Impact Factor
ISSN: 1449-1907
Int J Med Sci 2017; 14(9):804-810. doi:10.7150/ijms.19847 This issue Cite
Research Paper
Compensating effect of minor portal hypertension on the muscle mass loss-related poor prognosis in cirrhosis
1. Department of Gastroenterology, Chiba University Graduate School of Medicine, 1-8-1, Inohana, Chuou-ku, Chiba, 260-8670, Japan;
2. Department of Research Center for Frontier Medical Engineering, Chiba University, 1-33, Yayoicho, Inage-ku, Chiba, 263-8522, Japan.
Received 2017-2-27; Accepted 2017-6-18; Published 2017-7-19
Abstract
Background: To examine the influence of the severity of portal hemodynamic abnormality on the prognosis of cirrhosis with respect to the muscle mass loss (MML).
Methods: The study involved a subgroup analysis in 98 cirrhosis patients (63.5 ± 11.8 years) who prospectively underwent both Doppler ultrasound and hepatic venous catheterization. The prognostic influence of MML diagnosed by computed tomography using the L3 skeletal muscle index was evaluated (median observation period, 32.7 months).
Results: The cumulative survival rate showed difference between patients with MML (n = 34; 82.2%/1year, 41.2%/3years and 36.1%/5years) and those without (n = 64; 92.1%/1year, 74.9%/3years and 69.4%/5years; P = 0.005). When divided with respect to the portal velocity, the survival rate showed differences between patients with and without MML in the cohort < 12.8 cm/s (n=52, p=0.009) and ≥ 12.8 cm/s (n=44, p=0.041). The survival rate also showed differences between patients with MML (n = 24; 78.8%/1year, 40.6%/3years and 34.8%/5years) and those without (n = 45; 91.1%/1year, 71.3%/3years and 63.1%/5years; P = 0.008) in the cohort with hepatic venous pressure gradient (HVPG) > 12 mmHg. However, in the cohort with HVPG ≤ 12 mmHg, survival rate showed no difference between patients with MML (n=10; 100%/1year, 61.9%/3years and 61.9%/5years) and those without (n=19; 93.8%/1year, 71.2%/3years and 59.4%/5years; p = 0.493)
Conclusion: Lower HVPG has a compensating effect on the MML-induced poor prognosis of cirrhosis. Care should be taken in the evaluation of the influence of MML in consideration of the severity of portal hypertension.
Keywords: Muscle mass loss, portal hypertension, cirrhosis, hepatic venous pressure gradient
Introduction
Because of the increased prevalence of chronic liver disease, practical attention has been paid to the detection and diagnosis of cirrhosis [1]. This is the most advanced stage of liver disease, with a risk of developing various kinds of complications which may affect the prognosis of the disease as well as the quality of life [1, 2]. Clinical management of cirrhosis should be properly focused on the assessment of the severity of liver function and the prediction of long-term outcomes.
Muscle has a role to maintain energy metabolism and nutritional condition, and therefore the muscle mass loss (MML) may be a considerable impairment condition [3, 4]. Aging may increase the development of MML and further, cirrhosis is frequently associated with this muscular abnormality [5, 6]. Recent studies have shown the role of quantitative muscle assessment in cirrhosis, as a marker related to malnutrition and potential liver function [7], a prediction of post-operative outcomes [8, 9], and the prognosis for patients with cirrhosis or with hepatocellular carcinoma (HCC) [10-12].
Portal hemodynamics is another factor which shows prognostic influence on cirrhosis [13]. Investigators have reported the role of hepatic venous pressure gradient (HVPG), a surrogate marker of portal pressure, in the prediction of the risk of complications [14], development of HCC [15], and post-operative outcomes [16]. At the same time, portal flow appearance on Doppler ultrasound (US) may also represent the severity of portal hypertension [17, 18]. Recent studies have suggested the negative effect of decreased portal velocity in liver decompensation [19], and the risk of developing portal vein thrombosis [20].
Taken together, there might be an interrelationship between MML and portal hemodynamics, presented by portal pressure and/or portal velocity, on the prognosis of cirrhosis. However, an extensive literature search has not demonstrated the relationship between MML and portal hypertension or their compensating/synergistic effect on the prognosis of cirrhosis. Therefore, the present study aimed to examine the influence of the severity of portal hemodynamic abnormality on the prognosis of cirrhosis with respect to the MML.
Materials and Methods
Study design
This study is based on a subgroup analysis of the cohort which included prospectively enrolled subjects (April 2008 to March 2014, Number 2008-114) to examine the long-term outcomes in cirrhosis patients with portal hypertension. The study was approved by the ethical committee of our university hospital (Number 2015-73). Informed written consent was obtained from all participants.
The study enrolled the following patients:
i) Both inpatients and outpatients with cirrhosis whose diagnosis was made histologically or clinically by using US [21], which was performed for the surveillance of HCC, with laboratory data and clinical findings (varices or ascites).
ii) Patients who were scheduled to undergo hepatic venous catheterization for the detailed examination of portal hypertension because of the signs of gastroesophageal varices, moderate/severe ascites, and/or hepatic encephalopathy.
The computed tomography (CT) images which were taken for the routine surveillance of HCC were used for the evaluation of muscle mass volume and spleen volume. The latter was calculated by integrating all images for the spleen using image analysis software (HOPE/DrABLE-EX; Fujitsu, Tokyo, Japan) after manual tracing of an outline of the spleen in each slice at 5-mm intervals. In addition, Doppler US was performed on admission before performing angiography to evaluate portal hemodynamics.
The study excluded the following patients with:
i) Advanced HCC with the stage C/D using the Barcelona-Clinic Liver Cancer Staging System [22]
ii) Malignant disease which was not controlled by non-surgical treatment
iii) Vascular abnormality detected by Doppler US, such as portal vein thrombosis, intrahepatic shunt, and non-forward flow direction in the portal trunk
iv) Vasoactive drug use such as a beta-blocker
v) Transjugular intrahepatic portosystemic shunt (TIPS) or peritoneo venous shunt
vi) More than a 1-year interval between CT and HVPG measurement.
In addition, a patient was excluded from the study if Doppler US detected the presence of a paraumbilical vein, because of the shunt-related increase of portal venous flow [23], which may have a certain influence on the data analysis.
Definition of clinical presentations
Gastroesophageal varices were classified according to the General Rules for Recording Endoscopic Findings set by the Japanese Research Society for Portal Hypertension as small, medium, and large grades [24].
The degree of ascites was defined according to the established guidelines [25]: mild for ascites that were only detectable by US examination, moderate for ascites that caused moderate symmetrical distention of the abdomen, and severe for ascites that caused marked abdominal distension. Portal vein thrombosis was defined as an echogenic structure that partially or completely occupied the lumen of the portal vein. Hepatic encephalopathy was assessed using the West-Haven grading system [26], and grade II or above was classified as overt hepatic encephalopathy.
Diagnosis of MML
A CT scan was performed for all patients using a 16-detector CT scanner (Light Speed Ultrafast 16, GE Healthcare, WI; or Activion 16, Toshiba), a 128-detector CT scanner (Aquilion CX, Toshiba), or a 320-detector CT scanner (Aquilion ONE, Toshiba).
MML was diagnosed by using axial CT images at the L3 level according to the literatures [27, 28]. The Slice Omatic V5.0 software (Tomovision, Montreal, Quebec, Canada) was applied to identify the skeletal muscle while excluding other tissues by using Hounsfield unit thresholds (-29 to +150) [28]. This automatic process provides the sum of cross-section areas of the skeletal muscle (cm2), followed by the calculation of skeletal muscle index (SMI) at L3 level (L3-SMI) by the formula of cross-sectional muscle area/height2. The cutoff values for diagnosing MML used in this study were 38 cm2/m2 for women and 42 cm2/m2 for men, which were recently proposed for Japanese [29].
Hepatic venous catheterization
Hepatic venous catheterization was performed according to the literature [30]. After the insertion of the balloon catheter (5 Fr, 9 mm; Terumo Clinical Supply Co. Ltd, Gifu, Japan) into the main branch of the right hepatic vein, free and wedged hepatic venous pressures were measured, and HVPG was calculated as the difference between the 2 pressures.
US examination
The US examination was performed in the supine position, after fasting for more than 4 hours, using an SSA-770A or SSA 790A (Toshiba, Tokyo, Japan) with a 3.75-MHz convex probe; the operator was one of the authors, H. M. who had more than 20 years of experience. All patients were asked to breathe gently.
The portal hemodynamics was evaluated by both pulsed and color Doppler US [31]. Firstly, color Doppler was performed to screen the presence or absence of hemodynamic abnormalities, such as reversed portal flow, portal vein thrombosis, intrahepatic arterioportal shunt or portal venous shunt, which are exclusion criteria from the study. Then, measurement of the diameter (mm) and pulsed Doppler were performed at the portal trunk. Basically, the blood flow was measured with an angle < 60° between the US beam and the vessel, and the mean velocity (cm/s) was calculated by the integration of the average time value obtained from the waveform of the Doppler signal. Mean values of multiple measurements (2 to 4 times) were used for the data analysis. The collateral vessels with hepatofugal flow direction were also reported.
Statistical Analysis
All data are expressed as mean ± standard deviation or as percentages. When performing analysis, the cohort was divided into 2 groups based on the HVPG value of 12 mmHg which is the value to define severe portal hypertension [32, 33], or based on the portal trunk velocity of 12.8 cm/s according to the literature [19].
Student's t-test or Mann-Whitney U test was used for the comparison of continuous variables, and chi-square test or Fisher's exact test was used for the comparison of categorical variables. The Kaplan-Meier method was applied for the analysis of the survival rate using data of the date of death, liver transplantation, or final date confirmed to be alive during the study period. A log-rank test was used for the comparison of the differences, and P-values less than 0.05 were considered to be significant. Statistical analysis was performed using the SAS software package (version 9.3; SAS Institute Inc., Cary, North Carolina, USA).
Results
Patients
There were 98 patients with cirrhosis (male 64, female 34, with a mean age of 63.5 ± 11.8 years, and range of 21 to 83 years) (Table 1). The model for end-stage liver disease (MELD) score ranged from 6 to 17 (9.6 ± 2.7), and the Child-Pugh score ranged from 5 to 13 (7.2 ± 1.6) with Child-Pugh A in 33, B in 58, and C in 7. Ninety-four patients had accompanying gastroesophageal varices, and 13 patients were diagnosed with HCC. The median observation period was 32.7 months (range, 1.9 - 102.7).
Portal hemodynamics and prognosis
The HVPG ranged from 0.7 to 30.3 mmHg (14.8 ± 5.0), and 69 patients (70.4 %) showed HVPG > 12 mmHg (Table 2). Doppler US demonstrated forward flow direction in the portal trunk in all patients. The portal trunk showed a diameter from 5.0 to 17.8 mm (11.2 ± 1.9) and a velocity from 6.8 to 19.4 cm/s (12.7 ± 2.9) with measurement failure in 2 cases. Fifty-two patients (53.1%) presented with a velocity < 12.8 cm/s.
Patient characteristics
| Number | 98 |
|---|---|
| Age | 63.5 ± 11.8 (21-83) |
| Sex (male/female) | 64/34 |
| Etiology (HCV/alcohol/PBC/NASH/HBV/AIH/others) | 42/14/8/7/4/2/21 |
| Body mass index | 24.3 ± 4.8 (16.3-43.8) |
| Child-Pugh score | 7.2 ± 1.6 (5-13) |
| MELD score | 9.6 ± 2.7 (6-17) |
| Gastroesophageal varices (-/+) | 4/94 |
| Hepatocellular carcinoma (-/+) | 85/13 |
| Portal trunk diameter (mm) | 11.2 ± 1.9 (5.0-17.8) |
| Portal trunk velocity (cm/s) | 12.7 ± 2.9 (6.8-19.4) |
| Collateral vessel (-/+) | 12/86 |
| Spleen volume (cm3) | 469.9 ± 253.4 (103.5-1125.4) |
| HVPG (mmHg) | 14.8 ± 5.0 (0.7-30.3) |
| Diagnosis of cirrhosis (histology/imaging) | 42/56 |
HCV, hepatitis C virus; PBC, primary biliary cholangitis;
NASH, nonalcoholic steatohepatitis; HBV, hepatitis B virus
AIH, autoimmune hepatitis; MELD, model for end-stage liver disease
HVPG, hepatic venous pressure gradient
Patient characteristics with respect to HVPG
| HVPG | P value | ||
|---|---|---|---|
| ≤ 12 mmHg | 12 mmHg < | ||
| Number | 29 | 69 | - |
| Age | 62.0 ± 12.0 | 64.1 ± 11.7 | 0.44 |
| Sex (male / female) | 24/5 | 40/29 | 0.019 |
| Etiology* | 9/6/1/2/2/2/7 | 33/8/7/5/2/0/14 | 0.16 |
| Body mass index | 23.8 ± 4.5 | 24.4 ± 4.9 | 0.57 |
| Child-Pugh score | 6.6 ± 1.2 | 7.4 ± 1.6 | 0.011 |
| MELD score | 9.1 ± 2.3 | 9.8 ± 2.8 | 0.25 |
| Gastroesopageal varices (-/+) | 2/27 | 2/67 | 0.58 |
| Hepatocellular carcinoma (-/+) | 27/2 | 58/11 | 0.33 |
| Portal trunk diameter (mm) | 11.0 ± 1.9 | 11.3 ± 1.8 | 0.44 |
| Portal trunk velocity (cm/s) | 13.3 ± 3.4 | 12.4 ± 2.6 | 0.2 |
| Collateral vessel (-/+) | 7/22 | 5/64 | 0.038 |
| Spleen volume (cm3) | 473.3 ± 249.4 | 468.6 ± 256.8 | 0.94 |
| Diagnosis of cirrhosis (histology/imaging) | 14/15 | 28/41 | 0.49 |
HVPG, hepatic venous pressure gradient
*, Hepatitis C virus/alcohol/primary biliary cholangitis/nonalcoholic steatohepatitis/hepatitis B virus/autoimmune hepatitis/others
MELD, model for end-stage liver disease
Clinical findings in patients with and without muscle mass loss
| Muscle mass loss | |||
|---|---|---|---|
| - | + | P value | |
| Number | 64 | 34 | - |
| Age | 62.5 ± 12.8 | 65.3 ± 9.2 | 0.22 |
| Sex (male / female) | 45/19 | 19/15 | 0.16 |
| Etiology* | 28/8/4/6/2/0/16 | 14/6/4/1/2/2/5 | 0.12 |
| Body mass index | 26.1 ± 4.6 | 20.8 ± 2.6 | <0.0001 |
| Child-Pugh score | 7.1 ± 1.6 | 7.2 ± 1.6 | 0.85 |
| MELD score | 9.8 ± 2.9 | 9.2 ± 2.2 | 0.27 |
| Albumin | 3.4 ± 0.5 | 3.3 ± 0.6 | 0.56 |
| Effect of antiviral treatment† (-/+) | 25/3 | 13/2 | 1.000 |
| Gastroesopageal varices (-/+) | 2/62 | 2/32 | 0.61 |
| Hepatocellular carcinoma (-/+) | 55/9 | 30/4 | 1.000 |
| Portal trunk diameter (mm) | 11.4 ± 1.9 | 10.9 ± 1.7 | 0.21 |
| Portal trunk velocity (cm/s) | 12.5 ± 2.7 | 13.0 ± 3.2 | 0.46 |
| Collateral vessel (-/+) | 8/56 | 4/30 | 1.000 |
| Spleen volume (cm3) | 490.4 ± 257.2 | 432.0 ± 245.4 | 0.28 |
| HVPG (mmHg) | 14.6 ± 4.8 | 15.2 ± 5.4 | 0.56 |
*, Hepatitis C virus/alcohol/primary biliary cholangitis/nonalcoholic steatohepatitis/hepatitis B virus/autoimmune hepatitis/others
MELD, model for end-stage liver disease
†: Sustained virologic response for hepatitis C virus or no detection of hepatitis B virus DNA
HVPG, hepatic venous pressure gradient
The survival rate showed no difference between patients with HVPG > 12 mmHg (86.8% at 1 year, 60.9% at 3 years, and 53.3% at 5 years) and those with HVPG ≤ 12 mmHg (93.1% at 1 year, 71.8% at 3 years, and 71.8 at 5 years; P = 0.38). In addition, there was no significant difference in the survival rate between patients with portal velocity ≥ 12.8 cm/s (88.6% at 1 year, 65.6% at 3 years and 62.2% at 5 years) and those with portal velocity < 12.8 cm/s (90.3% at 1 year, 63.4% at 3 years and 55.7% at 5 years; P = 0.81).
MML and clinical findings
Thirty-four patients (34.7 %) were diagnosed with having MML. Clinical findings were compared between patients with and without the presence of MML (Table 3); significant difference was detected in the body mass index. Muscle volume presented by L3-SMI and HVPG showed correlation neither in the group with MML (r = 0.122, P = 0.49) nor in that without MML (r = -0.096, P = 0.45). Also, muscle volume showed no correlation with portal velocity (r = -0.171, P = 0.1). Prevalence of MML was not significantly different between the patients with HVPG ≤ 12 mmHg (10/29, 34.5%) and HVPG > 12 mmHg (24/69, 42%; P = 0.98) and those with portal velocity ≥ 12.8 cm/s (19/44, 43.2%) and portal velocity < 12.8 cm/s (15/52, 28.8%; P = 0.14). The median interval between US and CT was 4.5 days.
Impact of MML on disease prognosis with respect to portal hemodynamics
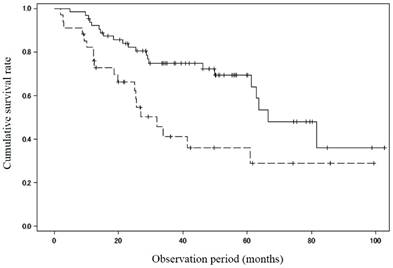
Thirty-eight patients died during the study period, 23 of hepatic failure, 6 of HCC, 1 of gastrointestinal bleeding, 1 of esophageal cancer, and 7 with unknown reason. In addition, 3 patients underwent liver transplantation. There was a significant difference in the survival rate between patients with MML (n = 34; 82.2%/1 year, 41.2%/3 years and 36.1%/5 years) and those without (n = 64; 92.1%/1 year, 74.9%/3 years and 69.4%/5years; P = 0.005) (Figure 1).
Cumulative survival rate between patients with and without muscle mass loss. There was a significant difference in the survival rate between patients with muscle mass loss (n = 34; 82.2%/1 year, 41.2%/3 years and 36.1%/5 years) and those without (n = 64; 92.1%/1 year, 74.9%/3 years and 69.4%/5 years; P = 0.005). Solid line: patients without muscle mass loss, dotted line: patients with muscle mass loss
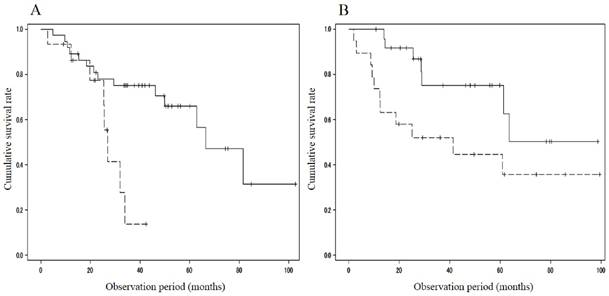
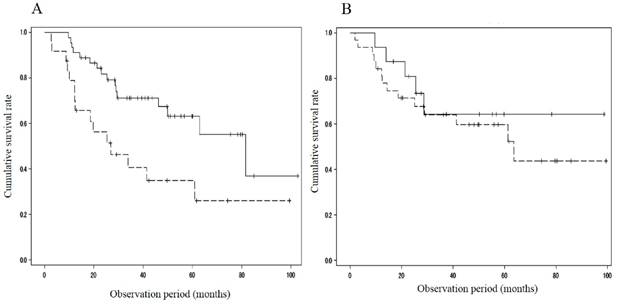
When divided with respect to the portal velocity, the survival rate showed differences between patients with MML (n = 15; 93.3%/1 year and 13.9%/3 years) and those without (n = 37; 89.2%/1 year, 75.1%/3 years and 65.9%/5 years; p=0.009) in the cohort with portal velocity < 12.8 cm/s, and between patients with MML (n = 19; 73.7%/1 year, 52.1%/3years and 44.7%/5 years) and those without (n = 25; 100%/1year, 75.3%/3 years and 75.3%/5 years; p=0.041) in the cohort with portal velocity ≥ 12.8 cm/s (Figure 2). The cumulative survival rate also showed significant difference between patients with MML (n = 24; 78.8%/1 year, 40.6%/3 years and 34.8%/5 years) and those without (n = 45; 91.1%/1 year, 71.3%/3 years and 63.1%/5 years; P = 0.008) in the cohort with HVPG > 12mmHg (Figure 3A). However, in the cohort with HVPG ≤ 12 mmHg, there was no significant difference in the survival rate between patients with MML (n=10, 100%/1 year, 61.9%/3 years and 61.9%/5 years) and those without (n=19, 93.8%/1 year, 71.2%/3 years and 59.4%/5 years; p = 0.493) (Figure 3B).
Comparison of cumulative survival rate between patients with and without muscle mass loss in the cohort with portal velocity < 12.8 cm/s or ≥ 12.8 cm/s. A. Portal velocity < 12.8 cm/s. Survival rate showed differences between patients with muscle mass loss (n = 15; 93.3%/1 year and 13.9%/3 years) and those without (n = 37; 89.2%/1 year, 75.1%/3 years and 65.9%/5 years; p=0.009). B. Portal velocity ≥ 12.8 cm/s. Survival rate showed differences between patients with muscle mass loss (n = 19; 73.7%/1 year, 52.1%/3 years and 44.7%/5 years) and those without (n = 25; 100%/1 year, 75.3%/3 years and 75.3%/5 years; p=0.041). Solid line: patients without muscle mass loss, dotted line: patients with muscle mass loss
Comparison of cumulative survival rate between patients with and without muscle mass loss in the cohort with HVPG > 12 mmHg or ≤ 12 mmHg. A. HVPG > 12 mmHg. Survival rate showed significant difference between patients with muscle mass loss (n = 24; 78.8%/1year, 40.6%/3years and 34.8%/5years) and those without (n = 45; 91.1%/1year, 71.3%/3years and 63.1%/5years; P = 0.008). B. HVPG ≤ 12 mmHg. There was no significant difference in the survival rate between patients with muscle mass loss (n=10, 100%/1year, 61.9%/3years and 61.9%/5years) and those without (n=19, 93.8%/1year, 71.2%/3years and 59.4%/5years; p = 0.493). HVPG: hepatic venous pressure gradient, solid line: patients without muscle mass loss, dotted line: patients with muscle mass loss
Discussion
The present study focused on the influence of portal hemodynamics on the MML-induced prognosis in cirrhosis. Interestingly, there is a compensating effect of lower HVPG against the poor prognosis, which may be the first to report, to the best of our knowledge. In other words, a presence of MML may have less influence in patients who do not have advanced portal hypertension, which indicates the importance of a multifactorial assessment in cirrhosis. The reason for the compensation may be because of the potent role of portal pressure on the long-term outcome in cirrhosis. Recent studies have shown the effect of non-invasive tools which might replace HVPG measurement, for example, elastography, serum markers, and the evaluation of parameters by magnetic resonance imaging or contrast-enhanced US [34]. The combined application of these markers may be the next strategy for forming a prediction formula of prognosis together with the diagnosis of MML.
The study highlighted two portal factors, portal pressure and portal velocity, and the cut-off value (≤ 12.8 cm/s) for the latter was derived from the velocity to predict the decompensation [19]. The reason for less effective results may be that portal slow velocity is an indirect factor for the severity of portal hypertension, reflecting various hemodynamics abnormality, such as intra/extrahepatic collaterals, intrahepatic vascular resistance and splanchnic inflow. However, survival rate in patients with both MML and portal velocity ≤ 12.8 cm/s was 93.3% at 1 year and 13.9% at 3 years. Also, in patients with both MML and HVPG > 12 mmHg, the survival rate was 78.8% at 1 year, 40.6% at 3 years and 34.8% at 5 years. These data appear comparable to or worse than the data in patients with MML in the literature; 85% at 1year and 63% at 3 years [6], and 63% at 1 year and 51% at 3 years [35], probably due to the synergistic effect of MML and advanced portal hypertension presented by slow portal velocity and/or higher portal pressure. Meanwhile, there was no difference in the survival rate between patients with HVPG > 12 mmHg and those with HVPG ≤ 12 mmHg in our study. Zipprich et al reported that age and HVPG were independent predictors of death in compensated patients, but not in decompensated patients [36]. In addition, recent two studies have failed to detect HVPG as a significant prognostic factor [37, 38]. Therefore, the prognostic influence of the HVPG is under debate, and may depend on the other confounding factors such as patient condition, supporting our results.
Grading of cirrhosis may offer a classification of patients, leading to an individualized management plan to improve the quality of medical care [39]. In addition to the existing models such as the Child-Pugh classification, MELD/MELD-Na, and compensation/decompensation, D'amico et al reported in-depth grading by combining data from 2 large studies including 1649 patients; the classification of stage 1 to 4 according to liver compensation/decompensation and the presence of portal hypertension was correlated with prognosis [40]. However, considering the results of our study, it may be the time to include the presence or absence of MML in such grading systems, in addition to the factors of portal hypertension.
Positive effects by TIPS result in apparent benefits in cirrhosis patients due to the reduction of portal pressure. Various changes in body composition can appear after TIPS, such as an improvement in fat-free mass and fluid-free or ascites-free body weight, and muscle strength [41]. A recent study has shown that total psoas and paraspinal muscle area increased significantly after TIPS, from 22.8 ± 0.9 to 25.1 ± 0.9 cm and 54.5 ± 1.3 to 57.9 ± 1.5 cm, respectively [42]. In addition, failure to reverse MML after TIPS was accompanied by higher mortality (43.5%) compared with patients in whom the total muscle area increased (9.8%). These data may help to explain the potential link between portal hemodynamics and MML presented by our study. Moreover, as investigators have suggested the benefit of an earlier application of TIPS [43, 44], we should examine whether the early intervention might contribute to a suppression of the negative synergistic effect by MML in cirrhosis.
There are some limitations to our study. The major one is that the present study is not a prospectively designed study. Next, the study used Japanese cut-off value for diagnosing sarcopenia [29], which may be relatively lower than the value in the Western research [28]. In addition, the number of patients with alcohol-related liver disease may be fewer than in Western studies due to the culture of our country. Along with the relatively small sample size, these issues need to be validated in the additional international studies including larger patient population.
In conclusion, the study clearly demonstrated the compensating effect by lower HVPG on the poor prognosis caused by an impaired muscle condition in cirrhosis. Care should be taken in the evaluation of the influence of MML in consideration of the severity of portal hypertension.
Abbreviations
MML, muscle mass loss; HCC, hepatocellular carcinoma; HVPG, hepatic venous pressure gradient; US, ultrasound; CT, computed tomography; SMI, skeletal muscle index; MELD, model for end-stage liver disease; TIPS, transjugular intrahepatic portosystemic shunt.
Competing Interests
The authors have declared that no competing interests exist.
References
1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761
2. Cárdenas A, Ginès P. Management of patients with cirrhosis awaiting liver transplantation. Gut. 2011;60:412-421
3. Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int. 2010;21:543-559
4. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F. et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39:412-423
5. Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225-237
6. Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A. et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193-199
7. Hiraoka A, Aibiki T, Okudaira T, Toshimori A, Kawamura T, Nakahara H. et al. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J Gastroenterol. 2015;50:1206-1213
8. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Fujimoto Y, Ogawa K. et al. Muscle steatosis is an independent predictor of postoperative complications in patients with hepatocellular carcinoma. World J Surg. 2016;40:1959-1968
9. Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G. et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640-648
10. Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J. et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151-1157
11. Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T. et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131-140
12. Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T. et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol. 2015;50:323-332
13. Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715-1728
14. Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R. et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488
15. Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A. et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923-928
16. Stremitzer S, Tamandl D, Kaczirek K, Maresch J, Abbasov B, Payer BA. et al. Value of hepatic venous pressure gradient measurement before liver resection for hepatocellular carcinoma. Br J Surg. 2011;98:1752-1758
17. Baik SK. Haemodynamic evaluation by Doppler ultrasonography in patients with portal hypertension: a review. Liver Int. 2010;30:1403-1413
18. Vizzutti F, Arena U, Rega L, Romanelli RG, Colagrande S, Cuofano S. et al. Performance of Doppler ultrasound in the prediction of severe portal hypertension in hepatitis C virus-related chronic liver disease. Liver Int. 2007;27:1379-1388
19. Kondo T, Maruyama H, Sekimoto T, Shimada T, Takahashi M, Okugawa H. et al. Impact of Portal Hemodynamics on Doppler Ultrasonography for Predicting Decompensation and Long-term Outcomes in Patients with Cirrhosis. Scan J Gastroenterol. 2016;51:236-244
20. Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, Ponziani F. et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol. 2009;51:682-689
21. Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection-analysis of 300 cases. Radiology. 2003;227:89-94
22. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524
23. Sacerdoti D, Bolognesi M, Bombonato G, Gatta A. Paraumbilical vein patency in cirrhosis: effects on hepatic hemodynamics evaluated by Doppler sonography. Hepatology. 1995;22:1689-1694
24. Tajiri T, Yosida H, Obara K, Onji M, Kage M, Kitano S. et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010;22:1-9
25. Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653
26. Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy-definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna 1998. Hepatology. 2002;35:716-721
27. Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-635
28. Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG. et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-173
29. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951-963
30. Debernardi-Venon W, Bandi JC, García-Pagán JC, Moitinho E, Andreu V, Real M. et al. CO2 wedged hepatic venography in the evaluation of portal hypertension. Gut. 2000;46:856-860
31. Chawla Y, Santa N, Dhiman RK, Dilawari JB. Portal hemodynamics by duplex Doppler sonography in different grades of cirrhosis. Dig Dis Sci. 1998;43:354-357
32. D'Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624
33. Albillos A, Banares R, Gonzalez M, Ripoll C, Gonzalez R, Catalina MV. et al. Value of the hepatic venous pressure gradient to monitor drug therapy for portal hypertension: a meta-analysis. Am J Gastroenterol. 2007;102:1116-1126
34. Thabut D, Moreau R, Lebrec D. Noninvasive assessment of portal hypertension in patients with cirrhosis. Hepatology. 2011;53:683-694
35. Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG. et al. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209-1216
36. Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. 2012;32:1407-1414
37. Ripoll C, Lastra P, Rincon D, Catalina V, Banares P. Comparison of MELD, HVPG, and their changes to predict clinically relevant endpoints in cirrhosis. Scand J Gastroenterol. 2012;47:204-211
38. Giannini EG, Savarino V, Farinati F, Ciccarese F, Rapaccini G, Marco MD. et al. Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int. 2013;33:1594-1600
39. Huo TI, Lee SD, Lin HC. Selecting an optimal prognostic system for liver cirrhosis: the model for end-stage liver disease and beyond. Liver Int. 2008;28:606-613
40. D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol. 2006;44:217-231
41. Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int. 2011;3:1250-1258
42. Tsien C, Shah SN, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013;25:85-93
43. García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A. et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379
44. Halabi SA, Sawas T, Sadat B, Jandali A, Halabi HA, Halabi FA. et al. Early TIPS versus endoscopic therapy for secondary prophylaxis after management of acute esophageal variceal bleeding in cirrhotic patients: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2016;31:1519-1526
Author contact
![]() Corresponding author: Hitoshi Maruyama, TEL: 81-43-2262083, FAX: 81-43-2262088, E-MAIL: maru-cibac.jp
Corresponding author: Hitoshi Maruyama, TEL: 81-43-2262083, FAX: 81-43-2262088, E-MAIL: maru-cibac.jp

 Global reach, higher impact
Global reach, higher impact