3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2017; 14(1):45-52. doi:10.7150/ijms.17202 This issue Cite
Research Paper
Steatosis influences the clinical profiles and long-term outcomes of interferon-treated chronic hepatitis C and liver cirrhosis patients
Division of Gastroenterology and Hepatology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan 30-1 Oyaguchi Kamicho, Itabashiku, Tokyo 173-8610, Japan.
Received 2016-8-15; Accepted 2016-11-1; Published 2017-1-1
Abstract
Objective: This study aimed to assess the relationship between steatosis and long-term outcomes of patients with chronic hepatitis C (CH) and liver cirrhosis (LC).
Patients and methods: The study population included 282 subjects with CH or LC who underwent liver biopsy at our institute. All patients achieved a sustained virological response (SVR) to interferon (IFN). Clinical characteristics, including age, gender and body mass index (BMI), were compared. The liver biopsy specimens of all patients were examined and scores were assigned to indicate the severity of each of the following features: inflammatory cell infiltration in the periportal, parenchymal and portal areas; F (fibrosis) stage; portal sclerotic change; perivenular fibrosis; pericellular fibrosis; bile duct damage; hepatic steatosis.
Results: Of the 282 patients, 112 (39.7%) were free of steatosis. The other 170 patients (60.3%) had steatosis. The blood biochemical parameters of the patients with hepatic steatosis were significantly poorer than those of patients free of steatosis. Inflammatory cell infiltration and F stage were both significantly more severe in patients with than in those without steatosis. The incidences of hepatocellular carcinoma differed significantly between the two groups. However, the incidences of hepatocellular carcinoma did not differ significantly between the groups with BMI above and below 25.
Conclusion: We consider hepatic steatosis to potentially affect the blood biochemical parameters and clinical profiles of Japanese patients with CH due to hepatitis virus type C. Patients with this form of CH showed favorable clinical responses to IFN. Furthermore, fibrosis and steatosis appear to affect the long-term outcomes of these patients. However, BMI alone cannot be used to predict HCC development.
Keywords: chronic hepatitis C, liver cirrhosis, sustained virological response, incidence of HCC, fibrosis, steatosis.
Introduction
The natural history of chronic hepatitis C involves progression to liver cirrhosis over a 30-year period in the majority of cases infected with the hepatitis C virus (HCV), and this group has an extremely high risk of developing hepatocellular carcinoma (HCC) [1, 2] .
Concomitant viral and host-associated factors, such as HCV genotype, serum HCV RNA load, age, and IL28B single nucleotide polymorphism are considered to impact both disease progression and responses to interferon (IFN) therapy [3-6] .
Recently, in developed nations, hepatic steatosis has been recognized as being associated with hyperlipidemia and obesity, reflecting an increase in lifestyle-related diseases such as diabetes mellitus (DM) [7-10] . Notably, hepatic steatosis is a relatively common feature of chronic hepatitis C infection [11] .
We evaluated the histological scoring of liver biopsy specimens obtained at our institute starting in 1992, and then prospectively observed the long-term outcomes of patients with steatosis. Although several studies have endeavored to determine if steatosis influences the long-term outcomes of patients with chronic hepatitis C [12, 13], whether steatosis is associated with liver injury in humans has not yet been clarified. Clinically, it is important to assess whether patients with hepatitis C who also have hepatic steatosis are at increased risk for developing HCC as compared to those who are infected with HCV but do not have hepatic steatosis.
Our present study aimed to assess the relationship between steatosis and the long-term outcomes of patients with chronic hepatitis C or liver cirrhosis who achieved a sustained virological response (SVR) to IFN.
Patients and Methods
Study population
Two hundred and eighty-two patients (174 males and 108 females) with type C chronic hepatitis or liver cirrhosis, who visited the Division of Gastroenterology and Hepatology, Nihon University Hospital to receive IFN therapy during the period from 1992 through 2013, were included in this study. We previously reported those treated during the period from 1992 through 2009 [14] . The patients treated during the period from 2009 through 2012, were administered combination therapy with Peg-IFN-α2a or r-α2b and ribavirin for 6-12 months. In 2013, patients were administered this combination therapy for 24 weeks and simeprevir for 12 weeks. (Figure 1) We considered a SVR to have been achieved in patients who remained negative for serum HCV RNA for > 24 weeks after the termination of IFN therapy. (Table 1)
Exclusion criteria were age less than 19 years or more than 75 years, habitual excessive alcohol intake (none of our subjects habitually drank alcohol in excess), the presence of HCC, the presence of hepatitis B surface antigen (determined by enzyme-linked immunosorbent assay [ELISA]; Abbott, Tokyo, Japan), the presence of anti-smooth muscle antibody (fluorescence antibody [Fluorescence antibody method; FA] method), the presence of anti-mitochondrial antibody (FA), current intravenous drug use, and a psychological state indicative of depression. A patient was diagnosed with hepatitis C if serum was positive for HCV antibody (second or third generation ELISA, Abbott Laboratories), and HCV RNA by reverse transcription (RT)-polymerase chain reaction (PCR). A serum sample was obtained at the time of liver biopsy and a portion was frozen at -80ºC until use.
Informed consent was obtained from all patients. Oral informed consent was obtained from those treated during the period from 1992 through 2000, while half of consents were oral and the other half written during the period from 2001 through 2013.
Two hundred and eighty-two patients (174 males and 108 females) with type C chronic hepatitis or liver cirrhosis were studied. Those managed during the period from 1992 through 2009 were previously reported [14]. Patients managed from 2009 through 2012 were administered combination therapy with Peg-IFN-α2a or r-α2b and ribavirin for 6-12 months. Patients managed in 2013 received this combination therapy for 24 weeks and simeprevir for 12 weeks.
Histology
Liver biopsy specimens were obtained by percutaneous needle biopsy (Tru-Cut soft tissue biopsy needles, 14 gauge; Baxter Healthcare, OK, USA), fixed in 10% buffered formalin and routinely embedded in paraffin. Paraffin-embedded specimens were cut into 3-4 μm sections and stained with hematoxylin and eosin. The liver biopsy specimens of all 282 patients were analyzed semi-quantitatively by assigning scores for each of the following features: the severity of inflammatory cell infiltration (0 for none, 1 for minimal, 2 for mild, 3 for moderate, and 4 for severe) in the periportal, parenchymal, and portal areas; the severity, or F stage, of fibrosis (0 for F0, indicating no fibrosis, 1 for F1, 2 for F2, 3 for F3, 4 for F4, indicating the most severe fibrosis) [15-17]; the severity scores (0 for none to 4 for severe) for portal sclerotic change, peri-venular fibrosis, and peri-cellular fibrosis; the severity of bile duct damage (0 for none to 4 for loss of all bile duct architecture); the presence of bridging necrosis (0 for none or 1 for existence); as described by Uchida [18, 19], Shibata et al. [20], and Ueno et al. [21]. Degree of liver steatosis was assessed according to the method of Brunt et al. [22] and Matteoni et al. [23]. The grade of steatosis in liver tissue was semi-quantitatively classified into 2 groups (absent; non-steatosis, scattered in several lobules to diffuse in all lobules; steatosis group). These two steatosis groups, consisting of patients with chronic hepatitis C or liver cirrhosis, were compared in terms of clinical characteristics, long-term outcomes and liver histological findings. The scores for all biopsy specimens were assigned independently by the first author and one of the co-authors (M. Moriyama). The latter had no knowledge of the patients' characteristics.
Clinical characteristics and blood and biochemical parameters
Clinical characteristics, including age, gender and body mass index (BMI), were compared between the steatosis and non-steatosis patients. The following levels indicating liver functions were measured employing a blood sample taken just before the liver biopsy: aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (γ-GTP), total bilirubin (T. Bil), total protein (TP), albumin (Alb), prothrombin time (PT), platelet counts and alfa fetoprotein (AFP).
Virology
HCV RNA was determined using a competitive RT-PCR method (Special Reference Laboratory, Co, Ltd, SRL, Tokyo, Japan), the DNA probe method (SRL, Tokyo, Japan) or the amplicor monitor method (Amplicor HCV Monitor, Roche Diagnostic K.K., Tokyo, Japan). The HCV genotype was determined by the method of Okamoto et al. [24], and described according to Simmond's classification [25].
Follow-up schedule
The subjects chosen for follow-up study were patients undergoing an initial liver biopsy. The subjects then underwent abdominal ultrasonography every 3 to 6 months, and abdominal dynamic computed tomography or Magnetic Resonance Imaging examination every 6 to 12 months, for detection of HCC. Furthermore, abdominal angiography or echo-guided tumor biopsies were performed for precise diagnosis in those who had an intrahepatic space occupying lesion.
Statistical Analysis
Gender and liver histology were compared between the groups with and without steatosis using the chi-square test for independence, while other parameters were compared using analysis of variance and Fisher's Protected Least Significant difference post hoc test with JMP 12 software (SAS Institute Inc., USA). Cumulative incidence curves were determined by the Kaplan-Meier method, and the differences between groups were assessed using the log-rank test. A p value of less than 0.05 was regarded as significant.
Multivariate analysis of the development of HCC from chronic hepatitis and liver cirrhosis
We carried out a multivariate regression analysis to assess the risk of HCC developing from CH due to HCV and/or LC. Clinical and laboratory findings of the 282 patients in whom steatosis had been identified based on the initial liver biopsy, or when HCC was diagnosed, were investigated. Inflammatory cell infiltration in the periportal, parenchymal and portal areas, portal sclerotic change, peri-venular fibrosis, peri-cellular fibrosis, bile duct damage, bridging necrosis, fibrosis, and steatosis, on the initial liver biopsy, were assessed. JMP 12 software (SAS Institute Inc., USA) was used to perform a stepwise logistic regression analysis of these parameters as independent risk factors for developing HCC.
Results
Clinical characteristics of patients with and without steatosis
Clinical characteristics of patients with and without steatosis are summarized in Table 1. There were no statistically significant differences between steatosis and non-steatosis patients in age (47±13 vs 49±11 p=0.12), gender (56.5% vs 65.2% p=0.16), T. Bil (0.75±0.96 vs 1.76±0.86 mg/dl p=0.21), TP (7.1±1.0 vs 7.2±0.59 g/dl p=0.39), Alb (5.0±6.2 vs 4.2±0.4 g/dl p=0.24), PT (94±8 vs 7.2±0.59 % p=0.64) or AFP (5±9.8 vs 114±887 ng/ml p=0.33). Average observation periods also did not differ significantly between the two groups (9.41±6.39 years vs 8.51±5.92 years p=0.23). However, BMI (22.3±3 vs 23.5±3.5 IU/L p<0.01), AST (43±31 vs 63±51 IU/L p<0.01), ALT (59±58 vs 86±65 IU/L p<0.01), ALP (211±86 vs 243±102 IU/L p<0.01), γ-GTP (49±89 vs 72±65 IU/L p<0.01), and platelet counts (20.0±6.2 vs 17.9±5.7 ×104/mm3 p<0.01) levels were significantly higher in those with steatosis.
Clinical characteristics of non-steatosis and steatosis patients.
| Non-steatosis | Steatosis | P | |
|---|---|---|---|
| Number | 112 | 170 | |
| Age (years) | 47±13 | 49±11 | 0.12 |
| Gender: Male (%) | 63(56.5%) | 111(65.2%) | 0.16 |
| Median observation period (years) | 9.41±6.39 | 8.51±5.92 | 0.23 |
| BMI | 22.3±3.3 | 23.5±3.5 | 0.01 |
| AST (IU/L) | 42±31 | 63±51 | 0.01 |
| ALT (IU/L) | 59±58 | 86±65 | 0.01 |
| ALP (IU/L) | 211±86 | 243±102 | 0.01 |
| γ-GTP (IU/L) | 49±89 | 72±65 | 0.01 |
| T. Bil (mg/dl) | 0.75±0.96 | 1.76±0.86 | 0.21 |
| TP(g/dl) | 7.1±1.0 | 7.2±0.59 | 0.39 |
| Alb (g/dl) | 5.0±6.2 | 4.2±0.4 | 0.24 |
| PT (%) | 94±8 | 95±11 | 0.64 |
| Platelet counts (×104/mm3) | 20.0±6.2 | 17.9±5.7 | 0.01 |
| AFP (ng/ml) | 5±9.8 | 114 ±887 | 0.33 |
| HCV genotype 1 b | 59 | 74 | 0.11 |
| genotype 2a or 2b | 53 | 96 |
Frequency of steatosis according to fibrosis stage
As to fibrosis, 5 of the non-steatosis patients (2.1%) had grade F0, 73 (54.2%) F1, 17 (22.3%) F2, 12 (13.4%) F3 and 5 (7.8%) F4. Of the patients with steatosis, one (0.6%) had F0, 80 (47.1%) F1, 46 (27.1%) F2, 26 (15.2%) F3 and 17 (10.0%) F4. (Table 1) When the patients were divided into groups according to F stage, it was revealed that the frequency of steatosis differed significantly according to the severity of fibrosis. (p<0.01).
Frequency of steatosis according to fibrosis stage.
| Stage | Number | Non-steatosis | Steatosis |
|---|---|---|---|
| F0 | 6 | 5(2.1%) | 1(0.6%) |
| F1 | 153 | 73(54.2%) | 80(47.1%) |
| F2 | 63 | 17(22.3%) | 46(27.1%) |
| F3 | 38 | 12(13.4%) | 26(15.2%) |
| F4 | 22 | 5(7.8%) | 17(10.0%) |
| Total | 282 | 112(100%) | 170(100%) |
Pathological findings
As shown in Table 3, inflammatory cell infiltration in the periportal (1.73±0.69 vs 2.1±0.72; p<0.01), parenchymal (1.86±0.65 vs 2.12±0.61 p<0.01) and portal (2.33±0.60 vs 2.62±0.54 p<0.01) areas, portal sclerotic change (0.46±0.69 vs 0.72±0.79 p<0.01), peri-venular fibrosis (1.03±0.97 vs 1.42±0.92 p<0.01), peri-cellular fibrosis (0.66±0.75 vs 1.20±0.94 p<0.01), bile duct damage (0.48±0.80 vs 0.79±0.90 p<0.01) and bridging necrosis (0.01±0.94 vs 0.41±0.23 p<0.01) were significantly more severe in patients with steatosis. The mean scores for F stage were significantly greater in patients with steatosis.
Histological analysis of steatotic and non-steatotic samples.
| Histological analysis | Non-steatosis | Steatosis | p |
|---|---|---|---|
| Inflammatory cells; periportal | 1.73±0.69 | 2.1±0.72 | 0.01 |
| Inflammatory cells; parenchymal | 1.86±0.65 | 2.12±0.61 | 0.01 |
| Inflammatory cells; portal | 2.33±0.60 | 2.62±0.54 | 0.01 |
| Portal sclerotic change | 0.46±0.69 | 0.72±0.79 | 0.01 |
| Peri-venular fibrosis | 1.03±0.97 | 1.42±0.92 | 0.01 |
| Peri-cellular fibrosis | 0.66±0.75 | 1.20±0.94 | 0.01 |
| Bile duct damage | 0.48±0.80 | 0.79±0.90 | 0.03 |
| Bridging necrosis | 0.01±0.94 | 0.41±0.23 | 0.02 |
| F stage | 1.58±1.03 | 1.82±1.06 | 0.02 |
Incidence of HCC in patients with chronic hepatitis and liver cirrhosis
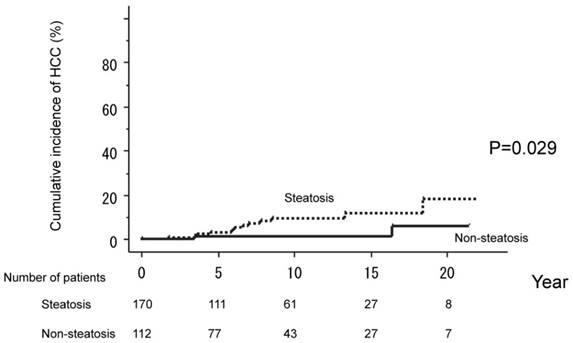
The 282 patients who achieved a SVR with IFN therapy were divided into two groups, based on serum parameters at the time of initial liver biopsy: non-steatosis (112 patients) and steatosis (170 patients). Cumulative HCC incidences were compared between these two groups. HCC occurred in only two patients (1.8 %) in the non-steatosis but in 12 (7.0%) in the steatosis group. The cumulative probability of HCC development in those without steatosis was 1.2% over five years, 1.2% over ten years, 1.2% over 15 years, and 5.9% over 20 years. The corresponding values for the steatosis group were 3.1%, 9.4%, 11.9%, and 18.2%. HCC incidences thus differed significantly between these two groups (P=0.029). (Figure 2)
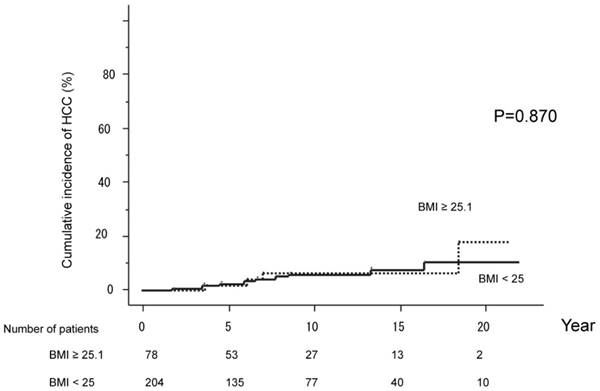
The cumulative HCC incidences were compared between groups with BMI under 25 (204 patients) versus 25.1 or higher (78 patients). HCC developed in 12 patients (10.5%) with BMI under 25, and 4 (17.8%) with BMI above 25. The cumulative probabilities of HCC development were 2.6%, 6.0%, 7.7% and 10.5% over five, ten, 15 and 20 years, respectively, in those with BMI under 25. The corresponding values for those with BMI of 25.1 or higher were 1.7%, 6.1%, 6.1%, 17.8% at five, ten, 15 and 20 years. HCC incidences did not differ significantly between the two BMI groups (P=0.870). (Figure 3)
Multivariate analysis of the development of HCC in chronic hepatitis and liver cirrhosis
In order to identify risk factors for the development of HCC, multivariate analyses were conducted using the Cox proportional hazard model.
Clinical and laboratory findings
BMI (Hazard ratio 1.04, 95%CI 0.88-1.21, P=0.63), AST (Hazard ratio 1.02, 95%CI 0.99-1.04, P=0.59), ALT (Hazard ratio 0.98, 95%CI 0.96-1.01, P=0.12), ALP (Hazard ratio 1.00, 95%CI 0.99-1.01, P=0.57), γ-GTP (Hazard ratio 0.98, 95%CI 0.99-1.01, P=0.87), and platelet counts (Hazard ratio 0.91, 95%CI 0.80-1.02, P=0.10) were not found to be significant risk factors (Table 4).
Inflammatory cell infiltration in the periportal (Hazard ratio 6.65, 95%CI 0.54-4.74, P=0.390), parenchymal (Hazard ratio 3.59, 95%CI 0.39-4.64, P=0.607), and portal (Hazard ratio 0.76, 95%CI 0.22-2.66, P=0.669) areas, portal sclerotic change (Hazard ratio 0.73, 95%CI 0.266-1.81, P=0.511), peri-venular fibrosis (Hazard ratio 0.32, 95%CI 0.06-1.30, P=0.121), peri-cellular fibrosis (Hazard ratio 1.67, 95%CI 0.40-6.58, P=0.479), bile duct damage (Hazard ratio 0.85, 95%CI 0.33-2.03, P=0.787) and bridging necrosis (Hazard ratio 0.71, 95%CI 0.02-7.39, P=0.802), assessed at the initial liver biopsy, were not found to be significant risk factors. However, these analyses revealed steatosis be a cardinal risk factor (Hazard ratio 4.92, 95%CI 0.13-3.54, P=0.031) and fibrosis to be a significant risk factor (Hazard ratio 2.74, 95%CI 1.61-4.93, P=0.001). The results are presented in Table 5.
Multivariate analysis of HCC development in chronic hepatitis and liver cirrhosis (BMI and laboratory data).
| Risk ratio | 95% Confidence interval | p | |
|---|---|---|---|
| BMI | 1.04 | 0.88 - 1.21 | 0.63 |
| AST | 1.02 | 0.99 - 1.04 | 0.59 |
| ALT | 0.98 | 0.96 - 1.01 | 0.12 |
| ALP | 1.00 | 0.99 - 1.01 | 0.57 |
| γ-GTP | 0.98 | 0.99 - 1.01 | 0.87 |
| Platelet counts | 0.91 | 0.80 - 1.02 | 0.10 |
Multivariate analysis of HCC development in chronic hepatitis and liver cirrhosis (Histological analysis).
| Histological analysis | Risk ratio | 95% Confidence interval | p |
|---|---|---|---|
| Inflammatory cells; periportal | 6.65 | 0.54-4.74 | 0.390 |
| Inflammatory cells; parenchymal | 3.59 | 0.39-4.64 | 0.607 |
| Inflammatory cells; portal | 0.76 | 0.22-2.66 | 0.669 |
| Portal sclerotic change | 0.73 | 0.266-1.81 | 0.511 |
| Peri-venular fibrosis | 0.32 | 0.06-1.30 | 0.121 |
| Peri-cellular fibrosis | 1.67 | 0.40-6.58 | 0.479 |
| Bile duct damage | 0.85 | 0.33-2.03 | 0.787 |
| Bridging necrosis | 0.71 | 0.02-7.39 | 0.802 |
| Fibrosis | 2.74 | 1.61-4.93 | 0.001 |
| Steatosis | 4.92 | 0.13-3.54 | 0.031 |
The 282 patients were divided into two groups, based on the absence of steatosis (112 patients) and the presence of steatosis (170 patients) at the time of liver biopsy. Serum parameters were compared between the two groups. HCC occurred in only two patients (1.8 %) in the non-steatosis but in 12 (7.0%) in the steatosis group. HCC incidences thus differed significantly between these two groups (P=0.029).
The cumulative HCC incidences were compared between the group with BMI under 25 (204 patients) and the group with BMI of 25.1 or higher (78 patients). HCC developed in 12 patients (10.5%) with BMI under 25 and 4 (17.8%) with BMI of at least 25.1. HCC incidences did not differ significantly between the two groups (P=0.870).
Discussion
Hepatic steatosis may affect the serological features of Type C chronic liver disease, according to the results of this study. These results are similar to those of a previous report [26-32]. However, we conducted further histopathologic examinations to determine whether or not steatosis influences the pathogenesis of liver disease and the long-term outcomes of patients with chronic hepatitis C.
Based on the results of our present histopathologic examinations, the prevalence of steatosis tends to increase with F stage progression. Histological fibrosis in chronic hepatitis C is reportedly significantly related to steatosis and, furthermore, steatosis accelerates hepatic fibrosis progression [14, 31, 33]. More detailed histopathological examinations are required to determine whether or not steatosis influences the pathogenesis of liver diseases and the long-term outcomes of chronic hepatitis C patients.
Our histopathological results are particularly interesting. First, inflammatory cell infiltration, portal sclerotic change, peri-venular fibrosis, peri-cellular fibrosis, bridging necrosis and bile duct damage were significantly more severe in the steatosis group. Most notably, the F stage, portal sclerotic change, bile duct damage and bridging necrosis parameters were more severe in the steatosis than in the non-steatosis group. Thus, different histopathological changes in the liver are associated with steatosis. Therefore, steatosis may influence the histopathological features observed in patients with chronic liver disease due to HCV infection. These parameters are suspected to have major involvement in the development of liver cancer [14, 20, 21]. Based on the observations in this study of the long-term outcomes of patients with chronic hepatitis, due to HCV, and liver cirrhosis, we speculate that steatosis may contribute to HCC development in patients with these liver disorders.
HCC is an important prognostic factor in HCV patients. Recently, it was reported that HCC develops in non-alcoholic hepatitis or non-alcoholic fatty liver disease patients. [34-37] In chronic hepatitis C patients, steatosis is an independent risk factor for HCC. [38] On multivariate analysis, the steatosis risk ratio was 4.92, with p=0.031, and the mean score ratio of fibrosis was 2.74, with p=0.001. Steatosis thus appears to be involved in carcinogenesis. The Kaplan-Meier analysis also revealed a significant difference in HCC incidence between the groups with and without steatosis (P=0.029). Steatosis affects the HCC incidence in patients with these liver disorders, according to the results of the cumulative probability of HCC occurrence and multivariate analysis of risk factors for HCC development in this study. Steatosis is thus suggested to influence the development of HCC in patients with chronic hepatitis due to HCV and/or with liver cirrhosis who achieve SVR in response to IFN. Our results also support those of prior reports in which it was concluded that steatosis might play a role in HCC occurrence [38-40].
Histologically, steatosis is present in 40%-86% of patients with chronic hepatitis C. The frequency of steatosis was 170 of 282 (60.0%) in this study. Our data were obtained under conditions similar to those of prior studies. HCV genotype 3 frequently induces non-alcoholic fatty liver disease. The HCV non-3 genotype promotes steatosis via metabolic factors and insulin resistance [26-32]. However, none of our patients had genotype 3.
Hepatic steatosis may affect the serological features of HCV-induced chronic liver disease, according to our present observations. ALT, AST and ALP levels were significantly higher in the steatosis group. These results are similar to those presented in a previous report [31]. On multivariate analysis, there were no significant differences in BMI, ALT, AST, ALP, γ-GTP or platelet counts between patients who did and did not develop HCC. We thus obtained no evidence suggesting that any of the clinical characteristics or laboratory parameters assessed herein is a risk factor for HCC occurrence.
Hourigan et al. reported hepatic steatosis to be associated age and BMI, and that steatosis is related to BMI [33] . In our dataset as well, patients with BMI of 25.1 or higher were significantly more likely to have hepatic steatosis. However, in patients with BMI of 25.1 or higher, there were no significant gender or age differences between those with and without HCC. Next, we compered HCC occurrence in groups with BMI under 25.0 versus 25.1 and higher, but no significant difference was detected between these two groups.
The present prospective study on the incidence of HCC in patients with chronic hepatitis C was performed from January 1992 to August 2015. Recently, direct acting antiviral drugs such as Sofosbuvir and Levipasvir have been shown to improve liver dysfunction, as indicated by reduced AST and ALT levels in the 95% of patients achieving SVR. Henceforth, we will continue to follow up our patients and will compare the frequencies of HCC occurrence after SVR achieved with direct acting antiviral drugs [41, 42].
In conclusion, we consider hepatic steatosis to potentially affect the blood biochemical parameters and clinical profiles of Japanese patients with CH due to hepatitis virus type C. Patients with CH due to the type C virus achieved a curative response to IFN. Furthermore, steatosis appears to affect the long-term outcomes of these patients. However, BMI alone cannot predict the occurrence of HCC.
Acknowledgements
The authors thank all members of the Division of Gastroenterology and Hepatology, Department of Medicine, Nihon University School of Medicine. The study protocol number of our institute is RK 10091015.
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
1. Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR. et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology (Baltimore, Md). 2001;34:809-16
2. Katayama K, Tanaka J, Komiya Y, Kumagai J, Yoshizawa H. [Significance of medical examination for HCV infection as a national project for prevention of hepatocellular carcinoma in Japan]. Nihon rinsho Japanese journal of clinical medicine. 2004;62(Suppl 7):248-52
3. McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK. et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. The New England journal of medicine. 1998;339:1485-92
4. Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N. et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nature genetics. 2009;41:1105-9
5. Aziz H, Gill U, Raza A, Gill ML. Metabolic syndrome is associated with poor treatment response to antiviral therapy in chronic hepatitis C genotype 3 patients. European journal of gastroenterology & hepatology. 2014;26:538-43
6. Adinolfi LE, Rinaldi L, Guerrera B, Restivo L, Marrone A, Giordano M. et al. NAFLD and NASH in HCV Infection: Prevalence and Significance in Hepatic and Extrahepatic Manifestations. International journal of molecular sciences. 2016:17
7. Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. International journal of molecular sciences. 2016:17
8. Chang Y, Jung HS, Yun KE, Cho J, Ahn J, Chung EC. et al. Metabolically healthy obesity is associated with an increased risk of diabetes independently of nonalcoholic fatty liver disease. Obesity (Silver Spring, Md). 2016
9. Shams ME, Al-Gayyar MM, Barakat EA. Type 2 Diabetes Mellitus-Induced Hyperglycemia in Patients with NAFLD and Normal LFTs: Relationship to Lipid Profile, Oxidative Stress and Pro-Inflammatory Cytokines. Scientia pharmaceutica. 2011;79:623-34
10. Alam S, Mustafa G, Alam M, Ahmad N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World journal of gastrointestinal pathophysiology. 2016;7:211-7
11. Kralj D, Virovic Jukic L, Stojsavljevic S, Duvnjak M, Smolic M, Curcic IB. Hepatitis C Virus, Insulin Resistance, and Steatosis. Journal of clinical and translational hepatology. 2016;4:66-75
12. Dyal HK, Aguilar M, Bartos G, Holt EW, Bhuket T, Liu B. et al. Diabetes Mellitus Increases Risk of Hepatocellular Carcinoma in Chronic Hepatitis C Virus Patients: A Systematic Review. Digestive diseases and sciences. 2016;61:636-45
13. Arrese M, Riquelme A, Soza A. Insulin resistance, hepatic steatosis and hepatitis C: a complex relationship with relevant clinical implications. Annals of hepatology. 2010;9(Suppl):112-8
14. Matsumura H, Nirei K, Nakamura H, Higuchi T, Arakawa Y, Ogawa M. et al. Histopathology of type C liver disease for determining hepatocellular carcinoma risk factors. World journal of gastroenterology. 2013;19:4887-96
15. Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology (Baltimore, Md). 1994;19:1513-20
16. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N. et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology (Baltimore, Md). 1981;1:431-5
17. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F. et al. Histological grading and staging of chronic hepatitis. Journal of hepatology. 1995;22:696-9
18. Uchida T. Pathology of hepatitis C. Intervirology. 1994;37:126-32
19. Uchida T. Small hepatocellular carcinoma: its relationship to multistep hepatocarcinogenesis. Pathology international. 1995;45:175-84
20. Shibata M, Morizane T, Uchida T, Yamagami T, Onozuka Y, Nakano M. et al. Irregular regeneration of hepatocytes and risk of hepatocellular carcinoma in chronic hepatitis and cirrhosis with hepatitis-C-virus infection. Lancet (London, England). 1998;351:1773-7
21. Ueno Y, Moriyama M, Uchida T, Arakawa Y. Irregular regeneration of hepatocytes is an important factor in the hepatocarcinogenesis of liver disease. Hepatology (Baltimore, Md). 2001;33:357-62
22. Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Seminars in liver disease. 2001;21:3-16
23. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-9
24. Okamoto H, Sugiyama Y, Okada S, Kurai K, Akahane Y, Sugai Y. et al. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. The Journal of general virology. 1992;73( Pt 3):673-9
25. Simmonds P, Alberti A, Alter HJ, Bonino F, Bradley DW, Brechot C. et al. A proposed system for the nomenclature of hepatitis C viral genotypes. Hepatology (Baltimore, Md). 1994;19:1321-4
26. Cheng FK, Torres DM, Harrison SA. Hepatitis C and lipid metabolism, hepatic steatosis, and NAFLD: still important in the era of direct acting antiviral therapy? Journal of viral hepatitis. 2014;21:1-8
27. Lonardo A, Adinolfi LE, Restivo L, Ballestri S, Romagnoli D, Baldelli E. et al. Pathogenesis and significance of hepatitis C virus steatosis: an update on survival strategy of a successful pathogen. World journal of gastroenterology. 2014;20:7089-103
28. Asselah T, Rubbia-Brandt L, Marcellin P, Negro F. Steatosis in chronic hepatitis C: why does it really matter? Gut. 2006;55:123-30
29. Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology (Baltimore, Md). 2002;36:729-36
30. Hickman IJ, Powell EE, Prins JB, Clouston AD, Ash S, Purdie DM. et al. In overweight patients with chronic hepatitis C, circulating insulin is associated with hepatic fibrosis: implications for therapy. Journal of hepatology. 2003;39:1042-8
31. Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology (Baltimore, Md). 2001;33:1358-64
32. Younossi ZM, McCullough AJ, Ong JP, Barnes DS, Post A, Tavill A. et al. Obesity and non-alcoholic fatty liver disease in chronic hepatitis C. Journal of clinical gastroenterology. 2004;38:705-9
33. Hourigan LF, Macdonald GA, Purdie D, Whitehall VH, Shorthouse C, Clouston A. et al. Fibrosis in chronic hepatitis C correlates significantly with body mass index and steatosis. Hepatology (Baltimore, Md). 1999;29:1215-9
34. Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet (London, England). 1998;351:214-5
35. El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. The New England journal of medicine. 1999;340:745-50
36. Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology (Baltimore, Md). 2002;36:1349-54
37. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology (Baltimore, Md). 2010;51:1820-32
38. Ohata K, Hamasaki K, Toriyama K, Matsumoto K, Saeki A, Yanagi K. et al. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036-43
39. Kumar D, Farrell GC, Kench J, George J. Hepatic steatosis and the risk of hepatocellular carcinoma in chronic hepatitis C. Journal of gastroenterology and hepatology. 2005;20:1395-400
40. Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007;109:2490-6
41. Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M. et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. The New England journal of medicine. 2014;370:1889-98
42. Mizokami M, Yokosuka O, Takehara T, Sakamoto N, Korenaga M, Mochizuki H. et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. The Lancet Infectious diseases. 2015;15:645-53
Author Biography
Dr. Kazushige Nirei is an assistant professor at the Division of Gastroenterology and Hepatology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan. He has coauthored more than 18 publications. The research interests of Dr. Nirei's group include Chronic Hepatitis type C, Chronic Hepatitis type B, hepatocellular carcinoma and diabetes mellitus.
![]() Corresponding author: Kazushige Nirei MD, PhD. Division of Gastroenterology and Hepatology, Department of Medicine, Nihon University School of Medicine, Japan. 30-1, Oyaguchikami-cho, Itabashi-ku, Tokyo 173-8610, Japan. Phone: +81-3-3972-8111 Ext.2424 Fax: +81-3-3956-8496 E-mail: nirei.kazushigeac.jp.
Corresponding author: Kazushige Nirei MD, PhD. Division of Gastroenterology and Hepatology, Department of Medicine, Nihon University School of Medicine, Japan. 30-1, Oyaguchikami-cho, Itabashi-ku, Tokyo 173-8610, Japan. Phone: +81-3-3972-8111 Ext.2424 Fax: +81-3-3956-8496 E-mail: nirei.kazushigeac.jp.

 Global reach, higher impact
Global reach, higher impact