3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2017; 14(8):711-720. doi:10.7150/ijms.20126 This issue Cite
Research Paper
Arterial Stiffness, Thickness and Association to Suitable Novel Markers of Risk at the Origin of Cardiovascular Disease in Obese Children
1. Research Area for Multifactorial Diseases, Children's Hospital Bambino Gesù, Rome, Italy;
2. Department of Clinical Biochemistry, Aalborg University Hospital and Clinical Institute, Faculty of Health, Aalborg University.
Received 2017-3-17; Accepted 2017-5-17; Published 2017-7-12
Abstract
Atherosclerosis origins early in childhood. Aim of the study was to investigate vascular signature and phenotypes of cardiovascular disease in obese children and adolescents identifying novel potential circulating markers of risk.
Cross-sectional study of intima-media-thickness (IMT), pulse wave velocity (PWV), augmentation index (AIX@75), circulating markers (E-selectin, soluble-intercellular-adhesion-molecule1_ICAM1, chemerin, fatty-acid-binding protein 4, sCD36, lipopolysaccharides_LPS, oxLDL, fetuin) in 123 obese (body mass index, BMI z-score >1.645 SD) children (N=55, age ≤10 years-old) and adolescents (N=68, age >10 years-old).
Adolescents had significantly higher uric acid (p=0.002), non-HDL-cholesterol (p=0.02), fasting glucose (p=0.04), systolic blood pressure (p=0.005) and PWV (p=0.02) than children.
Obese adolescent patients with metabolic syndrome (MetS) abnormalities had higher PWV (p<0.05) than peers without. No differences were observed in circulating biomarkers in relationship to age and MetS status. oxLDL, sCD36 and LPS were correlated to AIX@75 and/or IMTM in children and adolescents (p ranging from <0.05 to <0.0001). Total cholesterol, non-HDL-cholesterol, TG/HDL ratio, oxLDL, sCD36, ICAM1, LPS were significantly different across AIX@75 tertiles (p between 0.03 and 0.001).
Early phenotypes of cardiovascular alterations in young severely obese patients encompass increased IMT, stiffness of intermediate size and resistance vasculature. Novel biomarkers investigated in the present study were associated to estimates of stiffness and thickness not differently from traditional risk factor such as non-HDL-cholesterol and TG/HDL ratio.
Keywords: arterial stiffness, arterial thickness, children obesity, oxidized LDL, pulse wave velocity, sCD36.
Introduction and Background
Obesity-induced atherosclerosis starts early in life [1]. Due to the rise of childhood obesity prevalence, the assessment of determinants of atherosclerosis onset and progression in pediatric cohorts is challenging [2].
Arterial disease develops in a non-uniform fashion with arterial stiffening and/or thickening as result of excess adiposity [3]. Arterial stiffening is due to a complex pathophysiological process that encompasses adverse structural and functional alterations of the vascular wall. Indeed, the exposure to cardiovascular risk factors [i.e. high blood pressure, glucose, lipids including oxidized low density lipoproteins (oxLDL), etc.] promotes overproduction of collagen, reduces quantities of elastin, thus causing unorganized and dysfunctional fiber distribution, infiltration of vascular smooth muscle cells into the intima, and elevated smooth muscle tone [3,4]. Instead, arterial thickening is characterized by a steady accumulation of inflammatory molecules, complex lipids, and fibrin. Binding and recruitment of circulating monocytes to the vascular endothelium and further migration into the subendothelial space are key processes for atherosclerosis development also in children [5]. Once recruited monocytes infiltrate the intima by the help of cellular adhesion molecules that are expressed on the surface of vascular endothelial cells and whose circulating counterparts are endothelium-derived factors, such as the soluble intercellular adhesion molecule1 (ICAM-1) and E-selectin. This cascade of events causes monocyte differentiation into dendritic cells or CD36 positive macrophages that interact with atherogenic lipoproteins [5]. Macrophages CD36+ are important for scavenging and endocytosis of oxLDL and foam cell formation, which, in turn, produce and secrete pro-inflammatory chemokines and cytokines activating a vicious cycle (6). All these molecules released during the atherogenesis process can serve as markers of this condition [7].
Pulse wave velocity (PWV) and augmentation index (AIX) are measures of stiffness [8] while arterial intima media thickness (IMT) is estimated at the carotid site by B mode ultrasounds [9, 10].
In recent years, our research goal was the understanding of the origin of cardiovascular disease in overweight and obese children. We observed no association of arterial stiffness and thickness with degree of adiposity or cardiometabolic abnormalities early in preschoolers at the onset of obesity [2]. On the contrary, studies reported increased stiffness and thickness in obese adolescents with metabolic complications such as type 2 diabetes (T2D) [1, 11] and non-alcoholic fatty liver disease (NAFLD) [9] as compared to normal-weight controls. Few studies have investigated early atherosclerosis in school-age children but with inconsistent results [7, 12-14].
To fill this gap, we investigated brachial PWV, AIX and IMT as estimates of vascular health and examined their correlates with some circulating markers of endothelium dysfunction and inflammation and vessel wall thickening in a sample of obese (body mass index _BMI z-score_>1.645 SD) school-age children (≤10 years-old) and adolescents (>10 years-old).
Biomarkers were chosen as they represent distinct domains within the context of disturbed vascular biology associated with obesity. E-selectin and ICAM-1 associate with dysfunctional endothelium [7]; chemerin, fatty acid-binding protein 4 (FABP-4) and sCD36 are related to altered tissue lipogenesis, and enhanced insulin resistance (IR); sCD36 specifically to vessel wall cholesterol accumulation [15]; lipopolysaccharides (LPS) is a marker of low-grade inflammation that can interfere with development and stability of the plaque [16]; oxLDL is a signature of oxidative stress; fetuin is related to the risk of developing non-alcoholic fatty liver disease (NAFLD).
Hence, aim of the present study was to investigate vascular phenotypes and circulating markers of disturbed vascular biology in obese school-age children as compared to adolescents in a cross-sectional assessment. Association with metabolic syndrome (MetS) and single metabolic disturbances among high blood pressure, dyslipidemia, impaired glucose tolerance (IGT) and NAFLD was investigated.
Data Description
Patients
One-hundred twenty-three obese children (N=55) and adolescents (N=68) were enrolled among those consecutively referred for overweight and/or obesity by general pediatricians to the Units of Clinical Nutrition and Endocrinology at the ''Bambino Gesu'' Hospital (OPBG) between July 2012 and 2013. Children were randomly selected among those participating in the study “Profiling the genetic risk of complex diseases in the Italian population” which aims at identifying genetic profiles associated with increased risk of IGT [17].
Inclusion criteria were age ranging from 6 to 17.8 years; obesity (BMI z-score >1.645 SD), no previous treatment for obesity, no systemic and endocrine disease, no use of medication, alcohol or recreational drug.
Ethical Statement
The study protocol conformed to the 1975 Declaration of Helsinki and specifically to guidelines of the European Convention of Human Rights and Biomedicine for Research in Children. It was approved by the OPBG Ethics Committee.
Written informed consent was obtained from the parents/legal guardians, and patients' data was treated to guarantee confidentiality.
Anthropometric measurements and clinical examination
Weight was measured with an approved scale (90/384/EEC, SECA) with precision of 50 g and periodic calibration. Children were weighed with minimal dress and weight recorded to the last 100 g. Height (without shoes) was measured with a Holtain's stadiometer with precision of 0.1 cm and registered with approximation of 0.5 cm. The body mass index (BMI) and the BMI-z score (SDS) were calculated based on Italian age and sex-related standards [18]. Waist circumference (WC) was measured midway between the superior border of the iliac crest and the lower most margin of the ribs at the end of normal expiration and waist to height ratio (WTHR) calculated as rough estimate of visceral obesity.
Systolic (SBP) and diastolic blood pressure (DBP) were measured three times while the subjects were seated using an automated oscillatory system and appropriately sized arm cuffs (Dinamap; Criticon Inc), and the measurements were averaged.
Blood tests and biochemical assays
All the participants were asked to refrain from intensive physical activity in the 3 days prior to the study. Fasting blood samples were drawn after 8-12 h fast and concentrations of triglycerides (TG), high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL) cholesterol, and total cholesterol (TC) were assessed by using colorimetric kits (Roche/Hitachi Modular systems P/S, Can 433, Milan, Italy). Alanine aminotransferase (ALT), aspartate aminotransferase (ASP), γ -glutamyltransferase (γ-GT) and uric acid (UA) were measured (ADVIA 1650 Chemistry System; Bayer Diagnostics, Erlangen, Germany). Glucose was measured by the glucose oxidase technique (Cobas Integra, Roche, Rotkreuz, Switzerland) and insulin by chemiluminescent immunoassay method (ADVIA Centaur Analyzer; Bayer Diagnostics; Erlangen, Germany) on two fasting blood samples. For the measurement of circulating molecules blood samples were centrifuged at 8000 RPM for 12 minutes and stored at -80°C pending further analysis. Samples were thawed only once and measured according the manufacturer's procedures by using the following enzyme-linked immunosorbent assays (ELISA) kits: sICAM 1, sE-selectin, Fetuin, FABP4 (BioVendor, Modřice, Czech Republic); Chemerin, Lipocalin, (RayBiotech Inc, GA, USA); LPS by Limulus amoebocyte lysate chromogenic endpoint assay (Hycult Biotechnology, The Netherlands); oxLDL (Immundiagnostik AG, Germany; intra-assay-coefficient of variation 5.2 %).
Plasma sCD36 was measured by a well-established in-house ELISA essentially as described elsewhere [15]. Patients underwent standard oral glucose tolerance test (OGTT) and glucose (G) and insulin (I) levels were at baseline and every 30 min for 120 min.
Definition of metabolic abnormalities and calculation of metabolic indexes
Dyslipidemia was diagnosed as value of TC and/or TG higher than the 95th percentile and/or HDL-cholesterol lower than the 5th for age and sex [2, 17]. Hypertension (HT) was defined as SBP or DBP exceeding the 95th percentile for age, sex, and height [2, 17]. Impaired glucose tolerance (IGT) was diagnosed as 2-h glucose ≥140 mg/dl following the OGTT. NAFLD was suspected in the presence of ALT >40 U/l and ultrasound evidence of increased liver brightness after ruling out other conditions causing abnormalities of liver enzyme according to a standardized protocol [2].
Patients were grouped as affected by obesity with none of the metabolic abnormalities or obesity plus one or more abnormalities of the metabolic syndrome (MetS group) among hypertension, dyslipidemia (high TG or TC and/or low HDL-cholesterol), IGT and NAFLD.
HOMA-IR was calculated as average on two blood samples as [fasting glucose (mg/dl) × fasting insulin (µU/ml)/405]. Insulin sensitivity index (ISI) was calculated as:
[ISI =10,000/√(fasting glucose×fasting insulin)×(mean glucose×mean insulin)]
The TG to HDL-cholesterol ratio was calculated and a value ≥2.2 was considered as risky (“Atherogenic ratio”) [19]. Non HDL-cholesterol was calculated as TC minus HDL-cholesterol.
Arterial stiffness measures
Applanation tonometry was performed using SphygomoCor® (Atcor Medical, Sydney, NSW, Australia) with the subject in the supine position as described in Haller et al. [20]. The pulse wave of intermediate-sized arteries was recorded from the radial artery of the right arm (brachial PWV) with a high-fidelity micromanometer (SPC-301; Millar Instruments, Houston, TX, USA). All readings recorded met the manufacturer's quality control standards integrated into the software package. AIX was calculated as the difference between the second (caused by wave reflection) and the first systolic peak (caused by ventricular ejection). The average of three consecutive readings, each consisting of at least 20 sequentially recorded waveforms, was used for the analyses. AIX was adjusted for heart rate (AIX@75).
Measurement of Carotid IMT
Carotid artery ultrasound was performed (Siemens ACUSON X700™ equipped with VF10-5 linear array transducer, Siemens Erlangen, Germany). Subjects were placed in the supine position and images were taken from longitudinal sections of the carotid artery in a standardized fashion. Scans were stored digitally on the internal hard disk of the ultrasound system for subsequent analysis. The maximum value of IMT was taken as described in Pacifico et al. [10].
Statistical Analysis
Data are presented as median and inter-quartile range (IQR), unless otherwise stated (means and standard errors in figures 1 and 2) or as number and percentage. Distributions of continuous variables were examined for skewness and kurtosis and were logarithmically transformed when appropriate before analysis (G120, I120, SBP, DBP, non-HDL-cholesterol, TG/HDL ratio, PWV, oxLDL, HOMA, ISI, LPS, lipocalin, chemerin, FABP4). Differences between groups were tested for significance using independent samples t-test and analysis of variance (ANOVA) for quantitative variables, and chi-square test for qualitative variables. Pearson's correlation and linear regression coefficients were used to examine relationships between variables. The strength of these relations was expressed as coefficient and p value.
Stepwise linear regression analysis was performed to identify predictors of PWV.
A p value of <0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences (version 11.5; SPSS Inc., Chicago, IL, USA).
Results
Cardiovascular risk factors in children vs. adolescents
Adolescents had significantly different median BMI compared to children [28.9 (4) vs. 26.02 (4); p<0.0001] and WHTR [0.56 (0.06) vs. 0.59 (0.06), respectively; p=0.002] but not different BMI z-score. The same was for fasting glucose [79 (10) vs. 76 (11) mg/dl; p=0.037]; serum uric acid [7.9 (3.10) vs. 6.7 (2.4) mg/dl; p=0.002]; LDL-C [6 (2.2) vs. 5.3 (1.5) mg/dl; p=0.037] and non-HDL-cholesterol [97 (37) vs. 103 (37) mg/dl; p=0.02]; and SBP [105 (11) vs. 101 (7) mm/Hg; p=0.005].
No difference was found in circulating CVD biomarkers between age-groups.
Median PWV was significantly different in adolescents as compared to children [7.9 (3.1) vs. 6.7 (2.4) m/sec; p=0.02] while AIX@75 and MIMT were not.
MIMT, PWV, AIX@75 and circulating biomarkers of CVD risk in children and adolescents according to the MetS status
Number of children and adolescents with and without metabolic abnormalities was not significantly different (p=0.3). Metabolic abnormalities included high blood pressure (N=11, 8.9) and TG (N=19; 15.4%); low HDL-cholesterol (N=40; 32.5%); IGT (N=12, 9.7%); NAFLD (N=20; 16.2%) and prevalence of any single metabolic abnormality was not different in age-groups except for high TG (15% vs. 4%; p=0.002). Forty patients (32.5%) had HDL/TG ratio >2.2.
We reported in Table 1 anthropometrics, clinical characteristics and laboratory parameters of the whole sample (N=123; 61 males, 49.6%); of children versus adolescents with simple obesity (N=51, 41.5%) and of patients who were complicated by one or more metabolic abnormalities.
In the overall sample, median HDL-cholesterol (p<0.0001), TG/HDL-cholesterol ratio (p<0.0001); triglycerides (p<0.0001), fasting (p=0.01) and post load glucose (p<0.0001), uric acid (p=0.002), ALT (p=0.002) and AST (p<0.0001), HOMA-IR (0.009) ISI (0.001), PWV (p=0.03) and oxLDL (p=0.05) were statistically higher in cases with compared to patients without MetS abnormalities. Same differences were observed statistically significant in age-groups except for PWV that was higher only in adolescents with MetS abnormalities as compared to peers without (Table 1). Stepwise liner regression model identified BMI z-score as the best predictor (R2=0.290; β= 0.578; p<0.0001) of PWV in adolescents while TG/HDL ratio, PAS, ISI, uric acid and ALT did not.
sCD36 levels were significantly (p=0.05) higher in children with MetS than in peers with no abnormalities but not in adolescents.
Table 3 shows main correlations among atherogenic estimates. ICAM and E- selectin were significantly correlated each other (rho=0.289; p=0.006) as were sCD36 and oxLDL (rho=0.471; p<0.0001). sCD36 was the solo whose concentration was correlated to HOMA-IR (rho=0.197; p=0.03).
MIMT, and circulating biomarkers of CVD risk in cases with atherogenic ratio, high blood pressure, IGT and NAFLD
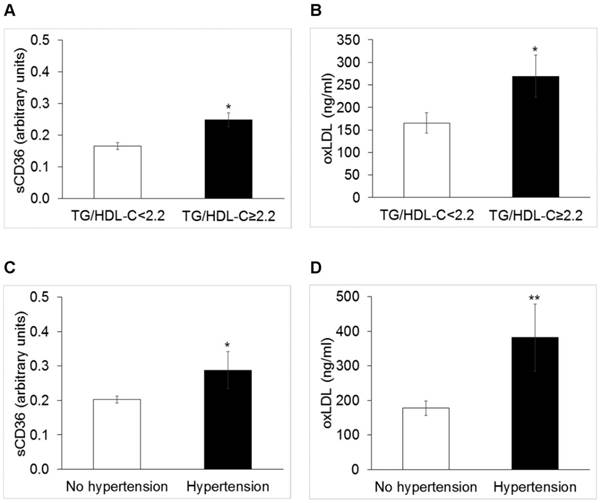
Cases with TG/HDL-C ≥ 2.2 (N=40) displayed circulating levels of sCD36 significantly higher than others [0.165 (0.010) vs. 0.24 (0.017) a.u., p=0.02]; oxLDL [156 (38.3) vs. 86 (18.6) mg/dl, p=0.05] (Figure 1), panels A and B), and HOMA-IR [2.62 (1.6) vs. 1.89 (1.9), p=0.03].
Cases with HT (N=11) showed significantly higher levels of sCD36 [0.26 (0.18) vs. 015 (0.17) a. u., p=0.05] and oxLDL [192 (67) vs. 69 (142) mg/dl, p=0.006] (Figure 1, panels C and D)].
IGT patients had significantly higher levels of lipocalin [19.8 (5) vs. 17.3 (1) vs. ng/ml; p=0.04].
Anthropometrics and cardiometabolic parameters of obese children and adolescents according to the metabolic syndrome (MetS) status.
| Whole sample (N=123) | Children (N=55) | Adolescents (N=68) | |||
|---|---|---|---|---|---|
| No MetS (N=18; 33%) | MetS (N=37; 67%) | No MetS (N=30; 44%) | MetS (N=38; 56%) | ||
| Age (years) | 10.4 (3) | 9 (1.09) | 8.6 (1.57) | 11.4 (2.95) | 12.5 (3.24) |
| Sex (M/F) | 58/65 | 9/9 | 15/22 | 15/15 | 19/19 |
| Body weight (kg) | 59.1 (20) | 50 (14) | 48.5 (14) | 60.8 (15) | 71 (26) |
| BMI z-score (SDS) | 2.20 (1) | 2.06 (0) | 2.28 (0.05)a | 2.02 (0) | 2.36 (1) a |
| WTHR | 0.58 (0.06) | 0.59 (0.05) | 0.60 (0.06) | 0.57 (0.07) | 0.56 (0.06) |
| Total cholesterol (mg/dl) | 145 (40) | 147 (51) | 151 (43) | 146.5 (47) | 139 (44) |
| HDL-cholesterol (mg/dl) | 44 (16) | 47 (7) | 41 (9) a | 50 (14) | 36 (12) c |
| TG/HDL-cholesterol ratio | 1.57 (0.98) | 1.39 (0.36) | 1.80 (1.08)b | 0.96 (0.52) | 1.90 (1.03) c |
| LDL-cholesterol (mg/dl) | 86 (35) | 88 (46) | 91 (26) | 78 (40) | 83 (36) |
| Non-HDL Cholesterol (mg/dl) | 99 (33) | 102 (47.5) | 106 (37) | 94.5 (39.7) | 98 (36) |
| Triglycerides (mg/dl) | 68 (36) | 66 (12) | 76 (49)* | 55 (27) | 76 (45) |
| Fasting glucose (mg/dl) | 78 (10) | 74 (9.8) | 79 (12.5) | 74 (11.5) | 81 (10)*** |
| Post load glucose (mg/dl) | 115 (20) | 114.5 (18.5) | 115 (26.5)* | 101.5 (22.3) | 124 (19)** |
| Uric acid (mg/dl) | 5.5 (2) | 4.8 (0.9) | 5.3 (2.0) | 5.50 (1.7) | 6.4 (2.4)** |
| ALT (uUI/ml) | 22 (12) | 23 (9) | 24 (7)** | 17.5 (7) | 25 (27)** |
| HOMA-IR | 2.2 (0.2) | 1.80 (1.4) | 2.4 (1.5) | 1.7 (2.1) | 3.3 (3.2)* |
| ISI | 3.8 (3.4) | 5.3 (3) | 3.68 (2.8)* | 4(2.7) | 2.57 (3.7)* |
| SBP (mm/hg) | 104 (9) | 102 (8) | 101 (9) | 103.5 (11) | 106 (15)** |
| DPB (mm/hg) | 69 (11) | 68 (6) | 69 (14) | 70 (11) | 70 (12) |
| PWV (m/s) | 7.4 (2.5) | 7.1 (1.75) | 6.4 (2.55) | 7.4 (1.55) | 8.1 (3.10)* |
| AIX@75 (%) | 5.75 (3.50) | 5.87 (3.69) | 6.5 (2.75) | 6 (3.7) | 5.50 (3.5) |
| MIMT (mm) | 0.5 (0.10) | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.09) | 0.5 (0.10) |
aData are expressed and median and interquartile range in parentheses. P refers to the statistical significance at the independent samples t-test between patients with and without abnormalities of the metabolic syndrome (MetS) belonging to the two class-age groups. * p≤0.05; ** ≤0.01; ***≤0.0001. ALT: alanine aminotransferase; AIX@75: augmentation index normalized by heart rate; BMI: body mass index; HOMA-IR: homeostatic model assessment of insulin resistance; ISI: insulin sensitivity index; LDL: low density lipoprotein; MIMT: maximum intima media thickness; MetS: metabolic syndrome; SBP and DBP: systolic and diastolic blood pressure; PWV: pulse wave velocity; TG/HDL-cholesterol: triglycerides to HDL-cholesterol; WHTR: waist to height ratio.
Circulating markers of cardiovascular disease in obese children and adolescents and without major metabolic syndrome (MetS) abnormalities
| Whole sample (N=123) | Children (N=55) | Adolescents (N=68) | |||
|---|---|---|---|---|---|
| No MetS (N=18; 33%) | MetS (N=37; 67%) | No MetS (N=30; 44%) | MetS (N=38; 56%) | ||
| Chemerin (ng/ml) | 1639 (58) | 1828 (56) | 1725 (68) | 1659 (63) | 1795 (57) |
| E-selectin (ng/ml) | 11 (0.82) | 7.81 (15) | 9.16 (8) | 14 (13) | 11.2 (11) |
| ICAM1 (ng/ml) | 289 (7.2) | 282 (149) | 291 (68) | 286 (61) | 280 (108) |
| FABP4 (ng/ml) | 19.3 (1.23) | 16.2 (13) | 16.4 (16) | 15.3 (20) | 13.3 (16) |
| Fetuin (µg/ml) | 1.09 (0.041) | 1.25 (1) | 1.12 (0) | 1.08 (0) | 0.85 (1) |
| Lipocalin (ng/ml) | 25.51 (3.9) | 17.34 (1) | 17.34 (21) | 15.9 (2) | 17.3 (2) |
| LPS (EU/ml) | 12.6 (0.88) | 12.25 (8) | 42 (74) | 11.43 (8) | 9.53 (5) |
| oxLDL (µg/ml) | 198 (22) | 42 (74) | 75 (150) | 85 (88) | 98 (343) |
| sCD36 (arbitrary units) | 0.209 (0.01) | 0.13 (0.06) | 0.17 (0.23)* | 0.14 (0.14) | 0.2(0.16) |
bData are expressed and median and interquartile range in parentheses. P refers to the statistical significance at the independent samples t-test between patients with and without abnormalities of the metabolic syndrome (MetS) belonging to the two class-age groups. *p≤0.05. ICAM1:soluble intercellular adhesion molecule1; FABP4: fatty acid-binding protein 4; LPS: lipopolysaccharide.
Correlates of PWV, Aix@75 and MIMT
| Whole sample (N=123) | Children (N=55) | Adolescents (N=68) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PWV | Aix@75 | MIMT | PWV | Aix@75 | MIMT | PWV | Aix@75 | MIMT | |
| Age (years) | 0.192* | - | - | - | - | - | - | - | - |
| BMI z-score | 0.431*** | - | - | 0.316* | - | - | 0.513*** | 0.276* | - |
| Uric acid (mg/dl) | 0.191* | - | - | 0.329* | - | - | - | 0.266* | - |
| Total Cholesterol (mg/dl) | - | 0.216* | - | - | - | - | - | - | 0.251* |
| LDL-cholesterol (mg/dl) | - | 0.222* | - | - | - | - | - | - | 0.268* |
| HDL/TG | - | 0.195* | - | - | - | - | - | - | 0.284* |
| Non HDL-Cholesterol (mg/dl) | - | - | - | - | 0.378 ** | 0.368 ** | - | 0.379 ** | 0.295* |
| LPS(EU/ml) | - | 0.242* | - | - | 0.341* | - | - | - | - |
| oxLDL (µg/ml) | - | 0.238 ** | 0.265 ** | - | 0.294* | - | - | 0.257* | |
| sCD36 (a.u.) | - | 0.184* | 0.278* | - | 0.365* | - | - | - | - |
cStatistical significant with *p<0.05; ** p<0.01 and *** p<0.001. BMI: body mass index; LPS: lipopolysaccharide. PWV: Pulse wave velocity; AIX@75 augmentation index normalized by heart rate, and MIMT: maximum intima media thickness.
Median values of sCD36 and oxidized low density lipoproteins (oxLDL) in obese patients with triglycerides to HDL-C ratio < 2.2 or ≥2.2 (Panel A and B, respectively; p<0.005 for both), and with and without hypertension (Panel C, p<0.05 and panel D p<0.01, respectively).
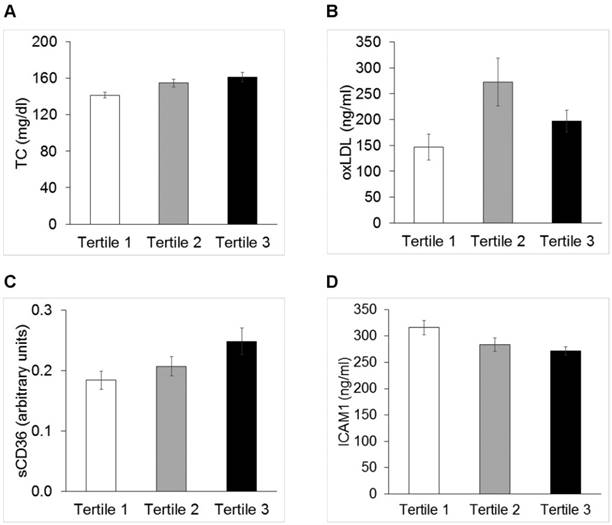
Tertiles stratification of MIMT, PWV, and AIX@75
Distribution of anthropometrics and circulating biomarkers of disturbed vascular biology were evaluated across tertiles of MIMT, PWV and AIX@75. BMI [26.0 (0.47) vs. 27.9 (0.41) vs. 29.5 (0.78) kg/m2, p<0.0001] and BMI z-score [1.93 (0.067) vs. 2.22 (0.05) vs. 2.41 (0.08) SDS; p<0.0001] were significantly different across PWV tertiles (P<0.0001 for both). Figure 2 shows mean values of TC, non HDL-cholesterol, oxLDL, sCD36, ICAM1, whose distributions were significantly different across tertiles of AIX@75.
Discussion
In the recent years, our research goal became the understanding of the origin of cardiovascular disease associated with early onset obesity. Metabolic abnormalities such as high blood pressure, dyslipidemia, impaired glucose metabolism and liver steatosis are diagnosed in preschoolers with overweight not later than one year after the onset of excess weight. At that time, arterial thickness and stiffness did not correlate with the degree of adiposity or metabolic derangement [2].
Findings of the present investigation suggest that associations between arterial thickness and/or stiffness with metabolic abnormalities manifest later in childhood and adolescence. They may be, however, weak at first (see Table 3) and their early recognition requires use of different techniques, in our case tonometry and ultrasounds combined with circulating biomarkers to look at different features and pathogenic culprits of the impaired vascular biology associated with obesity.
Cardiovascular risk factors in children vs. adolescents
In the present study, significant differences were observed in lipid profile, uric acid and blood pressure and brachial PWV between age-groups. No differences were found in circulating molecules associated with impaired vascular biology, AIX@75 and IMTM.
While the degree of obesity as measured by the BMI z-score was not different, the BMI and the waist to height ratio were significantly higher in children than in adolescents. The pubertal development influences the body shape and adiposity and indeed, large epidemiological studies show that prevalence of high WTHR is significantly higher in children than in adolescents [21].
Total cholesterol (Panel A, TC p=0.01,), oxidized low density lipoproteins (Panel B, oxLDL p<0.05), sCD36 (Panel C, p<0.05) and soluble intercellular adhesion molecule1 (Panel D, ICAM1 p<0.05) across tertiles of AIX@75.
While differences in the lipid profile between children and adolescents may be expected owing to the different age-dependent intake of carbohydrates and hence limiting their ability to serve as marker of risk [22], PWV seems depending on aging and body growth in a way that is mostly independent of the other cardiovascular risk factors. Aortic PWV, indeed, increased on average by 1 m/s from 3 to 18 years of age being largely influenced by the body growth at the passage from childhood to adolescence [23]. In a large study of 573 healthy children, the relationship between PWV and BMI was independent of other CV risk factors including degree of IR as estimated by the HOMA-IR [24].
PWV vs. AIX@75
In our series too, brachial PWV was correlated with BMI in the whole sample and in adolescents being higher in cases with metabolic abnormalities respect to those without. Conversely, in children we observed weak but significant correlation of traditional and not traditional CVD humoral markers with AIX@75 (Table 3).
PWV is a direct measure of stiffness while AIX is an indirect measure. AIX reflects the combined effect of magnitude and timing of the reflected waves that, in turn, are preliminarily related to peripheral vascular resistance and distensibility of the aortic wall [3]. Since it reflects the combination of all these features associated with obesity, AIX might represent a marker of altered vascular health more precocious than increased PWV [3]. Brachial PWV is a suitable stiffness index of intermediate-sized arteries and AIX of resistance arteries [3].
The relationship between PWV and AIX remains matter of debate. In our series, there was no correlation between the two parameters as previously seen in a study of hypertensive adults [25]. Increased stiffness may precede thickening hence altering the endothelial function, promoting the decline of nitric oxide (NO) synthase activity, favoring further arterial stiffness and increased thickness. [4]. In that, the lack of association between measures of stiffness and thickness does not surprise and, indeed, it confirms previous findings in healthy young individuals from the Cardiovascular Risk in Young Finns Study [26]. The study by Tounian et al. [7] also found no difference in IMT but increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children.
Stiffness and thickness in youth
Age [23], clinical [25] and preclinical hypertension [1, 25], LDL-cholesterol, hyperglycemia [1, 29] and inflammation are known risk factors for arterial stiffness [28] and increased IMT in pediatric ages [12-14]. Age, sex and body growth influenced both IMT and PWV.
Cross-sectional studies of normal-weight 7-years-old children [14], overweight vs. normal-weight prepubertal individuals [13] and overweight young people [12], all demonstrated that increase of IMT occurring in childhood obesity is strongly related to the cardiovascular risk factors of obesity.
Among risk factors, impaired glucose metabolism arose as one of the most important promoting factor of both increased thickness [29] and stiffness in youth [29,30].
In our series, less than 10% of patient presented with high blood pressure or IGT and this might have affected results. We cannot exclude that NAFLD was underestimated [31] in the present study since we considered as affected exclusively cases with ultrasound evidence of liver brightness and ALT levels higher than 40 UI/L. In keeping with a previous study of ours, no correlation was found between IMT and ALT levels [9].
Circulating oxLDL and sCD36 and LPSs
In our study, we focused on novel possible markers of CVD to investigate their association with arterial thickness and brachial stiffness. We excluded C-reactive protein since the lack of significant association with early onset of CVD [2, 32] and increased femoral and brachial stiffness [29] found in previous studies. We found of particular interest oxLDL, sCD36 and LPS since their involvement in the pathogenesis and progression of atherosclerotic lesions.
oxLDL can promote the progression from increased stiffening to thickening in several ways; by leading to endothelial dysfunction through the angiotensin II type 1 receptor, through the quenching of NO and the decrease of its production and by the promotion of foam cell formation. In children, oxLDL levels were inversely related to the arterial nitrate-mediated dilatation likely being involved in early atherosclerosis [26]. In other studies, oxLDL was associated with the incidence of MetS, the sum of obesity, hyperglycemia, and hypertriglyceridemia and the presence of T2D compared to normal-weight controls [27].
CD36 is a multifunctional membrane protein expressed by many cell types and important for fat uptake in the gut and accumulation in the liver. It serves as scavenger receptor for oxLDL and macrophage CD36 plays a crucial role in arterial cholesterol accumulation and early CVD [6]. sCD36, its non-cell bound circulating form, reflects tissue CD36 expression [15]. Circulating levels of sCD36 were associated with increased insulin resistance and arterial thickness in the healthy population of the Relationship between Insulin Sensitivity and Cardiovascular disease study [15]. Very recently, it a decrease of sCD36 in weight losing children in parallel with the amelioration of their IR and hepatic steatosis was reported [33]. The association between arterial dysfunction and sCD36 and oxLDL presented here supports cholesterol accumulation as an early process in the origin of obesity related CVD. Mechanistically, insulin resistance increases CD36 expression [15] and thereby the risk of CD36 mediated cholesterol accumulation in arteries.
LPSs have a role in the pathogenesis of CVD by promoting the release of proinflammatory cytokines, leading to severe endothelial dysfunction, plaque formation and rupture, oxidation of LDLs, and thrombogenesis as extensively reviewed elsewhere [16, 34].
In our series, there were weak but significant correlations between levels of these molecules and AIX@75 and/or IMTM. Nevertheless, these molecules did not perform better than known risk factors such as blood lipids and their ratio. Recent literature has put emphasis on the HDL to triglyceride ratio as risk factor and even marker of organ damage in obese youth [19].
Strength and weakness of the study
The strength of our study is the deep phenotyping with an extensive list of arterial functional, anthropological and biochemical parameters evaluated in the obese children and adolescents in a “quasi-longitudinal” (study of children vs. adolescents) study by the use of two different techniques to assess early atherosclerosis and the matching with circulating levels of various molecules that have been associated with impaired vascular biology. Major caveats are the lack of normal-weight controls, the cross-sectional design, the limited number of patients with each comorbidity that may have underpowered results; and the lack of information on the pubertal status that influences vascular structure and response [7]. Furthermore, we measured peripheral stiffness and aotic stiffness that has the best association with the CVD risk. We are also aware that measurement of oxLDL may be not always reliable as suggested by data distribution in the present series.
Despite weakness, findings of the present study can be informative for larger population studies of cardiovascular disease in young obese patients.
Conclusion
Our study responds to the need of investigations of early CVD associated with obesity in youth. Findings confirm that stigmata of atherosclerosis accompany obesity since its onset. Their recognition may require, nonetheless, simultaneous use of different measures and markers.
Abbreviations
ALT: alanine aminotransferase; AIX@75: augmentation index normalized by heart rate; ANOVA: analysis of variance; AST: aspartate aminotransferase; BMI: body mass index; CVD: cardiovascular disease; FABP-4: fatty acid-binding protein 4; HDL: High-density lipoprotein cholesterol; HOMA-IR: homeostatic model assessment of insulin resistance; ICAM1: soluble intercellular adhesion molecule1; IGT: impaired glucose tolerance; ISI: insulin sensitivity index; LDL: low-density lipoprotein cholesterol; LPS: lipopolysaccharides; MetS: metabolic syndrome; MIMT: maximum intima media thickness; OGTT: Oral glucose tolerance test; NAFLD: nonalcoholic fatty liver disease; oxLDL: oxidized LDL; SBP and DBP: systolic and diastolic blood pressure; PWV: pulse wave velocity; TG/HDL-C: triglycerides to HDL-cholesterol; WC: Waist circumference; WHTR: waist to height ratio.
Acknowledgements
The work was supported by grants from the Italian Ministry of Health (RF-OPG-2008-1142374 to MM; RC 201302R003008 to MM; ''Sviluppare profili genetici e trasferirli alla sanita` pubblica, in Italia''); by a grant to AH from the NovoNordisk Foundation (NNF11OC1014671 “Circulating CD36, Inflammation and the Metabolic Syndrome”.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contribution
Dr. Manco conceptualized and designed the study, analyzed data and interpreted results, wrote the first draft, and critically revised the manuscript; Dr. Nobili, Dr. Alisi and Dr. Panera contributed samples analysis and revised the manuscript for intellectual content, Dr Manco performed arterial ultrasounds and tonometry, Dr. Nobili enrolled patients, Dr. Handberg performed sCD36 tests, contributed the discussion and revised the manuscript for intellectual content.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Competing Interests
A Handberg is the inventor of two patent applications on sCD36 as a biomarker of the metabolic syndrome. The patent IP rights are owned by the Idea's Clinic of Aalborg University Hospital. The remaining authors have indicated they have no potential conflicts of interest to disclose. Other authors have indicated they have no financial relationships relevant to this article to disclose.
References
1. Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE. et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650-6
2. Shashaj B, Bedogni G, Graziani MP, Tozzi AE, DiCorpo ML. et al. Origin of cardiovascular risk in overweight preschool children: a cohort study of cardiometabolic risk factors at the onset of obesity. JAMA Pediatr. 2014Oct;168(10):917-24
3. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE. et al. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Pt 1):263-270
4. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932-943
5. Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013Oct;13(10):709-21 doi: 10.1038/nri3520
6. Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med. 2014Jun6;46:e99
7. Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B. et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400-1404
8. Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013Oct8;62(15):1309-19
9. Manco M, Bedogni G, Monti L, Morino G, Natali G. et al. Intima-media thickness and liver histology in obese children and adolescents with non-alcoholic fatty liver disease. Atherosclerosis. 2010Apr;209(2):463-8
10. Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, at al. Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res. 2008Apr;63(4):423-7
11. Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010Aug;28(8):1692-8
12. Reinehr T, Wunsch R, de Sousa G, Toschke AM. Relationship between metabolic syndrome definitions for children and adolescents and intima-media thickness. Atherosclerosis. 2008Jul;199(1):193-200
13. Giannini C, de Giorgis T, Scarinci A, Ciampani M, Marcovecchio ML, at al. Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre-pubertal children. Atherosclerosis. 2008Mar;197(1):448-56
14. Osiniri I, Sitjar C, Soriano-Rodríguez P, Prats-Puig A, Casas-Satre C. et al. Carotid intima-media thickness at 7 years of age: relationship to C-reactive protein rather than adiposity. J Pediatr. 2012Feb;160(2):276-280
15. Handberg A, Hojlund K, Gastaldelli A, Flyvbjerg A, Dekker JM. et al. Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J Intern Med. 2012;271:294-304
16. Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010Dec;31(6):817-44
17. Luciano R, Barraco GM, Muraca M, Ottino S, Spreghini MR. et al. Biomarkers of Alzheimer disease, insulin resistance, and obesity in childhood. Pediatrics. 2015Jun;135(6):1074-81
18. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000May6;320(7244):1240-3
19. Manco M, Grugni G, Di Pietro M, Balsamo A, Di Candia S. et al. Triglycerides-to-HDL cholesterol ratio as screening tool for impaired glucose tolerance in obese children and adolescents. Acta Diabetol. 2015 Dec 21
20. Haller MJ, Samyn M, Nichols WW, Brusko T, Wasserfall C. et al. Radial artery tonometry demonstrates arterial stiffness in children with type 1 diabetes. Diabetes Care. 2004Dec;27(12):2911-7
21. Hardy LL, Mihrshahi S, Gale J, Drayton BA, Bauman A. et al. 30-year trends in overweight, obesity and waist-to-height ratio by socioeconomic status in Australian children, 1985 to 2015. Int J Obes (Lond). 2016 Nov 29. doi: 10.1038/ijo.2016.204. [Epub ahead of print]
22. Knuiman JT, West CE, Katan MB, Hautvast JG. Total cholesterol and high density lipoprotein cholesterol levels in populations differing in fat and carbohydrate intake. Arteriosclerosis. 1987;7(6):612-9
23. Hidvégi EV, Illyés M, Benczúr B, Böcskei RM, Rátgéber L. et al. Reference values of aortic pulse wave velocity in a large healthy population aged between 3 and 18 years. J Hypertens. 2012Dec;30(12):2314-21
24. Urbina EM, Gao Z, Khoury PR, Martin LJ, Dolan LM. Insulin resistance and arterial stiffness in healthy adolescents and young adults. Diabetologia. 2012Mar;55(3):625-31
25. Matsui Y, O'Rourke MF, Ishikawa J, Shimada K, Kario K. Association of changes in ambulatory arterial stiffness index and pulse wave velocity during antihypertensive treatment: the J-CORE study. Am J Hypertens. 2012Aug;25(8):862-8
26. Koivistoinen T, Virtanen M, Hutri-Kähönen N, Lehtimäki T, Jula A. et al. Arterial pulse wave velocity in relation to carotid intima-media thickness, brachial flow-mediated dilation and carotid artery distensibility: the Cardiovascular Risk in Young Finns Study and the Health 2000 Survey. Atherosclerosis. 2012Feb;220(2):387-93
27. Järvisalo MJ, Lehtimäki T, Raitakari OT. Determinants of arterial nitrate mediated dilatation in children: role of oxidized low densitylipoprotein, endothelial function, and carotid intima-media thickness. Circulation. 2004Jun15;109(23):2885-9
28. Stringer DM, Sellers EA, Burr LL, Taylor CG. Altered plasma adipokines and markers of oxidative stress suggest increased risk of cardiovascular disease in First Nation youth with obesity or type 2 diabetes mellitus. Pediatr Diabetes. 2009Jun;10(4):269-77
29. Shah AS, Gao Z, Urbina EM, Kimball TR, Dolan LM. Prediabetes: the effects on arterial thickness and stiffness in obese youth. J Clin Endocrinol Metab. 2014Mar;99(3):1037-43
30. Wadwa RP, Urbina EM, Anderson AM, Hamman RF, Dolan LM. et al. Measures of arterial stiffness in youth with type 1 and type 2 diabetes: the SEARCH for diabetes in youth study. Diabetes Care. 2010Apr;33(4):881-6
31. Manco M, Alisi A, Nobili V. Risk of severe liver disease in NAFLD with normal ALT levels: a pediatric report. Hepatology. 2008Dec;48(6):2087-8 author reply 2088. doi: 10.1002/hep.22631
32. Moran A, Steffen LM, Jacobs DR Jr, Steinberger J, Pankow JS. et al. Relation of C-reactive protein to insulin resistance and cardiovascular risk factors in youth. Diabetes Care. 2005;28(7):1763-1768
33. Knøsgaard L, Kazankov K, Birkebæk NH, Holland-Fischer P, Lange A. et al. Reduced sCD36 following weight loss corresponds to improved insulin sensitivity, dyslipidemia and liver fat in obese children. European Journal of Clinical nutrition, 2016. 2016Sep;70(9):1073-7 doi: 10.1038/ejcn.2016.88
34. Manco M. Endotoxin as a missed link among all the metabolic abnormalities in the metabolic syndrome. Atherosclerosis. 2009Sep;206(1):36
Author contact
![]() Corresponding author: Melania Manco, MD, PhD, FACN, Scientific Directorate, Research Unit for Multifactorial Disease, Bambino Gesù Children's Hospital, Rome, Italy. E-mail: melania.manconet
Corresponding author: Melania Manco, MD, PhD, FACN, Scientific Directorate, Research Unit for Multifactorial Disease, Bambino Gesù Children's Hospital, Rome, Italy. E-mail: melania.manconet

 Global reach, higher impact
Global reach, higher impact