Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(11):853-857. doi:10.7150/ijms.16706 This issue Cite
Research Paper
Amelioration of estrogen deficiency-induced obesity by collagen hydrolysate
1. Department of Nursing, College of Medicine & Nursing, Hung Kuang University, Taichung, Taiwan;
2. School of Medicine, Chung Shan Medical University, Taichung, Taiwan;
3. Department of Orthopedic Surgery, Chung Shan Medical University, Taichung 402, Taiwan;
4. Center for Molecular Medicine, China Medical University Hospital, Taichung, Taiwan;
5. Department of Laboratory Medicine, Kuang Tien General Hospital, Taichung, Taiwan;
6. Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan;
7. Emergency department and center of Hyperbaric Oxygen Therapy, Tungs' Taichung MetroHarbor Hospital, Taichung Taiwan;
8. Department of Psychiatry, Chung Shan Medical Hospital, Taichung,Taiwan;
9. Graduate Institute of Cancer Biology, China Medical University, Taichung, Taiwan;
10. Institute of Biochemistry, Microbiology and Immunology, Medical College, Chung-Shan Medical University, Taichung, Taiwan;
11. Clinical Laboratory, Chung Shan Medical University Hospital, Taichung, Taiwan;
Received 2016-7-3; Accepted 2016-9-1; Published 2016-10-19
Abstract
Objectives: Menopausal transition with declining estrogen levels significantly affects the physiological properties of women and consequently contributes to a series of medical conditions, including obesity. Obesity is a crucial risk factor associated with cardiovascular diseases, diabetes mellitus, and breast cancer. Increasing dietary protein content improves satiety and energy expenditure. Thus, we hypothesize that supplementing with collagen, a common dietary protein, may alleviate menopause-induced obesity.
Methods: We used ovariectomized (OVX) rats to mimic a menopausal human. The body weight of OVX rats significantly increased compared with that of sham-operated rats (P<0.05), but uterus weight was decreased. Adipocyte size in perigonadal adipose tissue also increased (P<0.05).
Results: By contrast, OVX rats supplemented with aqueous collagen hydrolysate (2.5 mg/mL) exhibited significant attenuation in body weight gain and adipocyte enlargement (P<0.05), but insignificant change in uterus weight. Further investigation indicated that collagen hydrolysate supplementation insignificantly affected the levels of dorsal fat, serum total cholesterol, and serum triacylglycerol. Levels of serum biochemical factors, calcium, phosphorus, and glucose were also insignificantly altered by collagen hydrolysate supplementation.
Conclusion: Collagen hydrolysate supplementation reduced body weight gain and adipocyte enlargement in response to ovariectomy but slightly affected blood lipids, calcium, and glucose in both sham-operated and OVX rats. Collagen hydrolysate supplementation is beneficial in ameliorating estrogen deficiency-induced obesity and its associated risk factors.
Keywords: Collagen, Obesity, estrogen deficiency
Introduction
The change in body composition of post-menopausal women is considered a result of long-term interactions among energy intake, energy expenditure, and sex hormonal status or a combination thereof [1, 2, 3, 4,5]. Menopause is frequently associated with increased central (visceral) body fat [6,7], followed by an increasing incidence of hypertension [8] and the aggravation of the lipoprotein profile [9], which lead to metabolic syndrome [10] and the subsequent rise in the incidence of cardiovascular diseases [11]. During menopausal transition, women experience deleterious changes in circulating inflammatory markers and adipokines correlated with increased visceral adiposity [12]. A growing number of evidence has demonstrated that chronic low-grade/subclinical inflammation is a key element in the development of atherosclerotic cardiovascular disease and is closely associated with obesity, insulin resistance, and metabolic syndrome [13,14,15,16].
Dietary protein plays a role in controlling body weight, which is partially attributed to its effects on satiety. Among common dietary proteins, purified collagen hydrolysate has been clinically proven as an effective appetite suppressant that maintains a satiating effect to promote weight loss [17,18]. Insoluble fibrous protein collagen, the single most abundant protein in animals, is the major component of the extracellular matrix and connective tissues. At least 16 types of collagen have been identified in humans; among these, 80%-90% belong to types I, II, and III collagen. Although supplementing with collagens and their derived peptides has been demonstrated to improve cellulite and skin health [19], whether collagen hydrolysate supplementation affects menopause-induced obesity remains unclear.
In the present study, we aimed to investigate the effects of collagen hydrolysate supplementation on estrogen deficiency-induced obesity by using an ovariectomized (OVX) animal model. The body weight, uterus weight, fat mass, and serum biochemical factors of OVX rats were assessed. Adipocyte size was also determined.
Materials and Methods
Reagents
All chemicals used were obtained from Sigma-Aldrich (St. Louis, MO, USA) except when otherwise specified. Collagen hydrolysate was purchased from KVW Health Co. Ltd. (Taipei, Taiwan).
Ovariectomized rat model
All female Sprague-Dawley rats weighing approximately 200 g were purchased from the National Laboratory Animal Center (Taipei City, Taiwan) and maintained under the supervision of the Institutional Animal Care and Use Committee (IACUC) of the China Medical University. Animal experiment protocol was approved by the IACUC and performed in accordance with the guidelines.
For estrogen deficiency-induced obesity and collagen supplementation, 24 rats were divided into 4 groups for sham operation (group 1) and OVX operation (groups 2-4). Operation was performed under Nembutal (Pentobarbital sodium, 50 mg/kg body weight) anesthesia. Groups 1 and 2 were supplied with sterile water. Groups 3 and 4 were supplied with sterile water containing 1.25 mg/mL and 2.5 mg/mL collagen hydrolysate, respectively. All animals were maintained under 12 h/12 h light-dark cycles at controlled temperature (22 ± 0.5 ºC) and humidity (45%-50%). Body weight was measured daily at a specified time during the experiment. At the end of the experiment, blood and perigonadal adipose tissue were immediately obtained after the animals were sacrificed. The dorsal fat and uterus of each rat were dissected and weighed.
Histological analysis of adipose tissue and cell size determination
The perigonadal adipose tissues were fixed in zinc formaldehyde overnight at 4 °C and transferred into phosphate-buffered saline. The fixed tissues were then embedded into paraffin, sectioned, and stained with hematoxylin and eosin. Digital images were captured at 200× magnitude using an Olympus DX51 light microscope (Tokyo, Japan).
Measurement of serum biochemical makers
The blood was coagulated by maintaining it at room temperature for 15-20 min, followed by centrifugation for 10 min. The indicated parameters (calcium ions, glucose, inorganic phosphorus, total cholesterol, and triacylglycerol) were measured using a serum biochemistry analyzer (AU400, Olympus, Tokyo, Japan).
Statistical analysis
Data were expressed as means ± standard error. Statistical comparisons were made via ANOVA test using a statistical software (SigmaStat 3.5, Systat Software, Inc. San Jose, CA, USA).
Results
Collagen supplementation reduced OVX-associated increase in body weight but not uterine weight
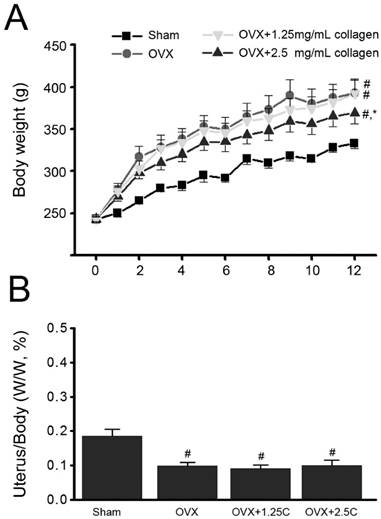
The effects of collagen uptake on estrogen deficiency-induced obesity were initially investigated. The increase in body weight of OVX rats (OVX) was significantly higher than that of sham-operated control rats (Sham) (P<0.01) (Figure 1A). After 12 weeks, the body weights of the Sham and OVX groups were 315 ± 7 g and 365 ± 16 g, respectively. The increase in body weight of the OVX with 2.5 mg/mL collagen supplement group was significantly lower than that of the OVX group but not that of the OVX with 1.25 mg/mL collagen supplement group (P<0.05).
Change in uterus weight in response to collagen supplementation was also monitored. The ratio of uterus/body weight in the OVX group significantly decreased compared with that of the Sham group (P<0.05) (Fig. 1B). Interestingly, the ratio of uterus/body weight in the OVX with collagen supplement groups (OVX+1.25C and OVX+2.5C) significantly decreased compared with that of the Sham group (P<0.01) but was insignificantly altered compared with that of OVX group.
Collagen supplementation decreased OVX-associated increase in adipocyte size
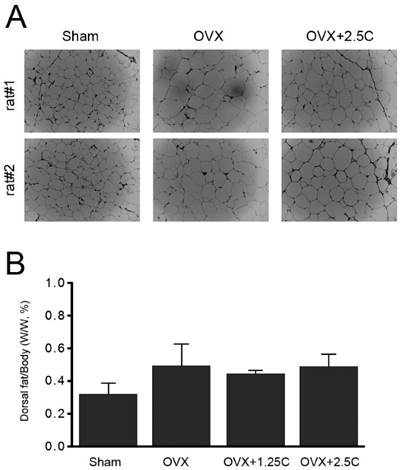
Collagen supplementation attenuated weight gain in OVX rats. Therefore, whether supplementation affected adipocyte properties was investigated. Adipocytes in the perigonadal adipose tissue from the OVX rats were clearly enlarged compared with those from the sham-operated control rats (Figure 2A). In addition, high collagen supplementation (2.5 mg/mL) reduced the enlarged adipocyte size in response to OVX treatment (Figure 1A, OVX+2.5C). Parallel with adipocyte size, the change in dorsal fat mass was analyzed. Although the dorsal fat mass in the OVX group appeared to be higher than that in the Sham group, the ratio of dorsal fat mass/body weight was insignificantly altered among the four experiment groups (Figure 2B).
Effects of collagen on blood biochemical factors
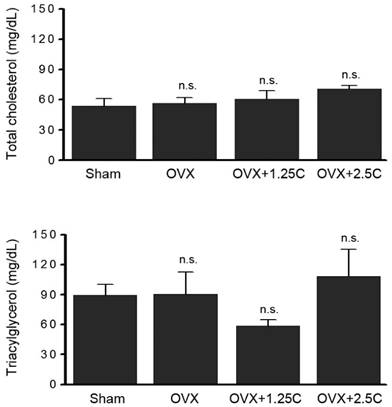
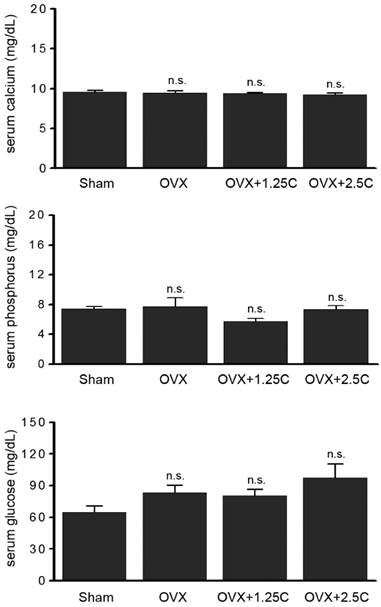
To clarify whether collagen supplementationt regulated lipid metabolism, the levels of blood lipids, total cholesterol, and triacylglycerol were determined. As shown in Figure 3 (upper panel), serum total cholesterol level was slightly regulated in the OVX and OVX with collagen supplement groups (P>0.05). A similar phenomenon was observed in serum triacylglycerol level (Figure 3, lower panel). In addition to blood lipids, other blood biochemical factors were monitored. The levels of serum calcium, phosphorus, and glucose were not altered in response to OVX treatment or OVX with collagen supplements (Figure 4).
Effects of collagen supplement on body weights. Rats were sham-operated or OVX, and then the OVX-rats were supplied with water containing collagen (1.25 and 2.5 mg/mL, 1.25C and 2.5C) or not. (A) Body weight was measured every week for 12 weeks. (B) After 12 weeks, rats were sacrificed, and then uteruses were obtained and weighed. #, P<0.05 as compared to Sham. *, P<0.05 as compared to OVX.
Effects of collagen supplement on adipocyte size and dorsal fat content. Rats were sham-operated or OVX, and then the OVX-rats were supplied with water containing collagen (1.25 and 2.5 mg/mL, 1.25C and 2.5C) or not. After 12 weeks, the rats were weighed, and then sacrificed to obtain periuterine adipose tissue and dorsal fat. (A) After H&E staining, the adipocytes were observed by using light microscope (200x). (B) Dorsal fat samples were collected and weighed.
Collagen supplement insignificantly affected blood lipids. Rats were sham-operated or OVX, and then the OVX-rats were supplied with water containing collagen (1.25 and 2.5 mg/mL, 1.25C and 2.5C) or not. After 12 weeks, blood samples were obtained for determination of serum total cholesterol and triacylglycerol. n.s., no statistical significance as compared to Sham or OVX.
Collagen supplement insignificantly affected blood lipids. Rats were sham-operated or OVX, and then the OVX-rats were supplied with water containing collagen (1.25 and 2.5 mg/mL, 1.25C and 2.5C) or not. After 12 weeks, sera were obtained for determination of calcium, phosphorus and glucose level. n.s., no statistical significance as compared to Sham or OVX.
Discussion
Longitudinal studies have demonstrated a high risk of developing impaired glucose tolerance [20], type 2 diabetes [20,21], and coronary heart disease [22,23,24] in subjects with visceral adiposity. Accordingly, visceral adiposity may have a significant pathophysiological role in the development of metabolic syndrome and its sequelae. Our findings showed that high collagen supplementation (2.5 mg/mL) significantly attenuated weight gain and reduced adipocyte enlargement in response to estrogen deficiency, thereby indicating that collagen supplementation is beneficial for menopause-induced obesity. Furthermore, collagen supplementation may also ameliorate the health of menopausal women by reducing risk factors attributed to menopause-induced obesity.
A previous study has reported that protein intake can prevent muscle mass loss associated with weight loss in obese older women [25]. Another study reports that using gelatin as a single diet protein results in appetite suppression in adults, which is more significant than using casein [26]. A recent review that investigated the effects of dietary protein intake on body composition changes also indicates that consuming high-protein diets contributes to the maintenance of lean mass and the loss of fat mass during weight loss in older adults [27]. Our findings indicate that supplementing with collagen hydrolysate, the digested gelatin, attenuates body weight gain and adipocyte enlargement in OVX rats. Collagen hydrolysate supplementation may not only alleviate menopause-induced obesity but also ameliorate muscle mass maintenance during menopause.
In obesity model animals, the association of abnormal fat mass has not been precisely investigated. [28] reported that fat mass was higher in obese, diabetic, leptin-resistant Zucker rats than in their homozygous lean controls. OVX treatment in rodents also leads to weight gain and fat deposition, whereas estradiol treatment restores these changes [29,30]. Our results show that collagen supplementation exhibits bioactivity in reducing body weight and adipocyte size similar to that of estradiol treatment. Thus, collagen supplementation may synergically act with estradiol to reduce the use of estradiol and consequently alleviate its side effects.
Conclusion
Our study provides evidence that collagen supplementation can attenuate body weight gain and decrease enlarged adipocyte size. Our findings also indicate that collagen supplementation insignificantly affects the levels of blood lipids, calcium, phosphorus, and glucose. Thus, collagen supplementation can complete its anti-obesity activity with minor side effects in menopausal women.
Acknowledgements
This work was supported by grants from the Ministry of Science and Technology, Republic of China (MOST 104-2320-B-039-032 and MOST 104-2632-B-040-002), as well as in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Poehlman ET, Goran MI, Gardner AW. et al. Determinants of decline in resting metabolic rate in aging females. The American journal of physiology. 1993;264:E450-455
2. Kritz-Silverstein D, Barrett-Connor E. Long-term postmenopausal hormone use, obesity, and fat distribution in older women. JAMA: the journal of the american medical association. 1996;275(1):46-49
3. Shinoda M, Latour MG, Lavoie JM. Effects of physical training on body composition and organ weights in ovariectomized and hyperestrogenic rats. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2002;26(3):335-343
4. Jensen LB, Vestergaard P, Hermann AP. et al. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2003;18(2):333-342
5. Mastorakos G, Valsamakis G, Paltoglou G, Creatsas G. Management of obesity in menopause: diet, exercise, pharmacotherapy and bariatric surgery. Maturitas. 2010;65(3):219-224
6. Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: a controlled longitudinal study. Annals of internal medicine. 1995;123(9):673-675
7. Lovejoy JC, Champagne CM, de Jonge L. et al. Increased visceral fat and decreased energy expenditure during the menopausal transition. International journal of obesity. 2008;32(6):949-958
8. Tremollieres FA, Pouilles JM, Cauneille C, Ribot C. Coronary heart disease risk factors and menopause: a study in 1684 French women. Atherosclerosis. 1999;142(2):415-423
9. Seed M, Knopp RH. Estrogens, lipoproteins, and cardiovascular risk factors: an update following the randomized placebo-controlled trials of hormone-replacement therapy. Current opinion in lipidology. 2004;15(4):459-467
10. Carr MC. The emergence of the metabolic syndrome with menopause. The Journal of clinical endocrinology and metabolism. 2003;88(6):2404-2411
11. Kannel WB, Wilson PW. Risk factors that attenuate the female coronary disease advantage. Archives of internal medicine. 1995;155(1):57-61
12. Lee CG, Carr MC, Murdoch SJ. et al. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. The Journal of clinical endocrinology and metabolism. 2009;94(4):1104-1110
13. Festa A, D'Agostino RJr, Howard G. et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102(1):42-47
14. Caballero AE. Endothelial dysfunction in obesity and insulin resistance: a road to diabetes and heart disease. Obesity research. 2003;11(11):1278-1289
15. Sjoholm A, Nystrom T. Endothelial inflammation in insulin resistance. Lancet. 2005;365(9459):610-612
16. Caballero AE, Bousquet-Santos K, Robles-Osorio L. et al. Overweight Latino children and adolescents have marked endothelial dysfunction and subclinical vascular inflammation in association with excess body fat and insulin resistance. Diabetes care. 2008;31(3):576-582
17. Veldhorst M, Nieuwenhuizen AG, Hochstenbach-Waelen A. et al. A breakfast with alpha-lactalbumin, gelatin, or gelatin + TRP lowers energy intake at lunch compared with a breakfast with casein, soy, whey, or whey-GMP. Clinical nutrition. 2009;28(2):147-155
18. Nieuwenhuizen AG, Hochstenbach-Waelen A, Veldhorst M A. et al. Acute effects of breakfasts containing alpha-lactalbumin, or gelatin with or without added tryptophan, on hunger, 'satiety' hormones and amino acid profiles. The British journal of nutrition. 2009;101(12):1859-1866
19. Schunck M, Zague V, Oesser S, Proksch E. Dietary Supplementation with Specific Collagen Peptides Has a Body Mass Index-Dependent Beneficial Effect on Cellulite Morphology. Journal of medicinal food. 2016;18(12):1340-1348
20. Hayashi T, Boyko EJ, Leonetti DL. et al. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes care. 2003;26(3):650-655
21. Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes care. 2000;23(4):465-471
22. Lamarche B, Lemieux S, Dagenais GR, Despres JP. Visceral obesity and the risk of ischaemic heart disease: insights from the Quebec Cardiovascular Study. Growth hormone & IGF research: official journal of the Growth Hormone Research Society and the International IGF Research Society. 1998;8(Suppl B):1-8
23. Fujimoto W Y, Bergstrom RW, Boyko EJ. et al. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes care. 1999;22(11):1808-1812
24. St-Pierre J, Lemieux I, Vohl MC. et al. Contribution of abdominal obesity and hypertriglyceridemia to impaired fasting glucose and coronary artery disease. The American journal of cardiology. 2002;90(1):15-18
25. Muscariello E, Nasti G, Siervo M. et al. Dietary protein intake in sarcopenic obese older women. Clinical interventions in aging. 2016;11:133-140
26. Hochstenbach-Waelen A, Westerterp-Plantenga MS, Veldhorst MA, Westerterp KR. Single-protein casein and gelatin diets affect energy expenditure similarly but substrate balance and appetite differently in adults. The Journal of nutrition. 2009;139(2):2285-2292
27. Kim JE, O'Connor LE, Sands LP. et al. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutrition reviews. 2006;74(3):210-224
28. Mathey J, Horcajada-Molteni MN, Chanteranne B. et al. Bone mass in obese diabetic Zucker rats: influence of treadmill running. Calcified tissue international. 2002;70(4):305-311
29. Laudenslager ML, Wilkinson CW, Carlisle HJ. et al. Energy balance in ovariectomized rats with and without estrogen replacement. The American journal of physiology. 1980;238(5):R400-R405
30. Richard D, Rochon L, Deshaies Y. Effects of exercise training on energy balance of ovariectomized rats. The American journal of physiology. 1987;253(5 Pt 2):R740-R745
Author contact
![]() Corresponding authors: Jer-Yuh Liu PhD., Graduate Institute of Cancer Biology, College of Medical, China Medical University, No 6, Hsueh-Shih Road, Taichung 404, Taiwan. Tel: +886-4-22052121 ext 7932; Fax: +886-4-22347028. E-mail: jylcmu.edu.tw; Li-Sung Hsu, Ph.D., Institutes of Biochemistry and Biotechnology, Chung Shan Medical University, No. 110, Sec. 1, Jian-Guo N. Road, Taichung 40201, Taiwan. Tel: +886-4-24730022 ext. 11682, Fax: +886-4-23248195, E-mail: lshsu405com.tw; Shao-Hsuan Kao, Ph.D. Institute of Biochemistry, Microbiology and Immunology, Medical College, Chung-Shan Medical University, Taichung 40201, Taiwan. Tel: 04-24730022 ext. 11681; E-mail: kaoshedu.tw.
Corresponding authors: Jer-Yuh Liu PhD., Graduate Institute of Cancer Biology, College of Medical, China Medical University, No 6, Hsueh-Shih Road, Taichung 404, Taiwan. Tel: +886-4-22052121 ext 7932; Fax: +886-4-22347028. E-mail: jylcmu.edu.tw; Li-Sung Hsu, Ph.D., Institutes of Biochemistry and Biotechnology, Chung Shan Medical University, No. 110, Sec. 1, Jian-Guo N. Road, Taichung 40201, Taiwan. Tel: +886-4-24730022 ext. 11682, Fax: +886-4-23248195, E-mail: lshsu405com.tw; Shao-Hsuan Kao, Ph.D. Institute of Biochemistry, Microbiology and Immunology, Medical College, Chung-Shan Medical University, Taichung 40201, Taiwan. Tel: 04-24730022 ext. 11681; E-mail: kaoshedu.tw.

 Global reach, higher impact
Global reach, higher impact