3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2017; 14(13):1317-1326. doi:10.7150/ijms.20984 This issue Cite
Research Paper
Ulcer Prevention Effect Of 3,4,5-Tihydroxy-N0-[(2-Methyl-1H-Indol-3yl)Methylidene]Benzohydrazide In HCl/Ethanol-Induced Gastric Mucosal Damage In Rats
1. Institute of Biological Sciences, Faculty of Science, University of Malaya;
2. Department of Chemistry, University of Malaya, Kuala Lumpur, Malaysia;
3. Department of Biomedical Science, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia;
4. Department of Obstetrics & Gynecology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia.
Received 2017-5-11; Accepted 2017-8-7; Published 2017-10-15
Abstract
The newly synthesized, 3,4,5-Trihydroxy-N 0-[(2-methyl-1H-indol-3-yl)-methylidene] benzohydrazide (TIBH), is an indole and gallic acid derivative. The aim of this research investigation was to evaluate the acute toxicity and the ulcer prevention potential of TIBH in HCl/Ethanol-induced gastric ulcer rat model. Six groups of rats were orally received 5ml/kg of vehicle (1 % Carboxy methyl cellulose) for the normal and ulcer control groups each, Omeprazole (20mg/kg) for positive control, 50 mg/kg, 100 mg/kg and 200 mg/kg of TIBH for experimental groups, respectively. After one hour, instead of rats in the normal group which received 5ml/kg of 1% CMC, other groups received 5ml/kg of HCl/Ethanol. All rats were sacrificed after one additional hour. Gastric juice, gastric mucosa, morphologies of gastric ulcers and protein expressions of both control and treatment groups were evaluated. TIBH showed a ulcer prevention potential by increase of the mucus secretion, decrease of the gastric acidity, up-regulation of HSP70 protein, down-regulation of Bax protein, decrease of the lipid peroxidation and the increase of the Superoxide dismutase (SOD) activity in gastric tissue homogenate. Acute toxicity assay exposed valuable information on the safety of this compound. TIBH had a dose dependent ulcer prevention potential against HCl/Ethanol-triggered gastric ulcer.
Keywords: Gastric ulcer, Gastro-protective activity, Acute toxicity, Immunostaining.
Introduction
Peptic ulcer occurs in more than 10% of world population [1]. It is one of the most important ailments of gastrointestinal tract in the world and it becomes a global problem due to its increasing morbidity and mortality [2]. Peptic ulcer defined as a mucosal damage with a length of more than 5 mm which is caused due to the demolition of the balance between defensive and aggressive factors. Defensive factors are like mucus and bicarbonate production while aggressive factors are caused by Helicobacter pylori, non-steroidal anti-inflammation drugs (NSID), alcohol consumption, overproduction of acid and pepsin, age-related decline of the prostaglandin level, stress and smoking [3].
Currently, researchers are studying on this common pathology and many drugs are available for instance agonists of the histamine H2 receptor (Ranitidine) and the irreversible proton pump inhibitors (Omeprazole) [2]. Conversely, sometimes anti-acid drugs are ineffective and weaken the absorption of calcium, iron, magnesium and vitamin B12 [4]. Thus, medicinal plants and synthetic compounds that can contribute in ulcer healing and in the prevention of the ulcer reoccurrence have been used as alternative treatments [3].
In former studies, numerous synthesized chemical compounds were discovered to have biological properties together with gastric ulcer prevention potential [5-12]. TIBH, 3,4,5-Trihydroxy-N 0-[(2-methyl-1H-indol-3-yl)-methylidene]benzohydrazide, is synthesized as a result of the reaction of the proper indole carboxaldehydes with gallic hydrazide. This compound was found to exhibit significant DPPH radical scavenging potential and also possessed inhibitory effect against lipid peroxidation [13]. The current study was carried out to determine the acute toxicity and ulcer prevention potential of TIBH against HCl/Ethanol induced gastric ulcer in rats.
Methods
Omeprazole
Omeprazole, as an anti-ulcer medicine and positive control was purchased from the University of Malaya, Medical Center. Omeprazole is a proton pump inhibitor drug that has being employed for the treatment of peptic ulcer ailment. It acts as enzymes inhibitor and stops the stomach from producing excessive acid that can harm the gastric wall. Omeprazole was dissolved in 1% w/v carboxymethyl cellulose (CMC) and administered orally to the rats in a dosage of 20 mg/kg body weight (5 mL/kg) (14).
TIBH
The TIBH was synthesized at Chemistry Department, Faculty of Science, University of Malaya. Microanalyses were performed on a Perkin-Elmer 2400 elemental analyzer. 1H-NMR and 13C-NMR spectra were evaluated with a Lambda JEOL 400 MHz FT-NMR (1H-NMR: 400 MHz and 13C-NMR: 100.4 MHz) spectrometer. TIBH is a newly synthesized compound by [13].
Carboxymethyl cellulose
Carboxymethyl cellulose (CMC) (Sigma Aldrich, Germany) was used as a vehicle and solvent for omeprazole and TIBH.
Animals
Adult (6 to 8 weeks) healthy Sprague-Dawley (SD) rats weighing 200-250 g were [14] purchased from the Animal Experimental Unit, Faculty of Medicine, University of Malaya. Female and male rats were respectively used for acute toxicity and ulcer prevention studies. The use of animals in this research study was approved by Ethics committee for Animal experiment of the Faculty of Medicine, University of Malaya, Malaysia (Ethic No. 2015-181201/BMS/R/MAAH) and the study was conducted according to the National Academy of Science's Guide for the Care and Use of Laboratory Animals [15].
Acute toxicity study
Healthy females SD rats (n= 18) were allotted equally between three groups (6 rats/group) and categorized as the vehicle (1% CMC) and 300 and 2000mg/kg of TIBH, respectively [16]. The rodents were fasted overnight (food but not water supply) before treatment. After feeding of the TIBH the animals were retained under monitoring for 30 minutes, 2, 4, 24, and 48 hours, for any toxicological or clinical signs. After 15 days, blood samples of all animals were taken and the animals were euthanized using an overdose of ketamine and xylazine anesthesia. Hematological evaluation of renal and liver function parameters were done by Clinical Diagnostic Laboratory of University Malaya. The organs (kidney and liver) were evaluated macroscopically and histologically [17].
Treatment and induction of gastric ulcer with HCl/Ethanol
The experiment was designed having 6 groups while 6 rats were randomly allotted to each group. The rats were fasted for 24 hours before the oral gavage pre-treatment (food but not water). Water supplement had been removed two hours before oral pre-treatment started. HCl/Ethanol-induced ulcer generation was conducted according to the model that previously described [18]. Accordingly rats in experimental groups were received 50, 100 and 200 mg/kg of TIBH, normal control group and ulcer control group rats were orally received 5 ml/kg of 1% CMC each and rats of positive control group were fed with 20 mg/kg of Omeprazole. After sixty minutes, rats of normal group were orally fed with 5 ml/kg of 1% CMC while 5ml/kg of 150 mM (HCl/absolute ethanol) 40:60 v/v were orally fed to the ulcer control, positive control and experimental groups.
After additional hour, all rats were sacrificed by overdose of ketamine and xylazine (150 and 15 mg/kg) [19]. The pyloric and the cardiac ends of the stomach were knotted. The stomach was removed and kept into cold phosphate buffer saline (PBS) for further experiments.
Evaluation of gastric juice acidity and gastric mucus
The gastric juice content of rat's stomachs were collected and centrifuged at 4000 rpm, 25°C for 10 min. The acidity of the resulted supernatant was measured with a digital pH meter that was titrated with 0.1 N NaOH solution. To measure the mucus secretion, the stomach was cut along the greater curvature, then the glandular portion of the stomach was lightly scrapped by a glass slide and the mucus was collected and weighed [20, 21].
Evaluation of stomach morphology
The stomach was cut along its greater curvature, distended over a clean and white background for better visualization and photographed. Ulcers showed as elongated bands of hemorrhagic lesions parallel to the long curve of the stomach. The ulcer area on the gastric mucosa was measured using Image J (1.50i). The inhibition percentage of ulcer area (I %) was determined using the subsequent formula where UA(mm2) was the measured ulcer area [3].
(I %) = [(UA control - UA treated) / UA control] × 100%
Evaluation of the gastric ulcer
Evaluation of gastric tissue homogenate
Finally the glandular portion of gastric tissue specimens of each rat's stomach was divided into two halves. The first half of the stomach was kept in PBS for tissue homogenate.
Evaluation of histology of gastric tissue
The other half was placed in the cassettes and was fixed in 10% buffered formalin. Later, these second halves of the stomach were processed by tissue-processing machine (Leica, Germany). Tissue embedding, sectioning and staining were performed for all of the tissue samples.
Hematoxylin and Eosin (H&E) staining
Sections of 5μm was stained with H&E dye to evaluate the tissue structure [10]. Two dyes, Hematoxylin (basic dye) and Eosin (acidic dye) were used for the detection of the nucleus and the cytoplasmic inclusions, respectively [22].
PAS staining
The sections of the 5μm thick glandular portion of the rat stomach was stained with Periodic acid-Schiff (PAS) as described [12].
Evaluation of Bax and HSP70 using Immunostaining
Tissue sections were heated at 60oC in a hot-air oven for 25 min (Venticell, MMM, Einrichtungen, Germany). The 5 µm gastric tissue sections were washed with xylene to remove the paraffin. They were then rehydrated by graded ethanol. Antigen recovery process was done using 10 mM of boiled sodium citrate buffer. Immunostaining was accomplished consistent with manufacturer's protocol (Dakocytomation, USA).
Evaluation of the Lipid peroxidation and Superoxide dismutase (SOD) activity
The first half of each stomach glandular portion was sliced into nearly 200 mg for tissue homogenate. One gram of tissue in 8 ml of phosphate-buffered saline (PBS) was homogenized using a Teflon homogenizer (Polytron, Heidolph RZR 1, Germany). The mixture then was centrifuged at 4,500 rpm for 15 min at 4oC. The resulted supernatant was divided into aliquots and retained at -80oC. Subsequently, the supernatant was used for the evaluation of the lipid peroxidation and SOD enzymatic assay using commercial kits. All processes were preceded in accordance to the manufacture's instruction.
Lipid peroxidation is the degradation of lipids happens due to the cellular injuries. It is an indicator of oxidative stress. Malondialdehyde (MDA) as a lipid peroxidation end product of the polyunsaturated lipids of the ulcer tissue was determined using a Cayman's TBARS assay kit.
Superoxide Dismutase is a mettalloenzyme catalyse the deactivation of the superoxide anions (O2) to molecular oxygen (O2) and hydrogen peroxide (H2O2) so it possesses a very vital function in the system of antioxidant defence. Activity of the superoxide dismutase (SOD) in the supernatant of all the gastric tissue homogenates was measured using a Cayman's assay kit as stated by the kit's instructions.
Statistical analysis
Values were expressed as mean ± SD. The statistical significance was obtained by comparing the control with experimental groups using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test using IBM SPSS statistics 20 software. Data obtained from the TIBH pre-treated animals were compared with the data obtained from the ulcer control animals.
Results
TIBH had no acute toxicity
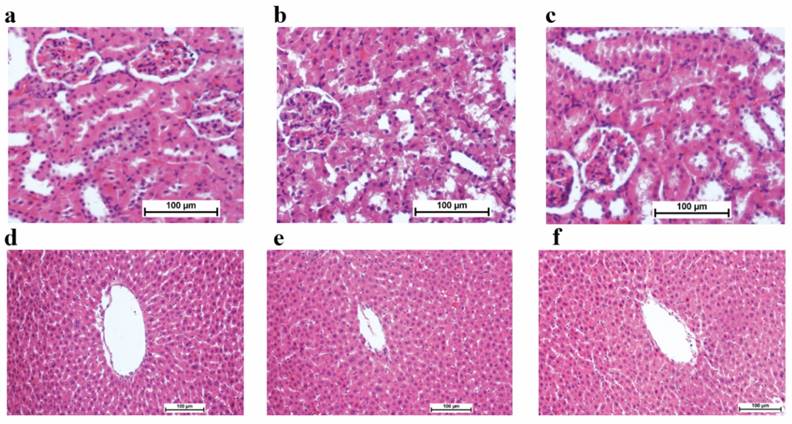
No significant abnormality, toxicity or death was observed among the experimental groups upon the oral gavage of 300 and 2000 mg/kg of TIBH in 0.1% CMC during 15 days of the experiment. Histological analysis of liver and kidney showed no hepatotoxicity or nephrotoxicity of the rats received 300 and 2000 mg/kg of the TIBH compared to the control group (Figure 1). Liver and renal function hematological parameters analysis did not reveal any significant differences among groups (Table 1).
Renal and liver sections of rats in acute toxicity study (H&E staining, 20x). a) Normal rat renal tissue; b) 300 mg/kg TIBH treated rat renal tissue, c) 2000 mg/kg TIBH treated rat renal tissue; d) Normal rat hepatic tissue; e) 300 mg/kg TIBH treated rat hepatic tissue; f) 2000 mg/kg TIBH treated rat hepatic tissue; No significant differences was observed in the analysed kidney and liver tissues between TIBH pre-treated and control groups (20× magnifications).
Effects of TIBH on Renal and liver function parameters in acute toxicity test in rats
| kidney function parameters in acute toxicity test in rats | ||||||||||
| Animal groups | Sodium (mM/L) | Potassium (mM/L) | Chloride (mM/L) | Carbon dioxide(mM/L) | Urea (mM/L) | Creatinine (µM/L) | ||||
| Normal control | 141.32 ± 0.33 | 5.40 ± 0.61 | 101.45 ± 0.88 | 31.67 ± 0.88 | 8.1 ± 0.68 | 27.67 ± 1.43 | ||||
| TIBH (300 mg/kg) | 142.56 ± 0.37 | 5.43 ± 0.26 | 102.98 ± 0.33 | 33.20 ± 0.51 | 7.41 ± 0.73 | 27.33 ± 0.67 | ||||
| TIBH (2000mg/kg) | 143.09 ± 0.51 | 5.5 ± 0.65 | 103.75 ± 0.88 | 32.05 ± 0.65 | 9.3 ± 0.47 | 28.58 ± 1.0 | ||||
| Liver function parameters in acute toxicity test in rats | ||||||||||
| Animal groups | Albumin (g/L) | Total bilirubin (µM/L) | Alkaline phosphatase (IU/L) | Alanine amino Transaminase (IU/L) | G-Glutaml. Transferase (IU/L) | |||||
| Normal control | 40.69 ± 0.88 | < 2 | 232 ± 9.87 | 64.25 ± 7.8 | -1.3 ± 0.6 | |||||
| TIBH (300 mg/kg) | 43.35 ± 1.2 | < 2 | 191 ± 12.73 | 65.67 ± 5.4 | -2.1 ± 0.2 | |||||
| TIBH (2000mg/kg) | 45.61 ± 1.45 | < 2 | 205 ± 10.35 | 68.55 ± 8.68 | -2.6 ± 0.5 | |||||
All values are expressed as mean ± SD (n = 6). * Shows the significant value was set at p < 0.05, compared with control group.
Evaluation of gastric juice acidity and gastric mucus
Administration of the HCl/Ethanol increased the gastric acidity in ulcer control group comparing to the normal control group. Gastric acidity reduced significantly in rats pre-fed with TIBH compared with the rats of the ulcer control group and the resulted pH was comparable for the omeprazole pre-treated rats (Table 2). The gastric mucus was significantly increased in the TIBH high dose and medium dose pretreated rats compared to the ulcer control rats (Table 2).
TIBH caused a significant reduction in ulcer area
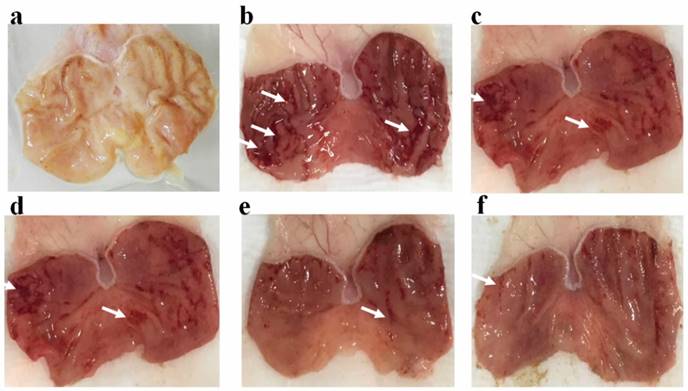
The macroscopic appearance of the stomach in TIBH pre-treated groups and control groups are shown in Figure 2. Ulcer area and the inhibition percentage of all groups are presented in Table 2. The rats pre-fed with the TIBH showed a significant reduction in ulcer area when compared to the ulcer control. Pretreatment of rats with the TIBH diminished the ulcer area generation which was comparable to the supportive effect of omeprazole.
TIBH displayed a gastric mucosal protection
H & E staining
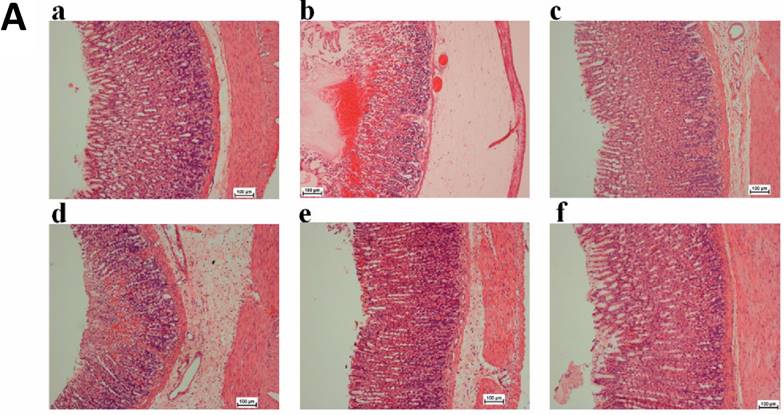
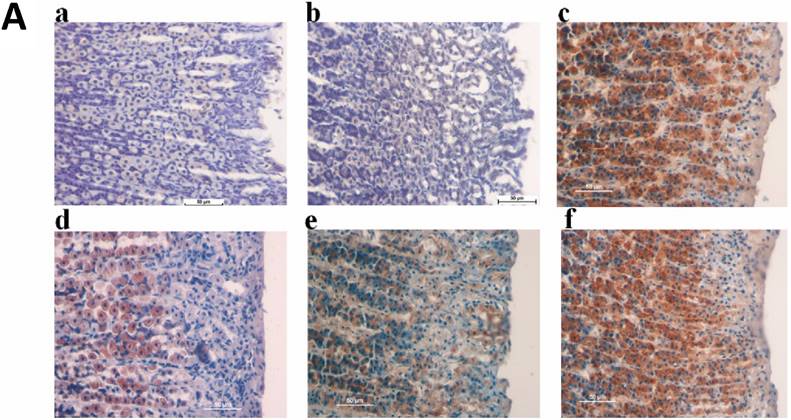
Microscopic analysis of the H&E stained gastric tissues revealed that ulcer control group possessed substantial injuries of the gastric mucosa and in some areas gastric submucosa layer was associated with leucocytes infiltration and severe edema comparing to the TIBH pre-treated groups. TIBH pretreated groups displayed a gastric mucosal protection and a decline in edema and leucocytes infiltration of the submucosal layer (Figure 3A).
TIBH increased gastric mucosal glycoproteins
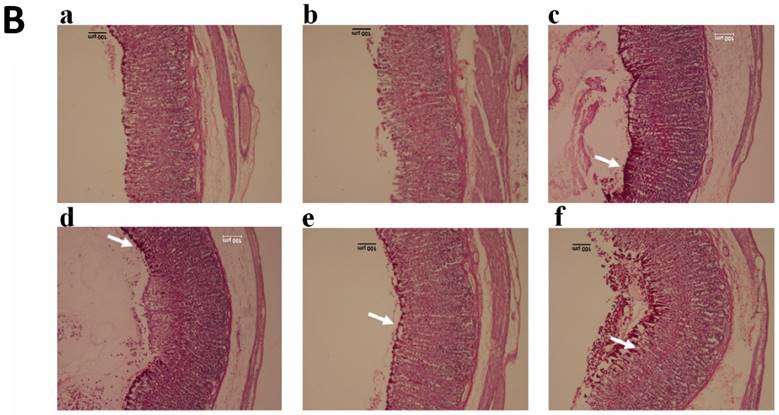
There was an increase in PAS staining of the gastric mucosa in rats pretreated with TIBH compared with the rats of ulcer control group, which specified an increase in the glycoprotein content of the gastric mucosa (Figure 3B) and indicated that TIBH increases gastric mucus secretion.
Expression of HSP70 protein in gastric tissue
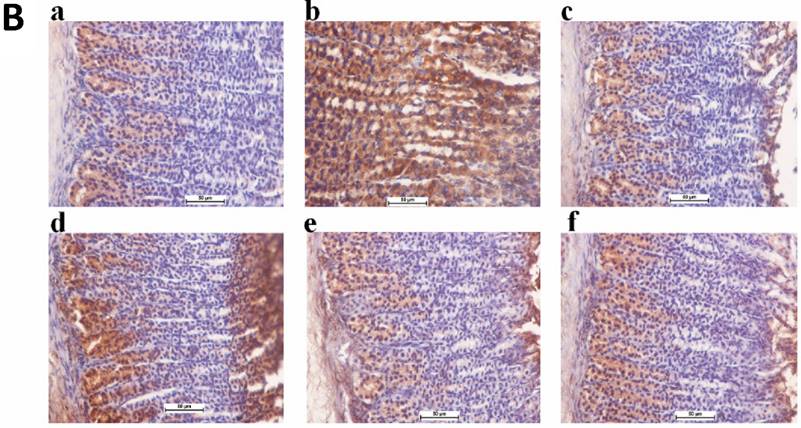
Immunostaining results indicated that rats fed with TIBH had over-expression of HSP70 protein compared to ulcer control group (Figure 4A).
Expression of Bax protein in gastric tissue
Immunohistochemical staining of Bax protein demonstrated that rats pre-treated with TIBH had a decrease in the expression of Bax protein (Figure 4B).
TIBH effects on SOD enzymatic activity and lipid peroxidation
The MDA level in the gastric tissues homogenate of the TIBH pre-treated groups was significantly decreased compared to the ulcer control group (Table 2). SOD activity was significantly enhanced in the TIBH pre-treated groups in comparison to ulcer control (Table 2).
Discussion
The results of the toxicity test of this study indicated that the TIBH was not toxic upon the oral administration of 2000 mg/kg. TIBH is a derivative of an indole and gallic hydrazide (a gallic acid derivative). A former study showed gallic acid is not toxic upon the oral administration of 5000 mg/kg (body weight) of the mice [23]. However indole derivatives have exhibited wide range of toxicity and have showed various LD50 from 1 up to 5000 mg/kg and above [14, 24, 25]. Our results showed that TIBH LD50 was greater than 2g/kg. TIBH used dosage for ulcer prevention was far lesser than the TIBH toxic dosage [26, 27].
Effects of the TIBH on gastric pH, mucus secretion, ulcer area, MDA level and SOD activity
| Animal Groups | Pre-treatment (5ml/kg) | Gastric pH | Mucus Weight (g) | Ulcer area (mm2) | Inhibition (%) | MDA (nmol/ml) | SOD (U/µl) |
|---|---|---|---|---|---|---|---|
| Normal control | 1% CMC | 5.8 ± 0.2 | 0.89 ± 0.03 | 0 | 0 | 17.43 ± 0.41 | 352 ± 9.15 |
| Ulcer control | 1% CMC | 3.2 ± 0.25 | 0.41 ± 0.01 | 246.4 ± 35 | 0 | 43.59 ± 0.83 | 222 ± 6.7 |
| Positive control | Omeprazole (20mg/kg) | 7.2 ± 0.22* | 0.7 ± 0.05* | 31.6 ± 4* | 87.2 | 32.85 ± 0.76* | 321 ± 8.1* |
| TIBH LD | TIBH (50mg/kg) | 6.4 ± 0.24 | 0.46 ± 0.02 | 111.2 ± 9* | 54.9 | 40.20 ± 0.93 | 230 ± 7.25 |
| TIBH MD | TIBH (100mg/kg) | 6.8 ± 0.31* | 0.68 ± 0.04* | 42.0 ± 2* | 83 | 30.80 ± 0.93* | 280 ± 5.1* |
| TIBH HD | TIBH (200mg/kg) | 7.2 ± 0.35* | 0.77 ± 0.06* | 24.4 ± 2* | 90.1 | 29.26 ± 0.49* | 306 ± 7.2* |
All values are expressed as mean ± SD (n = 6). * Shows the significant value was set at p < 0.05, compared with ulcer control group.
Effect of TIBH on macroscopic morphology of the rat's stomach in the HCl/ethanol-induced ulcer in the experimental groups (n = 6). a) Normal control group did not display any lesions in the gastric mucus membrane; b) in ulcer control group, strong ulceration was generated in the stomach; c) Positive control group (Omeprazole, 20 mg/kg) displayed minor gastric damages; d) low dose group pre-treated with 50 mg/kg of the TIBH also displayed moderate gastric damages; e) Medium dose and f)High dose pretreated groups with respectively 100 and 200 mg/kg of the TIBH revealed decrease of the gastric damages in comparison with the ulcer control. White arrows show the example of ulceration.
A. Histological analysis of gastric tissues obtained from TIBH pre-treated groups and control groups using H&E staining 10x. Histopathology of gastric tissue of 3a) Normal control group; 3b) Ulcer control group; 3c) Positive control (Omeprazole); 3d, 3e, 3f) TIBH low dose, medium dose and high dose pre-treated groups, respectively. B. Histological analysis of gastric tissues obtained from TIBH pre-treated groups and control groups using PAS staining 10×. Histopathology of gastric tissue of 3a) Normal control group; 3b) Ulcer control group; 3c) Positive control (Omeprazole); 3d, 3e, 3f) TIBH low dose, medium dose and high dose pre-treated groups, respectively. Medium (3e) and high dose (3f) TIBH pre-treated groups showed increment of magenta color (white arrow) compared to ulcer control groups (3b) which indicated the increase of the secreted mucus by gastric glands due to TIBH pre-treatment.
Ulcer model of this study was generated using the HCl/Ethanol. The effects of ethanol on the gastric mucus membrane are damaging and dose-dependent with higher concentrations being more harmful. The injury occurs 30 minutes after the intake and is maximized at about 60 minutes later [28]. Acidification of ethanol enhanced the severity of the development of the gastric lesions compared with equivalent concentrations without HCI. HCl/Ethanol-induced peptic ulcer has been frequently used as a simulated model of peptic ulcer for the evaluation of the gastro-protective and ulcer treatment potential of the various chemicals [11, 29, 30]. The harmful effect of the HCl on gastric mucosa has been known since years ago [31]. Ethanol on the other hand rapidly penetrates to submucosa, increases the generation of the reactive oxygen species (ROS) and decreases the mucus membrane secretion which together cause the damage of the gastric mucosal cells [7, 32]. Ethanol also inhibits cyclooxygenase and suppresses the secretion of prostaglandins from the non-parietal gastric epithelial cells [21, 33, 34]. Our study showed that administration of the TIBH significantly protects the gastric epithelium and suppresses the deleterious effects of the ethanol on the rat stomach gastric mucosa. This finding is consistent with previous studies [11, 35, 36]. We found that TIBH pre-treatment decreased the HCl/Ethanol produced ulcer area. The deduction of the damaged area caused by TIBH was comparable with healing effect of Omeprazole. Results derived from the macroscopic evaluation of the stomachs supported the protective effect of the TIBH against peptic ulcer and they were in agreement with the results of the previous studies [11, 24, 37].
A. Expression of HSP70 proteins in the gastric tissue obtained from TIBH pre-treated groups and control groups. 4a) Normal control group; 4b) Ulcer control group; 4c) Positive control group (Omeprazole); 4d) Low dose TIBH pre-treated group; 4e) Medium dose TIBH pre-treated group; 4f) High dose TIBH pre-treated group. Positive control, TIBH high dose and medium dose pre-treated rats show the over-expression of HSP70 protein (HSP70 stain 20x). B. Expression of Bax proteins in the gastric tissue obtained from TIBH pre-treated groups and control groups. 4a) Normal control group; 4b) Ulcer control group; 4c) Positive control group (Omeprazole); 4d) Low dose TIBH pre-treated group; 4e) Medium dose TIBH pre-treated group; 4f) High dose TIBH pre-treated group. Ulcer control group animals show Bax over-expression (brown color) while in positive control, TIBH high dose and medium dose pretreated rats, Bax expression decreased (Bax stain 20x).
Our results also presented that the oral administration of the TIBH before HCl/Ethanol administration significantly decreased the gastric acidity of the pre-treated groups compared to the ulcer control group. The capability to decrease the gastric acidity is considered to be a support for the treatment of the gastric ulceration and it has improved the management of peptic ulcer disease [38]. Omeprazole as a standard drug for peptic ulcer has shown healing effects against peptic ulcer due to its ability to minimize the degree of gastric acidity through the inhibition of the proton pumps in the parietal cells of the stomach [6, 39]. TIBH high dose pretreatment had a comparable effect with the Omeprazole pretreatment [10, 22, 40, 41].
Mucus is a protective gel layer attached to the mucosal surface. When the physiological conditions are normal, the mucus and bicarbonate defense mechanism is adequate to protect the gastric mucosa in contrast to acid and pepsin secretion. However, a number of agents such as ethanol and HCl can reduce the gastric mucus content and damage the gastric mucosa [36, 42].In our study, the mucus content of the stomach increased in TIBH medium and high dose pre-treated groups compared to ulcer control group. PAS staining was also used to localize and measure the degree of mucus secretion as a histological method. The Glycol functional groups presented in the mucus were oxidized by periodic acid into dialdehydes which on reaction with Schiff's reagent gave an insoluble purple magenta reagent [22, 43]. Increase of the magenta color of stomach tissue in the high dose and medium dose TIBH pretreated groups compared to the control group indicated that the mucus secretion increased due to TIBH pretreatment. The PAS staining histology and the mucus content evaluation were in agree with each other and both confirmed the promoting effect of the TIBH on mucus formation as a barrier against HCl/Ethanol triggered mucosal necrotic damages. Former investigators showed similar results for mucus production promoting effect of other chemicals [10, 20, 36, 40].
Oxidative stress contributes to the formation of numerous syndromes such as peptic ulcers and gastric carcinoma [33]. Ethanol metabolism in the stomach releases hydroxyl and superoxide radicals. Former investigations have confirmed that lipid peroxidation is involved in the occurrence of acute gastric damages triggered by ethanol [12, 21, 36]. A previous in vitro study showed that the TIBH has an inhibitory effect on lipid peroxidation [13]. The results also specified that the gastro-protective effects of the TIBH on the HCl/Ethanol induced ulcer model in the rat is correlated with its ability to decrease the lipid peroxidation. Studies showed that the ethanol exert a destructive effect on the rat gastric mucosa by the generation of ROS resulting in the lipid peroxidation and decrease of the SOD activity [10, 44]. Excessively produced superoxide damages the surrounding tissues. Sufficient amount of SOD in the cells and tissues keeps the superoxide anions at a very low concentration [45, 46]. A study demonstrated that the administration of the SOD to the rats could protect their gastric mucosa against the induced injury by ethanol [47]. Other studies showed that the SOD activity of the gastric tissue increased due to the exposure to various chemicals [5, 10, 36]. In this study, SOD activity was significantly decreased due to HCl/Ethanol administration. However, TIBH medium dose and high dose pretreatment could reverse the detrimental effects of the HCl/Ethanol on the gastric mucosa. Our study determined that the TIBH pretreatment has gastro-protective potential against the ROS induced gastric damages which is resulted from the HCl/Ethanol administration.
HCl/Ethanol administration resulted in the increase of the haemorrhagic ulcer, gastric submucosal oedema and infiltrated leucocytes [11, 20]. Hemorrhagic ulcer occurs due to the mucosal vasodilation as a result of endothelin-1 release into the blood under the effect of the ethanol. Furthermore, ethanol produces apoptosis that induces the cell death [48]. It also destroys the blood vessel resulting in the increased vascular permeability, the edema formation and the epithelial cell loss. Hemorrhagic ulcer, epithelial cell loss, submucosal edema and infiltrated leucocytes were observed in the histopathological evaluation of the stomach tissues. Our results revealed that all of the symptoms were efficiently suppressed by TIBH medium and high dose pre-treatment as it was shown by former investigations [7-9, 26].
HSP70 protein is expressed in mammalian cells and belongs to the family HSP proteins. It protects the cells by decreasing intracellular protein denaturation induced by oxidative stress or heat shock. This 70 kDa protein is a highly conserved and richly formed protein in the occurrence various forms of stress like heat, toxic substances, infection and proliferation [49]. Bax stimulates apoptosis, while BCL-2 prevents it. Apoptosis happens due to the imbalance of the expression of BCL-2 family anti-apoptotic proteins and apoptotic Bax proteins [50, 51]. Ethanol creates reactive oxygen species (ROS) which down regulates the HSP70 and up-regulates the Bax. Bax and HSP70 proteins are accountable to support intercellular homeostatic mechanism. They inhibit physiologic damages by conserving the structure of other normal proteins and by repairing or eliminating impaired proteins. Up-regulation of HSP70 proteins and down-regulation of Bax proteins resulted in this research study suggested these regulations as gastro-protective mechanism of the TIBH against ethanol-induced hemorrhagic of gastric mucosal membranes in rat. The obtained results of the present investigation confirmed the previous results on the protection of the mucosal damages through the regulations of the Bax and HSP70 proteins [11, 29] and showed that TIBH has a potential role in prevention of gastric ulcer by regulating these proteins.
Conclusion
TIBH is a safe compound in rats upon 2000 mg/kg oral administration. TIBH had a dose dependent ulcer prevention potential against HCl/Ethanol-triggered gastric ulcer which could be due to the increase of the mucosal secretion, decrease of the gastric acidity, up-regulation of HSP70 protein, down-regulation of Bax protein, decrease of the lipid peroxidation and the increase of the superoxide dismutase activity in gastric homogenate. Therefore, TIBH would be a useful gastric ulcer preventive compound, but future studies in human and clinical practice are needed.
Acknowledgements
The authors would like to thank the University of Malaya for supporting this project grants PG119-2015B and UMRG grants (RP021A-14AFR).The authors declare that they have no conflict of interest.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ishida K, Kojima R, Tsuboi M, Tsuda Y, Ito M. Effects of artichoke leaf extract on acute gastric mucosal injury in rats. Biological and Pharmaceutical Bulletin. 2010;33:223-9
2. Martins JLR, Rodrigues ORL, da Silva DM, Galdino PM, de Paula JR, Romão W. et al. Mechanisms involved in the gastroprotective activity of Celtis iguanaea (Jacq.) Sargent on gastric lesions in mice. Journal of ethnopharmacology. 2014;155:1616-24
3. Abdelwahab SI, Taha MME, Abdulla MA, Nordin N, Hadi AHA, Mohan S. et al. Gastroprotective mechanism of Bauhinia thonningii Schum. J of Ethnopharmacol. 2013;148:277-86
4. Ham M, Kaunitz JD. Gastroduodenal mucosal defence. Current opinion in Gastroenterol. 2008;24:665-73
5. Dhiyaaldeen SM, Amin ZA, Darvish PH, Mustafa IF, Jamil MM, Rouhollahi E. et al. Protective effects of (1-(4-hydroxy-phenyl)-3-m-tolyl-propenone chalcone in indomethacin-induced gastric erosive damage in rats. BMC Vet Res. 2014;10:1
6. Golbabapour S, Gwaram NS, Hassandarvish P, Hajrezaie M, Kamalidehghan B, Abdulla MA. et al. Gastroprotection studies of Schiff base zinc (II) derivative complex against acute superficial hemorrhagic mucosal lesions in rats. PloS One. 2013;8:e75036
7. Gwaram NS, Musalam L, Ali HM, Abdulla MA. Synthesis of 2'-(5-Chloro-2-Hydroxybenzylidene) benzenesulfanohydrazide Schiff base and its anti-ulcer activity in ethanol-induced gastric mucosal lesions in rats. Trop J Pharmaceut Res. 2012;11:251-7
8. Hajrezaie M, Golbabapour S, Hassandarvish P, Gwaram NS, Hadi AHA, Ali HM. et al. Acute toxicity and gastroprotection studies of a new schiff base derived copper (II) complex against ethanol-induced acute gastric lesions in rats. PloS One. 2012;7:e51537
9. Halabi MF, Shakir RM, Bardi DA, Al-Wajeeh NS, Ablat A, Hassandarvish P. et al. Gastroprotective activity of ethyl-4-[(3, 5-di-tert-butyl-2-hydroxybenzylidene) amino] benzoate against ethanol-induced gastric mucosal ulcer in rats. PloS One. 2014;9:e95908
10. Nazarbahjat N, Kadir FA, Ariffin A, Abdulla MA, Abdullah Z, Yehye WA. Antioxidant Properties and Gastroprotective Effects of 2-(Ethylthio) Benzohydrazones on Ethanol-Induced Acute Gastric Mucosal Lesions in Rats. PloS One. 2016;11:e0156022
11. Salama SM, Gwaram NS, AlRashdi AS, Khalifa SA, Abdulla MA, Ali HM. et al. A Zinc Morpholine Complex Prevents HCl/Ethanol-Induced Gastric Ulcers in a Rat Model. Scientific Reports. 2016:6
12. Salga MS, Ali HM, Abdulla MA, Abdelwahab SI. Gastroprotective activity and mechanism of novel dichlorido-zinc (II)-4-(2-(5-methoxybenzylideneamino) ethyl) piperazin-1-iumphenolate complex on ethanol-induced gastric ulceration. Chemico-Biological iInteractions. 2012;195:144-53
13. Khaledi H, Alhadi AA, Yehye WA, Ali HM, Abdulla MA, Hassandarvish P. Antioxidant, Cytotoxic Activities, and Structure-Activity Relationship of Gallic Acid-based Indole Derivatives. Arch Pharm. 2011;344:703-9
14. Health NIo. Guide for the care and use of laboratory animals; 1978.
15. Pahari N, Saha D, Jain VK, Jain B, Mridha D. Synthesis and evaluation of acute toxicity studies and analgesic characters of some novel indole derivatives. Int J Pharma Sci Res. 2010;1:399-408
16. Fard AA, Hajrezaie M, Kadir FA, Sefideh FA, Salama SM, Al-Najar ZA. et al. The Effects of Combined Adiponectin-Metformin on Glucose and Lipids Levels in Mice and Acute Toxicity and Anti-Ulcerogenic Activity of Adiponectin Against Ethanol-Induced Gastric Mucosal Injuries in Rat. Molecules. 2011;16:9534-52
17. Oecd. OECD Guidelines for the Testing of Chemicals: Organization for Economic; 1994.
18. Mizui T, Doteuchi M. Effect of polyamines on acidified ethanol-induced gastric lesions in rats. The Japanese J of Pharmacol. 1983;33:939-45
19. Wong J-Y, Abdulla MA, Raman J, Phan C-W, Kuppusamy UR, Golbabapour S. et al. Gastroprotective effects of Lion's Mane mushroom Hericium erinaceus (Bull.: Fr.) Pers.(Aphyllophoromycetideae) extract against ethanol-induced ulcer in rats. Evidence-Based Complementary and Alternative Medicine. 2013. 2013
20. Nordin N, Salama SM, Golbabapour S, Hajrezaie M, Hassandarvish P, Kamalidehghan B. et al. Anti-ulcerogenic effect of methanolic extracts from Enicosanthellum pulchrum (King) Heusden against ethanol-induced acute gastric lesion in animal models. PloS One. 2014;9:e111925
21. Rouhollahi E, Moghadamtousi SZ, Hamdi OAA, Fadaeinasab M, Hajrezaie M, Awang K. et al. Evaluation of acute toxicity and gastroprotective activity of curcuma purpurascens BI. rhizome against ethanol-induced gastric mucosal injury in rats. BMC Complementary and Alternative medicine. 2014;14:1
22. Bancroft JD, Gamble M. Theory and practice of histological techniques: Elsevier Health Sci; 2008.
23. Rajalakshmi K, Devaraj H, Devaraj SN. Assessment of the no-observed-adverse-effect level (NOAEL) of gallic acid in mice. Food Chem Toxicol. 2001;39:919-22
24. Ibrahim MM, Ali HM, Abdullah MA, Hassandarvish P. Acute toxicity and gastroprotective effect of the Schiff base ligand 1H-indole-3-ethylene-5-nitrosalicylaldimine and its nickel (II) complex on ethanol induced gastric lesions in rats. Molecules. 2012;17:12449-59
25. Venkataramana C, Sravani KR, Singh SS, Madhavan V. In-silico ADME and toxcity studies of some novel indole derivatives. J. appl. pharm. sci. 2011;1:159
26. Ketuly KA, Hadi AHA, Golbabapour S, Hajrezaie M, Hassandarvish P, Ali HM. et al. Acute toxicity and gastroprotection studies with a newly synthesized steroid. PloS One. 2013;8:e59296
27. Salga MS, Ali HM, Abdullah MA, Abdelwahab SI, Hussain PD, Hadi AHA. Mechanistic studies of the anti-ulcerogenic activity and acute toxicity evaluation of dichlorido-copper (II)-4-(2-5-Bromo-benzylideneamino) ethyl) piperazin-1-ium phenolate complex against ethanol-induced gastric injury in rats. Molecules. 2011;16:8654-69
28. Stermer E. Alcohol consumption and the gastrointestinal tract. Isr. Med. Assoc. J. 2002;4:200-2
29. AlRashdi AS, Salama SM, Alkiyumi SS, Abdulla MA, Hadi AHA, Abdelwahab SI. et al. Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/ethanol-induced gastric mucosal injury in rats. Evidence-Based Complementary and Alternative Medicine. 2012. 2012
30. Asai M, Kawashima D, Katagiri K, Takeuchi R, Tohnai G, Ohtsuka K. Protective effect of a molecular chaperone inducer, paeoniflorin, on the HCl-and ethanol-triggered gastric mucosal injury. Life sc. 2011;88:350-7
31. Mahl GF. Anxiety, HCl secretion, and peptic ulcer etiology. Psychosomatic Medicine. 1950;12:158-69
32. Mustafa IM, Hapipah M, Abdulla MA, Ward TR. Synthesis, structural characterization, and anti-ulcerogenic activity of schiff base ligands derived from tryptamine and 5-chloro, 5-nitro, 3, 5-ditertiarybutyl salicylaldehyde and their nickel (II), copper (II), and zinc (II) complexes. Polyhedron. 2009;28:3993-8
33. Tandon R, Khanna R, Dorababu M, Goel R. Oxidative stress and antioxidants status in peptic ulcer and gastric carcinoma. Indian j Physiol Pharmacol. 2004;48:115-8
34. Warzecha Z, Ceranowicz P, Dembinski M, Cieszkowski J, Ginter G, Ptak-Belowska A. et al. Involvement of cyclooxygenase-1 and cyclooxygenase-2 activity in the therapeutic effect of ghrelin in the course of ethanol-induced gastric ulcers in rats. J Physiol Pharmacol. 2014;65:95-106
35. Al Batran R, Al-Bayaty F, Ameen Abdulla M, Al-Obaidi J, Mazen M, Hajrezaei M. et al. Gastroprotective effects of Corchorus olitorius leaf extract against ethanol-induced gastric mucosal hemorrhagic lesions in rats. J gastroenterol hepatol. 2013;28:1321-9
36. Hajrezaie M, Salehen N, Karimian H, Zahedifard M, Shams K, Al Batran R. et al. Biochanin A gastroprotective effects in ethanol-induced gastric mucosal ulceration in rats. PloS One. 2015;10:e0121529
37. Moghadamtousi SZ, Rouhollahi E, Karimian H, Fadaeinasab M, Abdulla MA, Kadir HA. Gastroprotective activity of Annona muricata leaves against ethanol-induced gastric injury in rats via Hsp70/Bax involvement. Drug Design, Develop Therapy. 2014;8:2099
38. Proctor MJ, Deans C. Complications of peptic ulcers. Surgery (Oxford). 2014;32:599-607
39. Brijnner G, Luna P, Thiesemann C. Drugs for pH control in upper gastrointestinal bleeding. Alimentary pharmacol therapeut. 1995;9:47-50
40. Ibrahim IAA, Abdulla MA, Hajrezaie M, Bader A, Shahzad N, Al-Ghamdi SS. et al. The gastroprotective effects of hydroalcoholic extract of Monolluma quadrangula against ethanol-induced gastric mucosal injuries in Sprague Dawley rats. Drug design, develop therapy. 2016;10:93
41. Liju VB, Jeena K, Kuttan R. Gastroprotective activity of essential oils from turmeric and ginger. J Basic Clin Physiol Pharmacol. 2015;26:95-103
42. Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Amer J Physiol Cell Physiol. 2005;288:C1-C19
43. Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nature Rev Gastroenterol Hepatol. 2013;10:352-61
44. Kwiecien S, Brzozowski T, Konturek S. Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 2002;53:39-50
45. Bannister JV, Bannister WH, Rotilio G. Aspects of the structure, function, and applications of superoxide dismutas. CRC Critical Rev Biochemist. 1987;22:111-80
46. Kurahashi T, Fujii J. Roles of antioxidative enzymes in wound healing. J Develop Biol. 2015;3:57-70
47. Terano A, Hiraishi H, Ota S-i, Shiga J, Sugimoto T. Role of superoxide and hydroxyl radicals in rat gastric mucosal injury induced by ethanol. Gastroenterologia Japonica. 1989;24:488-93
48. Gulia Y, Choudhary M. PEPTIC ULCER DISEASE: A REVIEW. Pharmacologyonline. 2011;3:48-70
49. Shichijo K, Ihara M, Matsuu M, Ito M, Okumura Y, Sekine I. Overexpression of heat shock protein 70 in stomach of stress-induced gastric ulcer-resistant rats. Digestive Diseases and Sci. 2003;48:340-8
50. Konturek PC, Brzozowski T, Konturek S, Pajdo R, Konturek J, Kwiecień S. et al. Apoptosis in gastric mucosa with stress-induced gastric ulcers. Journal of Physiol and Pharmacol: an Official Polish Physiological Society. 1999;50:211-25
51. Emily H-YC, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T. et al. BCL-2, BCL-X L sequester BH3 domain-only molecules preventing BAX-and BAK-mediated mitochondrial apoptosis. Molecular Cell. 2001;8:705-11
Author contact
![]() Corresponding author: Dr.Gokula Mohan A/L Duchiyanda Mohan, Institute of Biological Sciences, Faculty of Science, University of Malaya University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: g.mohanedu.my
Corresponding author: Dr.Gokula Mohan A/L Duchiyanda Mohan, Institute of Biological Sciences, Faculty of Science, University of Malaya University of Malaya, 50603 Kuala Lumpur, Malaysia. E-mail: g.mohanedu.my

 Global reach, higher impact
Global reach, higher impact