. For presenting quantitative data, significantly in TIE2-R849W embryos, there was a near 50% decrease in the area and loop numbers at CVP (Figure 1E-F). According to these results, the direct effect of TIE2-R849W on early angiogenesis was identified in vivo.
Expression of humanized TIE2-R849W causes obvious eye defects of zebrafish
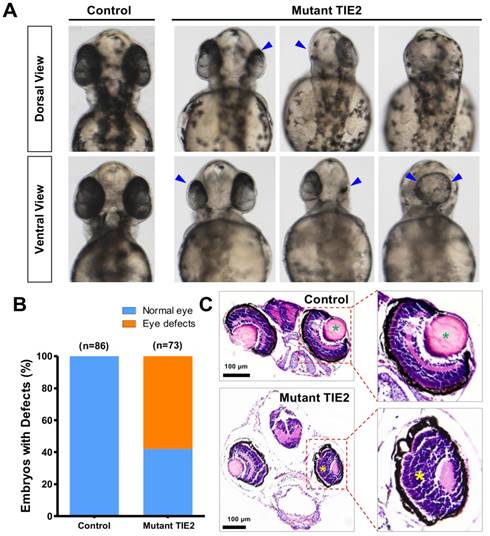
With the development of transgenic zebrafish, the number, size and location of eyes affected by TIE2-R849W could also be determined at the same time. With TIE2-R849W expression, serious and frequent eye defects were triggered. From the dorsal view and ventral view, there existed apparently unilateral or bilateral eyeless fish, with reduction of size in one side and eye-fusion at the dorsal position (Figure 2A). Compared to normal eye embryogenesis in the control group, at 52 hours after fertilization (hpf), 58.5% (42/73) of the TIE2-R849W group exhibited diverse eye defect types (Figure 2A-B). To detect more optic differences and details between control and mutant group, histological studies on normal eyes in the control group and typical small eyes in mutant group were performed. Considering the feasibility of paraffin sectioning, adult zebrafish are more suitable; however, in the mutant group, there was a high death rate and severe difficulty in further growth because of severe malformations (eye defects and other malformations mentioned below). Usually, zebrafish larva survived without feeding for 7 days. Thus, transgenic zebrafish at 7 dpf were collected. Compared to the control group, reduced optic size in one side was evident. Under the influence of TIE2-R849W expression, other defects in optic histological construction included loss of lens, abnormal plexiform layer, and a still recognizable separated layer structure (Figure 2C). In short, humanized TIE2-R849W caused obvious eye defects in zebrafish.
Expression of TIE2-R849W causes malformed forebrain and abnormal jaw developmental extension in zebrafish
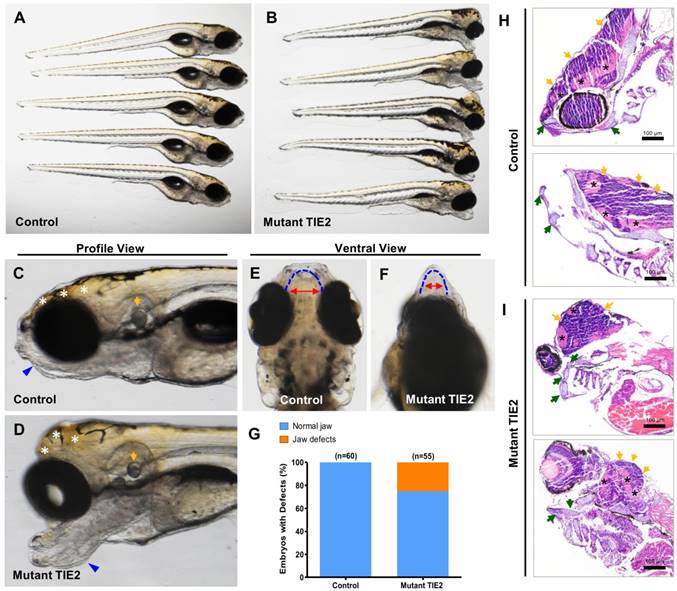
As mentioned above, there was a high death rate and severe difficulty in further growth because of severe malformations in the R849W group, except for eye defects. At 7 dpf in the mutant group (n = 55), significantly malformed forebrain and jaw were observed, while embryos in the control group all developed into the normal phenotype (Figure 3A-D). Among various individuals in the mutant group, there was a slightly different but almost similar degree of alteration (Figure 3A-B). From the profile view and comparison (Figure 3C-D), despite the obvious perturbations in the forebrain and mandibular structures, the otocysts developed normally. Furthermore, based on morphological analysis, TIE2-R849W resulted in mandibular hypermorphosis towards the anterior inferior part, reduction of mandibular width, holistic morphological abnormalities and mild bimaxillary deviation (Figure 3E-F). The bar graph (Figure 3G) shows that 25.4% of embryos (14/55) in the mutant group displayed significant developmental jaw defects compared to the control group. As there was high mortality of injected embryos because of jaw malformations, this also limited us from adopting more detailed studies or intervention in the adult phase. As another important pattern, based on the histological research on malformed head (7 dpf), zebrafish in the TIE2-R849W group were characterizeed by abnormal extension of mandibular cartilage, ectopic proliferation of myelencephalon and aberrant location of neurogliocytes (Figure 3H-I). According to previous studies, the fact that expression of TIE2-R849W could lead to jaw and forebrain defects implies the important relationship between TIE2 and the WNT pathway. This hypothesis or implication guided us in performing further verification.
Obvious eye defects of zebrafish caused by humanized TIE2-R849W. (A) At 52 hpf, compared to normal embryogenesis of eyes in the control group, from the angle of dorsal view and ventral view, there exist apparently unilateral or bilateral eyelessness, reduction of size in one side and eye-fusion at dorsal position (impaired eyes are indicated by blue arrow). (B) Quantification of eye defects in control and TIE2-R849W group at 52 hpf. (C) Histological research (HE) on reduced size eye (7 dpf) indicates loss of lens (indicated by green asterisk), abnormal plexiform layer (indicated by yellow asterisk), and still-recognizable separated layer structure (showed by partial enlargement). (HE, hematoxylin and eosin staining; scale bars are 100 μm for C.)
Up-regulated gene expression of pik3r2, foxo1b and CVP-related egfl7 caused by TIE2-R849W
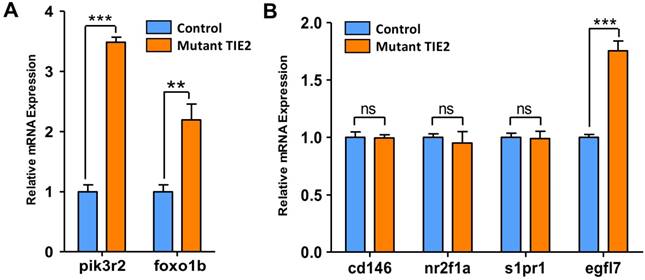
Results mentioned above are phenotypes related to TIE2-R849W. For detecting more details and mechanism, qRT-PCR analysis on potential involved genes has been a common approach to explore and confirm potential theories in zebrafish-related studies. Before performing more experiments, it is important to get direct support for this zebrafish model. For TIE2-R849W, consistent with commonly accepted results in vitro[9], the high gene expression state of pik3r2 and foxo1b was also verified via this animal model in vivo (Figure 4A). Among diverse malformations, CVP defects were direct and optimal phenotypes reflecting TIE2-R849W-related vascular malformations. To investigate potential reasons for abnormal CVP, according to previous studies on CVP and TIE2-R849W, the formation of CVP could be regulated by signaling mechanisms. Other research groups had found that nr2f2[20], nr2f1a[21], s1pr1[22], egfl7[23] and other transcription factors all play important roles in angiogenesis patterning. Abnormal expression of these genes would induce obvious CVP defect formation. According to our qRT-PCR analysis, except for egfl7, the expression of cd146, nr2f1a, and s1pr1 did not display significant differences with control group (Figure 4B). As a novel factor thought to participate in CVP formation[23], EGFL7, which is synthesized and secreted by endothelial cells, can attach to the extracellular matrix (ECM) and activate the INTEGRIN-NOTCH signaling pathway, thereby regulating cellular migration, invasion and angiogenesis[23-25]. Thus, the unique and high level of egfl7 in the mutant group further suggests its potential key role in the formation of CVP defects, or venous malformation.
wnt-related ligands and intercellular signaling molecules increase above normal expression levels under the influence of TIE2-R849W
Malformed head structure was the most aberrant characteristic of TIE2-R849W expression in zebrafish. Previous studies suggested that abnormal expression of wnt-related molecules, such as wnt8a[26, 27], gsk-3β[28, 29], dkk1b[20] or β-catenin[28], could induce similar phenotypes, including malformed forebrain, eyelessness or jaw malformations. Via qRT-PCR analysis, the expression levels of wnt-pathway related genes were analyzed (Figure 5A). The expression of TIE2-R849W induced significant upregulation of not only down-stream but also up-stream signals. Notably, as ligands belonging to the classical wnt pathway, wnt8a and wnt9a were obviously up-regulated. Interestingly, an important wnt-related negative regulation signal, dkk1b, was also up-regulated in the TIE2-R849W group. Almost all tested wnt-related ligands and intercellular signal molecules increased above normal expression levels. A schematic diagram was proposed to display visualized relationship between expression levels and roles in pathway (Figure 5B). The high expression level of β-catenin downstream of the wnt pathway may be the basis of different malformations. This implies unique regulation of TIE2-R849W on the WNT signal pathway, via not only phosphorylated PI3K[30], but also upregulated WNT. For confirming our data obtained in vivo, in one previous research[9], a comparison of transcriptional profiles of human umbilical vein endothelial cells (HUVECs) expressing wild-type versus VM-causative mutant forms of TIE2, related gene-expression microarrays had been performed. The expression conditions of related genes in this array profile (GSE46684) were obtained from NCBI and analyzed once more (Figure 5C). According to this array, in mutant-1 group, consistent with accepted and common high expression of pik3, high level of egfl7, foxo1, wnt9a, lrp5 and dkk1 could also be confirmed. However, compared with control group, there was no remarkable difference in mutant-2 and mutant-3. From this point, although EGFL7 and WNT pathway in vitro or in vivo had been indicated to perform important roles in TIE2-R849W related regulatory pathway, more work need be continued and discussed.
Discussion
Previous studies on TIE2-R849W were based on cellular models; this is the first animal model to discuss its induced phenotype and mechanism. For making direct observations on early pattern formation and potential pathogenic mechanisms of TIE2-R849W, the CVP of zebrafish is considered an important model based on the visible vascular endothelial cells. CVP formation involves venous fate specification, differentiation, migration, cell death, venous sprouting and remodeling, making it suitable for studying venous vessel formation[21, 31, 32]. In the present study, we found that expression of mutant TIE2 led to CVP defects (Figure 1C-D). The formation of CVP is regulated by signaling mechanisms. Other research groups have found that nr2f2[20], nr2f1a[21], s1pr1[22], egfl7[23] and other transcription factors all play important roles in angiogenesis patterning. First, according to published gene array and cellular researches[9], pik3r2 and foxo1b have been proven to involve in vascular integrity and angiogenesis. Consistently, upregulated pik3 and foxo1b are also identified in our zebrafish study (Figure 4A). However, unfortunately, there has been no direct evidence to prove the phenotype influence of mutant pik3r2 and foxo1b in zebrafish. Then, for detecting the mechanisms by which TIE2-R849W related CVP defects, the expression level of egfl7 in qRT-PCR was confirmed to be distinctly upregulated in different groups, which is also in keeping with the severity and tendency of CVP defects. As a novel factor shown to participate in CVP formation[23], EGFL7, synthesized and secreted by endothelial cells, can attach to ECM and activate the INTEGRIN-NOTCH signal pathway to regulate cellular migration, invasion and angiogenesis[23-25]. In venous malformations, irregular structure of the vascular lumen is a significant characteristic that induces progressive lumen enlargement. This discovery implies a new point, early formation pattern of vascular endothelial lumen, to discuss the formation of venous malformations.
In addition to CVP defects, overexpression of TIE2-R849W mRNA causes obvious eye defects (including unilateral or bilateral eye loss, reduction of size and eye fusion) and malformed forebrain in zebrafish (Figure 2 and 3). Histological research on reduced-size eyes (7 dpf) indicates loss of lens, abnormal plexiform layer, and still recognizable separated layer structure (Figure 2C). Based on comparison with diverse zebrafish models, the TIE2-R849W mutant zebrafish model has a pleiotropic phenotype resembling wnt-related zebrafish models discussed below. Our qRT-PCR confirmed the abnormal expression of the wnt signaling pathway in this condition (Figure 5).
TIE2-R849W-related malformed forebrain and abnormal jaw developmental extension in zebrafish. (A, B) Injection of TIE2-R849W mRNA causes malformed forebrain and abnormal jaw developmental extension by 7 dpf. There is a slightly different but almost similar degree of alteration among different individuals. (C, D) From the profile view, despite the obvious perturbations in the forebrain (indicated by white asterisk) and mandibular structures (indicated by blue triangle), the otocysts develop normally (indicated by orange arrow). (E, F) In the mutant group, there exists mandibular hypermorphosis towards the anterior inferior part, reduction of mandibular width, holistic morphological abnormalities and mild bimaxillary deviation. (G) Quantification of jaw developmental defects in control and TIE2-R849W group at 52 hpf. (H, I) In contrast to the control group (I), zebrafish in the TIE2-R849W group were histologically characterized by abnormal extension of mandibular cartilage (indicated by green arrow), ectopic proliferation of myelencephalon (indicated by orange arrow) and aberrant location of neurogliocytes (indicated by black asterisk) (HE, hematoxylin and eosin staining; scale bars are 100 μm for H and I).
Expression pattern of pik3r2, foxo1b and CVP-related genes in control and TIE2-R849W expression assessed by qRT-PCR. TIE2-R849W results in supra-physiological expression level of pik3r2 and foxo1b (A). In the mutant group, except for egfl7, the expression of other CVP-related genes, cd146, nr2f1a and s1pr1, do not display significant differences with the control group (B). Asterisks denote statistically significant differences (*P<0.05; **P<0.001; ***P<0.0001; ns, not significant).
Expression pattern of wnt-related genes in control and TIE2-R849W group assessed by qRT-PCR and gene array. (A) Expression pattern of wnt-related genes in control and TIE2-R849W group assessed by qRT-PCR. Asterisks denote statistically significant differences (*P<0.05; **P<0.001; ***P<0.0001; ns, not significant). (B) A schematic diagram for mutation-induced expression changes of wnt-related genes, pik3r2 and foxo1b. The quantity of arrows is consistent with statistical significance level, while not significant is indicated by a blue dash. (C) Heat map of 18 genes, which shows expression differences between control and mutant TIE2-R849W HUVECs.
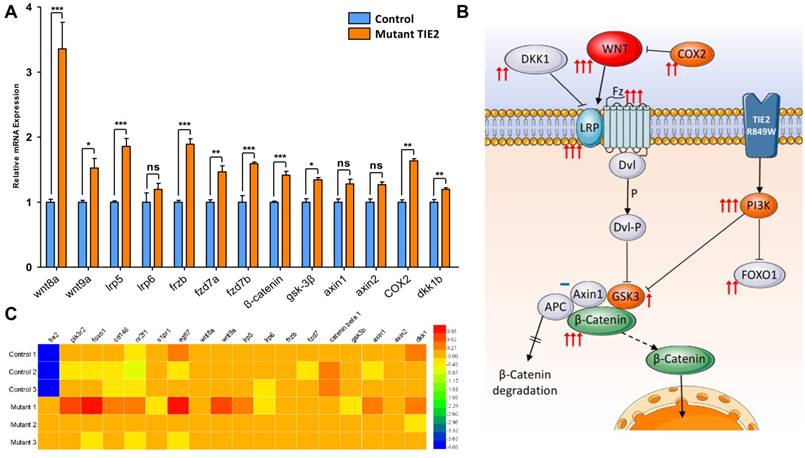
Wnt signaling pathway is one of the most important regulatory mechanisms in early developing stages of zebrafish. Multiple molecules participate in the Wnt pathway and occupy diverse positions; thus, different activation models may display different phenotypes. Wnt8a in zebrafish is one of the main Wnt pathway ligands, and a vital candidate involved in dorsal axis specification. When excess wnt8 mRNA was injected into zebrafish embryos, 55-79% of embryos failed to form normal dorsoanterior structures, develop either one or both eyes (reduced eye cup size and/or loss of lens), and lost the prominent midbrain-hindbrain boundary compared to uninjected embryos[26, 27]. As a terminal executant and core downstream molecule involved in the Wnt pathway, β-catenin is a cadherin-associated protein expressed maternally in zebrafish. Unphosphorylated β-catenin translocates into the nucleus, where it modulates target gene transcription by interacting with members of the TCF/LEF1 family of transcription factors. Unlike wnt8a, overexpression of β-catenin induces similar but unique dorsal axis specification, such as malformed head structure, ectopic eyes, well-developed lens and fused trunk midway[28]. For normal activation of β-catenin accompanied with Axin1 and adenomatous poliposis coli (APC), glycogen synthase kinase-3 (Gsk-3) is the most important suppression signaling molecule involved in the canonical Wnt pathway, inducing modification and degradation of β-catenin. The kinase dead mutant of gsk-3 or inhibition of Gsk-3β also mimics some phenotypes, including loss of eyes or reduction in their size and partial loss of forebrain, that arise from overexpression of wnt8 in zebrafish[28, 29]. Loss of telencephalon and eyes can also be induced by overexpression of mutant L399Q axin1 in wild-type embryos16,18. Thus, as a negative regulatory molecule, embryos in which dkk1 was ectopically expressed exhibited opposite phenotypes, including enlarged forebrain, eyes, and axial mesendoderm[20]. Coexpression of dkk1 with wnt8 suppressed the phenotypes elicited by the wnt8 plasmid[27, 29].
Any change in the Wnt signaling pathway could induce similar but slightly different phenotypes, including eye and forebrain defects. Until now, there also has been discussion about potential dialogue between TIE2 and WNT. ANGPT1-TIE2 stimulates the β-catenin-dependent inhibition of GSK-3β via PI3K/Akt, inducing Notch ligand Delta-like 4 (Dll4) up-regulation, which stimulates the extracellular deposition of collagen type IV[30]. In our study, overexpression of TIE2-R849W induced high level of Wnt8a and β-catenin such that even with relatively high dkk1 expression, it could not fully weaken the function of β-catenin and obvious activated state of the Wnt signaling pathway. However, more work is needed to uncover the exact mechanism by which TIE2-R849W triggers and affects this sophisticated process, especially the activation of wnt ligands and receptors. Another point is the conflict between data in vivo and gene array in vitro. This implies limitations of simple cellular assays and complex mechanisms beneath TIE2-R849W expression.
In summary, there are still unclear points to be uncovered in this research. Due to the vital role of tie2 in zebrafish, the lethality of transgenic zebrafish is an important obstacle for long-term observation and studies applied to mature tissues. Due to the tiny physique of early embryonic zebrafish, it is difficult to get satisfying and identical slice angles for the H-E stain process. Most importantly, lack of highly specific antibody for zebrafish protein further restricts related progress in mechanism research and verification, such that genetic studies are insufficient. Finally, there is still more work to do to identify the explicit regulatory relationship between TIE2-R849W and the molecules mentioned.
Conclusion
Transgenic expression of a venous malformation-related mutation, TIE2-R849W, significantly induces multiple malformations of zebrafish, including CVP defects, eye defects, malformed forebrain and abnormal jaw developmental extension. According to analysis on gene expression levels in vivo and in vitro, in this process, EGFL7 can be considered a potential reason for the CVP defect caused by TIE2-R849W, and the Wnt pathway also performs an important role in the TIE2-R849W related regulatory pathway as a key trigger for eye defects and jaw malformations. However, there is still more work needed to uncover more details and mechanisms.
Supplementary Material
Supplementary tables.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81371164).
Author Contributions
Yan-An Wang, Jia-Wei Zheng and Zhi-Yuan Zhang made substantial contributions to conception and design. Zhong Du and Hai-Long Ma analyzed experimental results. Zhong Du wrote the manuscript. All authors have given approval to the final version of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B. et al. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996;87:1181-90
2. Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Archives of dermatology. 2004;140:971-6
3. Natynki M, Kangas J, Miinalainen I, Sormunen R, Pietila R, Soblet J. et al. Common and specific effects of TIE2 mutations causing venous malformations. Human molecular genetics. 2015;24:6374-89
4. Yadav P, De Castro DK, Waner M, Meyer L, Fay A. Vascular anomalies of the head and neck: a review of genetics. Seminars in ophthalmology. 2013;28:257-66
5. Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB. et al. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet. 2009;41:118-24
6. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171-80
7. Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T. et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nature cell biology. 2008;10:513-26
8. Soblet J, Limaye N, Uebelhoer M, Boon LM, Vikkula M. Variable Somatic TIE2 Mutations in Half of Sporadic Venous Malformations. Molecular syndromology. 2013;4:179-83
9. Uebelhoer M, Natynki M, Kangas J, Mendola A, Nguyen HL, Soblet J. et al. Venous malformation-causative TIE2 mutations mediate an AKT-dependent decrease in PDGFB. Human molecular genetics. 2013;22:3438-48
10. Morris PN, Dunmore BJ, Brindle NP. Mutant Tie2 causing venous malformation signals through Shc. Biochemical and biophysical research communications. 2006;346:335-8
11. Huang YH, Wu MP, Pan SC, Su WC, Chen YW, Wu LW. STAT1 activation by venous malformations mutant Tie2-R849W antagonizes VEGF-A-mediated angiogenic response partly via reduced bFGF production. Angiogenesis. 2013;16:207-22
12. Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M. et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70-4
13. Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio). Eugene, OR: M. Westerfield. 2007
14. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental dynamics: an official publication of the American Association of Anatomists. 1995;203:253-310
15. Piotrowski T, Schilling TF, Brand M, Jiang YJ, Heisenberg CP, Beuchle D. et al. Jaw and branchial arch mutants in zebrafish II: anterior arches and cartilage differentiation. Development. 1996;123:345-56
16. Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Developmental biology. 2002;248:307-18
17. Barettino D, Feigenbutz M, Valcarcel R, Stunnenberg HG. Improved method for PCR-mediated site-directed mutagenesis. Nucleic acids research. 1994;22:541-2
18. Ito K, Yoshiura Y, Ototake M, Nakanishi T. Macrophage migration inhibitory factor (MIF) is essential for development of zebrafish, Danio rerio. Developmental and comparative immunology. 2008;32:664-72
19. Deng W, Wang Y, Liu Z, Cheng H, Xue Y. HemI: a toolkit for illustrating heatmaps. PloS one. 2014;9:e111988
20. Kashiwada T, Fukuhara S, Terai K, Tanaka T, Wakayama Y, Ando K. et al. beta-Catenin-dependent transcription is central to Bmp-mediated formation of venous vessels. Development. 2015;142:497-509
21. Wu BJ, Chiu CC, Chen CL, Wang WD, Wang JH, Wen ZH. et al. Nuclear receptor subfamily 2 group F member 1a (nr2f1a) is required for vascular development in zebrafish. PloS one. 2014;9:e105939
22. Mendelson K, Zygmunt T, Torres-Vazquez J, Evans T, Hla T. Sphingosine 1-phosphate receptor signaling regulates proper embryonic vascular patterning. The Journal of biological chemistry. 2013;288:2143-56
23. Nikolic I, Stankovic ND, Bicker F, Meister J, Braun H, Awwad K. et al. EGFL7 ligates alphavbeta3 integrin to enhance vessel formation. Blood. 2013;121:3041-50
24. Massimiani M, Vecchione L, Piccirilli D, Spitalieri P, Amati F, Salvi S. et al. Epidermal growth factor-like domain 7 promotes migration and invasion of human trophoblast cells through activation of MAPK, PI3K and NOTCH signaling pathways. Molecular human reproduction. 2015;21:435-51
25. Takeuchi K, Yanai R, Kumase F, Morizane Y, Suzuki J, Kayama M. et al. EGF-like-domain-7 is required for VEGF-induced Akt/ERK activation and vascular tube formation in an ex vivo angiogenesis assay. PloS one. 2014;9:e91849
26. Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development. 1995;121:1787-99
27. Nakamura Y, Weidinger G, Liang JO, Aquilina-Beck A, Tamai K, Moon RT. et al. The CCN family member Wisp3, mutant in progressive pseudorheumatoid dysplasia, modulates BMP and Wnt signaling. The Journal of clinical investigation. 2007;117:3075-86
28. Kelly GM, Erezyilmaz DF, Moon RT. Induction of a secondary embryonic axis in zebrafish occurs following the overexpression of beta-catenin. Mechanisms of development. 1995;53:261-73
29. van de Water S, van de Wetering M, Joore J, Esseling J, Bink R, Clevers H. et al. Ectopic Wnt signal determines the eyeless phenotype of zebrafish masterblind mutant. Development. 2001;128:3877-88
30. Zhang J, Fukuhara S, Sako K, Takenouchi T, Kitani H, Kume T. et al. Angiopoietin-1/Tie2 signal augments basal Notch signal controlling vascular quiescence by inducing delta-like 4 expression through AKT-mediated activation of beta-catenin. The Journal of biological chemistry. 2011;286:8055-66
31. Choi J, Mouillesseaux K, Wang Z, Fiji HD, Kinderman SS, Otto GW. et al. Aplexone targets the HMG-CoA reductase pathway and differentially regulates arteriovenous angiogenesis. Development. 2011;138:1173-81
32. Kim JD, Kang H, Larrivee B, Lee MY, Mettlen M, Schmid SL. et al. Context-dependent proangiogenic function of bone morphogenetic protein signaling is mediated by disabled homolog 2. Developmental cell. 2012;23:441-8
Author contact
![]() Corresponding author: Yan-An Wang, (Email: wangyan_ancom)
Corresponding author: Yan-An Wang, (Email: wangyan_ancom)

 Global reach, higher impact
Global reach, higher impact