3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2017; 14(11):1080-1087. doi:10.7150/ijms.20452 This issue Cite
Research Paper
Serum 1,25-dihydroxyvitamin D Better Reflects Renal Parameters Than 25-hydoxyvitamin D in Patients with Glomerular Diseases
1. Division of Nephrology, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea;
2. Division of Nephrology and Hypertension, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA.
Received 2017-4-7; Accepted 2017-7-24; Published 2017-9-4
Abstract
Background: Impaired vitamin D metabolism may contribute to the development and progression of chronic kidney disease. The purpose of this study was to determine associations of circulating vitamin D with the degree of proteinuria and estimated glomerular filtration rate (eGFR) in patients with biopsy-proven glomerular diseases.
Methods: Clinical and biochemical data including blood samples for 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) levels were collected from patients at the time of kidney biopsy.
Results: Serum 25(OH)D levels were not different according to eGFR. However, renal function was significantly decreased with lower serum 1,25(OH)2D levels (P < 0.001). The proportions of nephrotic-range proteinuria and renal dysfunction (eGFR ≤ 60 mL/min/1.73 m2) progressively increased with declining 1,25(OH)2D but not 25(OH)D. Multivariable linear regression analysis showed that 25(OH)D was significantly correlated with serum albumin and total cholesterol (β = 0.224, P = 0.006; β = -0.263, P = 0.001) and 1,25(OH)2D was significantly correlated with eGFR, serum albumin and phosphorus (β = 0.202, P = 0.005; β = 0.304, P < 0.001; β = -0.161, P = 0.024). In adjusted multivariable linear regression, eGFR and 24hr proteinuria were independently correlated only with 1,25(OH)2D (β = 0.154, P = 0.018; β = -0.171, P = 0.012), but not 25(OH)D. The lower level of 1,25(OH)2D was associated with the frequent use of immunosuppressive agents (P < 0.001).
Conclusion: It is noteworthy in these results that circulating 1,25(OH)2D may be superior to 25(OH)D as a marker of severity of glomerular diseases.
Keywords: Vitamin D, Biopsy, Glomerular disease, Proteinuria, Glomerular filtration rate.
Introduction
Vitamin D has been recognized for decades as a key player in the control of bone metabolism through regulating calcium and phosphate homeostasis [1]. Vitamin D is hydroxylated to 25-hydroxyvitamin D (25(OH)D) in the liver and converted into its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D), by the enzyme 1α-hydroxylase [2]. The fact that 1α-hydroxylase is predominately, although not exclusively, found in renal tubular epithelial cells has suggested renal involvement in the process of vitamin D metabolism [3]. Indeed, the kidney plays a central role in vitamin D metabolism and in regulating its circulating levels, and thus any form or severity of renal disease may affect vitamin D metabolism through reduced 1α-hydroxylase activity, subsequent loss of renal capacity to generate 1,25(OH)2D and resultant decreases in serum 1,25(OH)2D, tissue vitamin D receptor (VDR) content and their actions [3-5]. On the contrary, given that a number of experimental studies suggest that vitamin D axis has a renoprotective role [6-8], prosurvival vitamin D activity such as inhibiting the renin-angiotensin system (RAS), attenuating interstitial inflammation and reducing proteinuria help to maintain kidney health [3]. Thus, impaired vitamin D metabolism may contribute to the development and progression of kidney disease.
It is known that vitamin D-deficient individuals with normal renal function have low serum 25(OH)D levels in spite of normal glomerular filtration rates (GFRs) [3]. These low serum 25(OH)D levels result in marked reduction of the levels of 25(OH)D filtered and available for uptake by proximal kidney tubular cells, thereby compromising the activation of 25(OH)D to 1,25(OH)2D and the VDR induction of renal megalin for urinary protein reabsorption [2, 3]. These findings may lay the foundation for pursuing serum vitamin D levels as potential markers of renal injury. Currently, both serum 25(OH)D and 1,25(OH)2D can be measured to evaluate vitamin D status. Because the 25-hydroxylation of vitamin D is mainly substrate dependent and 25(OH)D has a longer half-life than 1,25(OH)2D, circulating levels of 25(OH)D are used to determine vitamin D status and the biological effects of vitamin D in clinical practice [9]. Some epidemiological studies have placed emphasis on monitoring serum 25(OH)D levels, because serum 25(OH)D has been thought to correlate well with clinical parameters including bone mineral density and immune system function [2]. However, some data have shown no definite association between serum 25(OH)D and kidney function after adjustment for confounders [10, 11]. Rather, the level of 1,25(OH)2D has been reported to decline even in the early stage of chronic kidney disease (CKD), and this finding indicates that serum 1,25(OH)2D levels are closely associated with renal dysfunction [11].
The purpose of the present study was to investigate the relationships between circulating vitamin D levels and severity of glomerular diseases confirmed by kidney biopsy. Until now, few studies have been conducted to determine the usefulness of serum vitamin D levels as a renal injury indicator in patients with pathologically confirmed renal diseases. We compared levels of 25(OH)D and 1,25(OH)2D to determine which better reflected renal function parameters such as proteinuria and GFRs in patients with non-diabetic glomerular diseases.
Materials and Methods
Study design
A total of 199 adult patients underwent percutaneous native renal biopsies at The Catholic University of Korea Yeouido St. Mary's Hospital during the period from September 2011 to February 2015. The indications for kidney biopsy were isolated hematuria, proteinuria or renal dysfunction of unexplained cause. Percutaneous kidney biopsy was done by nephrologists under ultrasonographic guidance using an automated biopsy gun as previously described [12]. All subjects gave written informed consent before we obtained their kidney samples. Final histopathologic diagnosis on each sample was made comprehensively based on all the clinical data and pathologic findings. Cases showing diabetic nephropathy and tubular or interstitial diseases including acute tubular necrosis, tubulointerstitial nephritis and cast nephropathy were excluded in this study. Patients were divided into three groups according to their serum 25(OH)D and 1,25(OH)2D levels. This study was approved by the Institutional Review Board of The Catholic University of Korea Yeouido St. Mary's Hospital (SC16RISI0003) and performed in accordance with the principles of the Helsinki Declaration.
Data collection
Baseline demographic and clinical data at enrolment included age, sex, body mass index (BMI), presence of diabetes mellitus and hypertension, and medication history before and after kidney biopsy, including and RAS blockers and immunosuppressive agents such as steroids, cyclosporine and cyclophosphamide. In order to adjust for seasonal variation in vitamin D levels, we classified time points into four seasons as follows: spring (March to May); summer (June to August); autumn (September to November); and winter (December to February). We determined the serum levels of 25(OH)D, 1,25(OH)2D, creatinine, albumin, sodium, potassium, corrected calcium, phosphorus, magnesium, intact parathyroid hormone (iPTH) and total cholesterol from blood samples. We calculated estimated GFR (eGFR) using the Modification of Diet in Renal Disease equations [13]. We corrected the measured serum calcium for albumin according to the following formula: serum corrected calcium = calcium+ 0.8× (4-albumin) (if albumin < 4.0 g/dL) [14]. For fractional excretion (FE) of sodium, calcium, uric acid, phosphorus and magnesium, we applied the following formula [15]: FE α = [urine α (mEq/L) × serum creatinine (mg/dL) / serum α (mEq/L) × urine creatinine (mg/dL)] × 100 (α: sodium, calcium, uric acid, phosphorus or magnesium).
Statistical analysis
Data for continuous variables with normal distributions are expressed as mean ± standard deviation, and those without normal distributions are presented as the median and interquartile range. For multiple comparisons of the three groups, we used ANOVAs followed by post hoc correction for the continuous variables and used the χ2 test to compare the differences in categorical variables. Variables that were not normally distributed were log-transformed to achieve normality. We conducted univariable and stepwise multivariable linear regression analyses for independent variables adjusted for age, sex, and seasonal variation versus 25(OH)D and 1,25(OH)2D for dependent variables. We also conducted stepwise multiple linear regression analyses for independent variables versus 24hr proteinuria and eGFR for dependent variables and entered variables with P < 0.1 on univariable analyses into the multivariable regression models. We considered P < 0.05 to be statistically significant.
Results
Baseline characteristics
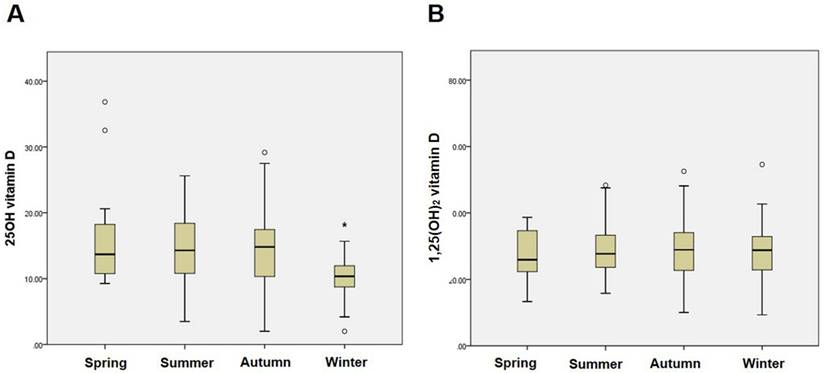
The present study included a total of 173 patients with non-diabetic glomerular diseases for the analysis. The pathologic kidney biopsy diagnoses were IgA nephropathy (41.0%) followed by nonspecific mesangial proliferative glomerulonephritis (23.7%), focal segmental glomerulosclerosis (13.8%), minimal change disease (6.3%), membranous nephropathy (4.0%), membranoproliferative glomerulonephritis (2.9%), lupus nephritis (1.7%), Henoch-Schönlein purpura nephritis (1.7%), and others (4.9%). The mean serum 25(OH)D and 1,25(OH)2D were 13.7 ± 5.8 ng/mL (Range: 3.7-39.5 ng/mL) and 29.1 ± 10.0 pg/mL (Range: 9.3-75.9 pg/mL), respectively. Serum 25(OH)D was significantly correlated with serum 1,25(OH)2D by partial correlation coefficient adjusted by age (r = 0.179; P = 0.02). Fig. 1 shows the seasonal variations in 25(OH)D and 1,25(OH)2D in the study population. Levels of 25(OH)D were significantly lower in winter (P < 0.001), whereas 1,25(OH)2D did not differ by season.
The baseline characteristics of the study population segregated by baseline 25(OH)D and 1,25(OH)2D levels are shown in Table 1. There were no significant differences among age, sex, BMI, the presence of diabetes mellitus and hypertension, serum potassium, serum phosphorus, corrected calcium, serum magnesium and iPTH levels in analyses with tertiles of both 25(OH)D and 1,25(OH)2D. Individuals with higher 25(OH)D had on average higher serum albumin and sodium but lower total cholesterol and 24hr proteinuria than did those with lower 25(OH)D concentrations. On average, patients with higher 1,25(OH)2D had higher serum albumin and eGFR but lower total cholesterol and 24hr proteinuria.
Seasonal variations in 25(OH)D and 1,25(OH)2D status in non-diabetic glomerular diseases. (A) Seasonal variation in 25(OH)D. *P < 0.001 vs. other seasons. (B) Seasonal variation in 1,25(OH)2D.
Baseline characteristics according to 25(OH)D and 1,25(OH)2D tertiles in glomerular diseases
| 25(OH)D | 1,25(OH)2D | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Tertile (T1, n = 58) | 2nd Tertile (T2, n = 58) | 3rd Tertile (T3, n = 57) | P | 1st Tertile (T1, n = 58) | 2nd Tertile (T2, n = 58) | 3rd Tertile (T3, n = 57) | P | ||
| Age (yr) | 44 ± 18 | 45 ± 16 | 45 ± 17 | 0.983 | 45 ± 18 | 47 ± 17 | 42 ± 15 | 0.242 | |
| Sex (male, %) | 28(48.3) | 29(50.0) | 37(64.9) | 0.145 | 30(51.7) | 32(55.2) | 32(56.1) | 0.882 | |
| DM (%) | 8 (13.8) | 4 (6.9) | 6 (10.5) | 0.477 | 6 (10.3) | 9 (15.5) | 3 (5.3) | 0.198 | |
| HTN (%) | 15 (25.9) | 21 (36.2) | 19 (33.3) | 0.467 | 17 (29.3) | 24 (41.4) | 14 (24.6) | 0.136 | |
| BMI (kg/m2) | 23.8 ± 3.9 | 24.2 ± 3.7 | 23.5 ± 3.8 | 0.641 | 24.3 ± 4.4 | 24.3 ± 3.4 | 22.9 ± 3.6 | 0.083 | |
| Serum Creatinine (mg/dL) | 1.6 ± 2.6 | 1.1 ± 0.4 | 1.2 ± 1.0 | 0.244 | 1.7 ± 2.3 | 1.3 ± 1.6 | 0.9 ± 0.2 | 0.043 | |
| eGFR (mL/min/1.73m2) | 77.2 ± 31.0 | 80.3 ± 26.0 | 80.7 ± 31.8 | 0.786 | 69.0 ± 34.3 | 78.6 ± 27.9 | 90.8 ± 21.3 | <0.001 | |
| Serum Albumin (g/dL) | 3.7 ± 1.1 | 4.1 ± 0.6 | 4.1 ± 0.6 | 0.004 | 3.6 ± 1.0 | 4.1 ± 0.6 | 4.3 ± 0.5 | <0.001 | |
| Serum Sodium (mEq/L) | 140.0 ± 3.4 | 141.0 ± 2.2 | 141.3 ± 1.8 | 0.022 | 140.9 ± 3.1 | 140.9 ± 2.8 | 140.5 ± 2.0 | 0.759 | |
| Serum Potassium (mEq/L) | 4.1 ± 0.5 | 4.0 ± 0.3 | 4.1 ± 0.3 | 0.167 | 4.1 ± 0.5 | 4.1 ± 0.4 | 4.1 ± 0.3 | 0.971 | |
| Corrected Calcium (mg/dL) | 9.0 ± 0.6 | 9.0 ± 0.4 | 9.0 ± 0.4 | 0.797 | 9.0 ± 0.5 | 9.0 ± 0.4 | 9.0 ± 0.4 | 0.732 | |
| Serum Phosphorus (mg/dL) | 3.9 ± 0.9 | 3.7 ± 0.6 | 3.9 ± 0.7 | 0.329 | 4.1 ± 0.8 | 3.8 ± 0.9 | 3.7 ± 0.6 | 0.051 | |
| Serum Magnesium (mg/dL) | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 0.619 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 0.727 | |
| Intact PTH (pg/mL) | 30.6 ± 26.7 | 26.8 ± 14.5 | 27.1 ± 26.9 | 0.634 | 33.9 ± 34.0 | 25.8 ± 17.4 | 25.0 ± 12.3 | 0.081 | |
| Total Cholesterol (mg/dL) | 212.8 ± 84.1 | 186.3 ± 41.8 | 183.2 ± 38.4 | 0.013 | 218.4 ± 81.5 | 183.4 ± 43.8 | 180.5 ± 37.0 | 0.001 | |
| 24hr Proteinuria (g/day) | 2.7 ± 4.29 | 9.84 ± 1.69 | 1.07 ± 1.94 | 0.002 | 2.99 ± 4.22 | 1.34 ± 2.03 | 0.50 ± 0.76 | <0.001 | |
Abbreviations: 25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; BMI: body mass index; BP: blood pressure; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; HTN: hypertension; PTH; parathyroid hormone
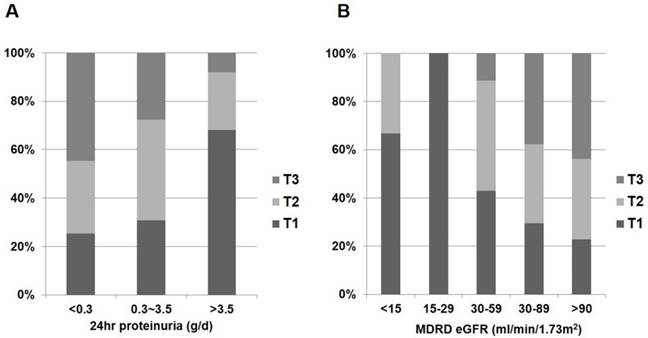
Distribution of 1,25(OH)2D status according to renal function and proteinuria in non-diabetic glomerular diseases. (A) 1,25(OH)2D and 24hr proteinuria. (B) 1,25(OH)2D and eGFR.
Vitamin D and renal biochemical parameters
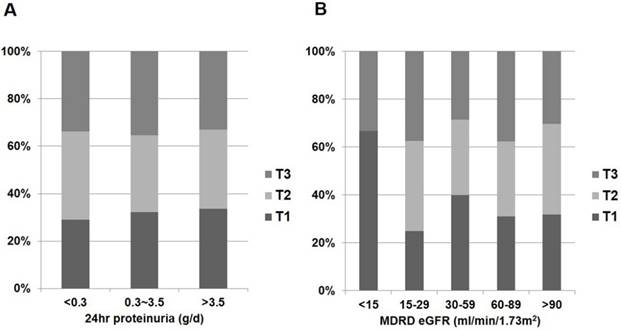
In the whole patient group, eGFR was negatively correlated with log-transformed 24hr proteinuria when calculated using partial correlation coefficient adjusting for age (r = -0.335; P < 0.001). Correlations between vitamin D metabolites and clinical parameters in patients with glomerular diseases are shown in Fig. 2 and Table 2. Patients with nephrotic range proteinuria (24hr proteinuria > 3.5 g/day) or moderate to severe renal dysfunction (eGFR ≤ 60 mL/min/1.73 m2) were likely to have low 1,25(OH)2D (P < 0.001 and P < 0.001, respectively) (Fig. 2). However, the amount of proteinuria and severity of renal dysfunction were not associated with 25(OH)D (P = 0.184 and P = 0.898, respectively) (Fig. 3).
Table 2 summarises the relationships between vitamin D metabolites and biochemical parameters on the basis of linear regression analysis. Log 25(OH)D was positively correlated with serum albumin (β = 0.374; P < 0.001) and serum magnesium (β = 0.159; P = 0.029) but negatively correlated with total cholesterol (β = -0.392; P < 0.001) and log 24hr proteinuria (β = -0.317; P < 0.001). Meanwhile, log 1,25(OH)2D was positively correlated with eGFR (β = 0.292; P < 0.001) and serum albumin (β = 0.378; P < 0.001) but negatively correlated with serum creatinine (β = -0.245; P < 0.001), serum phosphorus (β = -0.245; P < 0.001), iPTH (β = -0.223; P < 0.001), total cholesterol (β = -0.257; P = 0.001) and log 24hr proteinuria (β = -0.361; P < 0.001). In stepwise multivariable linear regression, log 25(OH)D was independently correlated with serum albumin (β = 0.224; P = 0.006) and total cholesterol (β = -0.263; P = 0.001), and log 1,25(OH)2D was independently correlated with eGFR (β = 0.202; P = 0.005), serum albumin (β = 0.304; P < 0.001) and serum phosphorus (β = -0.161; P = 0.024).
Additionally, log 25(OH)D was positively correlated with log FE of phosphorus (r = 0.214; P = 0.006), whereas log 1,25(OH)2D was negatively correlated with log FE of magnesium (r = -0.202; P = 0.010). Otherwise, there were no significant differences in FE of other ions according to either 25(OH)D or 1,25(OH)2D (data not shown).
Vitamin D and severity of glomerular diseases
Table 3 shows the relationships between 25(OH)D or 1,25(OH)2D and eGFR or proteinuria in glomerular diseases. In stepwise multivariable linear regression, we detected significant associations between eGFR and log 1,25(OH)2D (β = 0.237; P < 0.001) successively adjusted for age, sex, BMI, presence of diabetes mellitus and hypertension and 24hr proteinuria. When further adjusted for serum total cholesterol, corrected calcium and phosphorus, eGFR was significantly associated with log 1,25(OH)2D (β = 0.154; P = 0.018). For proteinuria, both log 25(OH)D and log 1,25(OH)2D were significantly associated with log 24hr proteinuria (β = -0.221; P = 0.002 and β = -0.227; P < 0.001, respectively) when we adjusted for age, sex, BMI, presence of diabetes mellitus and hypertension and eGFR. However, in the fully adjusted model, only log 1,25(OH)2D was significantly associated with log 24hr proteinuria (β = -0.171; P = 0.012).
Table 4 shows the distribution of patients on antiproteinuric therapy after kidney biopsy. Overall, there were no significant differences in use of RAS blockers by 25(OH)D and 1,25(OH)2D levels, although the patients with the highest 1,25(OH)2D but not 25(OH)D were likely to use less immunosuppressive agents (P < 0.001).
Relationships between clinical parameters and 25(OH)D and 1,25(OH)2D in glomerular diseases
| Log (25(OH)D) | Log (1,25(OH)2D) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| β | P | β | P | β | P | β | P | |
| BMI (kg/m2) | -0.063 | 0.391 | -0.170 | 0.027 | ||||
| Serum Creatinine (mg/dL) | -0.039 | 0.589 | -0.245 | 0.001 | ||||
| eGFR (mL/min/1.73 m2) | 0.082 | 0.259 | 0.292 | <0.001 | 0.202 | 0.005 | ||
| Serum Albumin (g/dL) | 0.374 | <0.001 | 0.224 | 0.006 | 0.378 | <0.001 | 0.304 | <0.001 |
| Serum Sodium (mEq/L) | 0.118 | 0.105 | 0.012 | 0.882 | ||||
| Serum Potassium (mEq/L) | 0.008 | 0.112 | -0.031 | 0.699 | ||||
| Corrected Calcium (mg/dL) | -0.051 | 0.485 | 0.090 | 0.249 | ||||
| Serum Phosphorus (mg/dL) | -0.045 | 0.54 | -0.245 | 0.001 | -0.161 | 0.024 | ||
| Serum Magnesium (mg/dL) | 0.159 | 0.029 | 0.017 | 0.826 | ||||
| Intact PTH (pg/mL) | -0.029 | 0.683 | -0.223 | 0.003 | ||||
| Total Cholesterol (mg/dL) | -0.392 | <0.001 | -0.263 | 0.001 | -0.257 | 0.001 | ||
| 24hr Proteinuria (g/day) | -0.317 | <0.001 | -0.361 | <0.001 | ||||
Adjusted for age, sex, and seasonal variation. Abbreviations: 25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; BMI: body mass index; eGFR: estimated glomerular filtration rate; PTH: parathyroid hormone
Distribution of 25(OH)D status according to renal function and proteinuria in non-diabetic glomerular diseases. (A) 25(OH)D and 24hr proteinuria. (B) 25(OH)D and eGFR.
Multivariable linear regression between 25(OH) D or 1,25(OH)2D and estimated glomerular filtration rate or proteinuria
| Log (25(OH)D) | Log (1,25(OH)2D) | |||
|---|---|---|---|---|
| β | P | β | P | |
| eGFR | ||||
| Unadjusted | 0.066 | 0.388 | 0.292 | <0.001 |
| Model 1 | 0.062 | 0.345 | 0.232 | <0.001 |
| Model 2 | 0.015 | 0.808 | 0.237 | <0.001 |
| Model 3 | 0.015 | 0.808 | 0.154 | 0.018 |
| 24hr proteinuria | ||||
| Unadjusted | -0.254 | 0.001 | -0.361 | <0.001 |
| Model 1 | -0.23 | 0.002 | -0.322 | <0.001 |
| Model 2 | -0.221 | 0.002 | -0.277 | <0.001 |
| Model 3 | -0.095 | 0.153 | -0.171 | 0.012 |
Model 1 adjusted for age, sex and BMI, Model 2 adjusted for model 1+ diabetes mellitus, hypertension and eGFR (or 24hr proteinuria), Model 3 adjusted for model 2 + total cholesterol, calcium and phosphorus. Abbreviations: 25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; eGFR: estimated glomerular filtration rate
Discussion
In the current study, we demonstrated a significant negative relationship between serum baseline 1,25(OH)2D and proteinuria and a significant positive relationship between 1,25(OH)2D and eGFR in patients with biopsy-proven glomerular diseases. On the contrary, the baseline level of 25(OH)D was not associated with renal function and proteinuria. In addition, more patients with low serum 1,25(OH)2D received immunosuppressive agents than did those with high serum 1,25(OH)2D. To our knowledge, the data shown herein are novel in their longitudinal link between serum 1,25(OH)2D concentrations and disease severity in patients with non-diabetic glomerular diseases confirmed by kidney biopsy.
Although diabetic nephropathy is currently the most common cause of end-stage renal disease [16], non-diabetic glomerulonephritis remains a major cause of morbidity and mortality from CKD in many regions of the world, particularly Asian countries [17]. As with any other causes of CKD, secondary hyperparathyroidism can begin relatively early in the course of glomerulonephritis and steadily progress as GFR declines. The pathogenic factors that could contribute to the development and maintenance of secondary hyperparathyroidism are multiple but principally involve the closely related consequences of phosphate retention and abnormalities in vitamin D metabolism [18]. Few studies have shown a relationship between renal function and abnormal calcium, abnormal phosphorus or vitamin D status in patients with non-diabetic glomerulonephritis. According to a Chinese study of 2,924 patients who had been newly diagnosed with primary glomerulonephritis, there was a significant decline in serum 25(OH)D in patients with CKD stage 5 [17]. However, the serum 25(OH)D was normal in patients with early-stage CKD. In our study, both 25(OH)D and 1,25(OH)2D levels were inversely proportional to the amount of urinary protein but only 1,25(OH)2D was proportional to eGFR. This finding suggests that serum 1,25(OH)2D is a more sensitive biomarker of renal function in glomerular diseases.
There are some grounds that serum 1,25(OH)2D reflects the severity of glomerular diseases. The pleiotropic effects of 1,25(OH)2D beyond controlling parathyroid function or mineral metabolism may extend to other areas in the course of renal disease [18]. One of these non-calcemic effects of vitamin D is suppression of RAS [19]. It has been well-known that vitamin D is a potent negative endocrine regulator of RAS and works predominantly as a suppressor of renin synthesis and angiotensin II accumulation in the kidney [18-20]. In cultured juxtaglomerular-like cells, the administration of active vitamin D reduces renin expression by 90% by blocking the cyclic adenosine monophosphate response element in the renin gene promoter [1]. Earlier clinical studies established a significant relationship between low circulating levels of 1,25(OH)2D and elevated serum renin [5]. Another explanation of vitamin D's effect on glomerular pathology is that vitamin D has intrarenal immunomodulating effects. In a previous study using 111 frozen kidney biopsy tissue samples, urinary monocyte chemoattractant protein (MCP)-1 and renal macrophage infiltration were each inversely correlated with serum 1,25(OH)2D levels [21]. This supports that 1,25(OH)2D inhibits the MCP-1 driven inflammatory process by blocking nuclear factor-κB activation [1, 22]. Numerous studies have investigated the effects of 1,25(OH)2D on glomerular pathologies both in vivo and in vitro. The administration of 1,25(OH)2D diminished both proliferation of cultured mouse and human mesangial cells and secretion of transforming growth factor (TGF)-β in human mesangial cells [23]. Furthermore, the administration of 1,25(OH)2D resulted in improved renal parameters such as proteinuria, mesangial proliferation and podocyte injury in a number of animal models of non-diabetic renal diseases including Heymann nephritis, cyclosporine A nephrotoxicity, subtotal nephrectomy, anti-Thy1.1 glomerulonephritis and puromycin aminonucleoside-induced nephrosis [1, 23, 24]. Based on data on the possible effects of vitamin D on glomerular health, one previous study evaluated the efficacy of vitamin D on proteinuria in patients with chronic glomerulonephritis. In a study of 10 patients with IgA nephropathy and persistent proteinuria despite use of RAS blockades, twice-weekly oral calcitriol therapy for 12 weeks demonstrated a modest antiproteinuric effect in these patients [25].
Distribution of treatment after kidney biopsy according to the levels of vitamin D metabolites in glomerular diseases
| 25(OH)D | 1,25(OH)2D | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | P | T1 | T2 | T3 | P | |
| RAS blockers | 0.35 | 0.23 | ||||||
| No | 11(19.0) | 8(13.8) | 4(7.0) | 11(19.0) | 6(10.3) | 6(10.5) | ||
| Yes (Before biopsy) | 14(24.1) | 12(20.7) | 17 (29.8) | 16(27.6) | 17(29.3) | 10(17.5) | ||
| Yes (After biopsy) | 33(56.9) | 38(65.5) | 36(63.2) | 31(53.4) | 35(60.3) | 41(71.9) | ||
| Immmunosuppressive agents | 0.61 | <0.001 | ||||||
| No | 32(55.2) | 42(72.4) | 42(73.7) | 31(53.4) | 34(58.6 | 51(89.5) | ||
| Yes | 26(44.8) | 16(29.8) | 15(26.3) | 27(46.6) | 24(41.4) | 6(10.5) | ||
Abbreviations: 25(OH)D: 25-hydroxyvitamin D; 1,25(OH)2D: 1,25-dihydroxyvitamin D; RAS: renin-angiotensin system; T: tertile
There is also a possibility that serum 1,25(OH)2D in subjects with non-diabetic glomerulonephritis reflects early tubulointerstitial injury. Because the renal tubuleinterstitium is the primary site of hydroxylation of 25(OH)D to 1,25(OH)2D, early dysfunction or loss of tubular and peritubular cells could impair the synthesis of 1,25(OH)2D, causing the decreased local and systemic effects of the hormone. Furthermore, this could potentially result in further compromise to the functional and structural integrity of the renal parenchyma and contribute to the gradual decline of renal function [26]. Although much less was known about the effect of vitamin D on tubular interstitial fibrosis, it was found that 1,25(OH)2D suppressed the myofibroblast activation from interstitial fibroblasts, TGF-β1-induced α-smooth muscle actin expression, type I collagen and thrombospondin-1 triggered by TGF-β1 and β-catenin signalling [27]. Unlike serum calcium and phosphorus, serum magnesium is not regulated by a known hormone including vitamin D, and most magnesium reabsorption occurs mainly in the thick ascending limb of loop of Henle [28]. Fractional excretion of magnesium increases as CKD evolves, maintaining normal serum magnesium levels until advanced CKD [29]. Considering the increased renal excretion of magnesium in subjects with low serum 1,25(OH)2D in our study, 1,25(OH)2D might be a marker for the diagnosis and monitoring of early tubular injury.
The major strength of our study is that we only included subjects who had percutaneous kidney biopsy and then were diagnosed with non-diabetic glomerular diseases, thereby setting an equal baseline for the effects of the underlying disease. An additional strength is that both 25(OH)D and 1,25(OH)2D with multiple renal functional parameters were simultaneously determined. Despite the presence of normal 25(OH)D levels, many patients with CKD tend to have low serum 1,25(OH)2D, indicating the functional vitamin D deficiency in these patients [30]. Therefore, it may be more meaningful to measure serum 1,25(OH)2D with 25(OH)D at the same time in patients in the early stages of CKD. We should also note a number of limitations of the present study. First, the results are cross-sectional analyses and thus do not provide evidence of causation. Second, our sample size was rather small and all patients were from a single institution, so there may have been some selection bias. Third, the possible of residual confounding factors could not be excluded. Fourth, we did not measure other biomarkers associated with mineral metabolism, such as fibroblast growth factor 23 or Klotho. Finally, even though this study included only patients with non-diabetic glomerular diseases, a certain degree of heterogeneity might exist among different glomerulopathies.
Conclusions
In this study, we showed that circulating serum 1,25(OH)2D was superior to 25(OH)D as a marker of renal disease severity in patients with biopsy-proven glomerular disease. This finding may provide a thorough grounding in choosing vitamin D supplementation in these patients.
Abbreviations
1,25(OH)2D: 1,25-dihydroxyvitamin D; 25(OH)D: 25-hydroxyvitamin D; BMI: body mass index; BP: blood pressure; CKD: chronic kidney disease; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; ESRD: end-stage renal disease; FE: fractional excretion; HTN: hypertension; iPTH: intact parathyroid hormone; MCP-1: monocyte chemoattractant protein-1; RAS: renin-angiotensin system; T: tertile; TGF-β: transforming growth factor-β; VDR: vitamin D receptor
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (NRF-2015R1C1A1A02037258). We thank Dr. Jong Hee Chung (Department of Statistics, The Graduate School of Ewha Womans University, Seoul, Republic of Korea) for her statistical advice.
Authors' contributions
S. Chung and Y. A. Hong designed the research. S. Chung, M. Kim, E. S. Koh, H. S. Hwang, Y. K. Chang, C. W. Park, S. Y. Kim, Y. S. Chang and Y. A. Hong collected and reviewed the data. S. Chung and Y. A. Hong performed the statistical analysis. S. Chung and Y. A. Hong wrote the paper. All authors read and approved the final manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Doorenbos CR, van den Born J, Navis G, de Borst MH. Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol. 2009;5:691-700
2. Jones G. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3). Semin Dial. 2007;20:316-24
3. Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int. 2011;79:715-29
4. Wang XX, Jiang T, Shen Y, Caldas Y, Miyazaki-Anzai S, Santamaria H. et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59:2916-27
5. Andress DL. Vitamin D in chronic kidney disease: a systemic role for selective vitamin D receptor activation. Kidney Int. 2006;69:33-43
6. Ma J, Zhang B, Liu S, Xie S, Yang Y, Ma J. et al. 1,25-dihydroxyvitamin D(3) inhibits podocyte uPAR expression and reduces proteinuria. PLoS One. 2013;8:e64912
7. Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J. et al. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73:163-71
8. Zou MS, Yu J, Nie GM, He WS, Luo LM, Xu HT. 1, 25-dihydroxyvitamin D3 decreases adriamycin-induced podocyte apoptosis and loss. Int J Med Sci. 2010;7:290-9
9. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266-81
10. LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q. et al. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026-33
11. Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA. et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31-8
12. Chung S, Koh ES, Kim SJ, Yoon HE, Park CW, Chang YS. et al. Safety and tissue yield for percutaneous native kidney biopsy according to practitioner and ultrasound technique. BMC Nephrol. 2014;15:96
13. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-70
14. Payne RB, Little AJ, Williams RB, Milner JR. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. 1973;4:643-6
15. Musso CG, Juarez R, Vilas M, Navarro M, Rivera H, Jauregui R. Renal calcium, phosphorus, magnesium and uric acid handling: comparison between stage III chronic kidney disease patients and healthy oldest old. Int Urol Nephrol. 2012;44:1559-62
16. Jin DC, Yun SR, Lee SW, Han SW, Kim W, Park J. et al. Lessons from 30 years' data of Korean end-stage renal disease registry, 1985-2015. Kidney Res Clin Pract. 2015;34:132-9
17. Li Y, Zhang W, Ren H, Wang W, Shi H, Li X. et al. Evaluation of anemia and serum iPTH, calcium, and phosphorus in patients with primary glomerulonephritis. Contrib Nephrol. 2013;181:31-40
18. Al-Badr W, Martin KJ. Vitamin D and kidney disease. Clin J Am Soc Nephrol. 2008;3:1555-60
19. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229-38
20. Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci U S A. 2008;105:15896-901
21. Zehnder D, Quinkler M, Eardley KS, Bland R, Lepenies J, Hughes SV. et al. Reduction of the vitamin D hormonal system in kidney disease is associated with increased renal inflammation. Kidney Int. 2008;74:1343-53
22. Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X. et al. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193-201
23. Panichi V, Migliori M, Taccola D, Consani C, Giovannini L. Effects of calcitriol on the immune system: new possibilities in the treatment of glomerulonephritis. Clin Exp Pharmacol Physiol. 2003;30:807-11
24. Matsui I, Hamano T, Tomida K, Inoue K, Takabatake Y, Nagasawa Y. et al. Active vitamin D and its analogue, 22-oxacalcitriol, ameliorate puromycin aminonucleoside-induced nephrosis in rats. Nephrol Dial Transplant. 2009;24:2354-61
25. Szeto CC, Chow KM, Kwan BC, Chung KY, Leung CB, Li PK. Oral calcitriol for the treatment of persistent proteinuria in immunoglobulin A nephropathy: an uncontrolled trial. Am J Kidney Dis. 2008;51:724-31
26. Singh DK, Winocour P, Summerhayes B, Viljoen A, Sivakumar G, Farrington K. Are low erythropoietin and 1,25-dihydroxyvitamin D levels indicative of tubulo-interstitial dysfunction in diabetes without persistent microalbuminuria? Diabetes Res Clin Pract. 2009;85:258-64
27. Tian J, Liu Y, Williams LA, de Zeeuw D. Potential role of active vitamin D in retarding the progression of chronic kidney disease. Nephrol Dial Transplant. 2007;22:321-8
28. Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol. 2015;10:1257-72
29. Felsenfeld AJ, Levine BS, Rodriguez M. Pathophysiology of Calcium, Phosphorus, and Magnesium Dysregulation in Chronic Kidney Disease. Semin Dial. 2015;28:564-77
30. Agarwal R. Vitamin D, proteinuria, diabetic nephropathy, and progression of CKD. Clin J Am Soc Nephrol. 2009;4:1523-8
Author contact
![]() Corresponding author: Yu Ah Hong, MD, Address: Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Daejeon St. Mary's Hospital, 64, Daeheung-ro, Jung-gu, Daejeon, 34943, Republic of Korea. Phone: +82-42-220-9329, Fax: +82-42-220-9473 E-mail address: amorfatiac.kr
Corresponding author: Yu Ah Hong, MD, Address: Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Daejeon St. Mary's Hospital, 64, Daeheung-ro, Jung-gu, Daejeon, 34943, Republic of Korea. Phone: +82-42-220-9329, Fax: +82-42-220-9473 E-mail address: amorfatiac.kr

 Global reach, higher impact
Global reach, higher impact