3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(9):680-685. doi:10.7150/ijms.16267 This issue Cite
Research Paper
Suppression of Myostatin Stimulates Regenerative Potential of Injured Antigravitational Soleus Muscle in Mice under Unloading Condition
1. Laboratory of Physiology, School of Health Sciences, Toyohashi SOZO University, Toyohashi, Aichi, Japan;
2. Department of Orthopaedic Surgery, St. Marianna University School of Medicine, Kawasaki, Kanagawa, Japan;
3. Department of Regenerative Medicine, National Center for Geriatrics and Gerontology, Obu, Aichi, Japan;
4. Faculty of Education, Yamaguchi University, Yamaguchi City, Yamaguchi, Japan;
5. Graduate School of Health and Sports Science, Doshisha University, Kyotanabe, Kyoto, Japan;
6. Hirosaki Gakuin University, Hirosaki, Aomori, Japan;
7. Department of Physiology, Graduate School of Health Sciences, Toyohashi SOZO University, Toyohashi, Aichi, Japan.
Received 2016-5-23; Accepted 2016-7-20; Published 2016-8-10
Abstract
Effects of myostatin (MSTN)-suppression on the regeneration of injured skeletal muscle under unloading condition were investigated by using transgenic mice expressing a dominant-negative form of MSTN (MSTN-DN). Both MSTN-DN and wild-type (WT) mice were subjected to continuous hindlimb suspension (HS) for 6 weeks. Cardiotoxin (CTX) was injected into left soleus muscle under anesthesia 2 weeks after the initiation of HS. Then, the soleus muscles were excised following 6-week HS (4 weeks after CTX-injection). CTX-injection caused to reduce the soleus fiber cross-sectional area (CSA) in WT mice under both unloading and weight-bearing conditions, but not in MSTN-DN mice. Under unloading condition, CTX-injected muscle weight and fiber CSA in MSTN-DN mice were significantly higher than those in WT mice. CTX-injected muscle had many damaged and regenerating fibers having central nuclei in both WT and MSTN-DN mice. Significant increase in the population of Pax7-positive nuclei in CTX-injected muscle was observed in MSTN-DN mice, but not in WT mice. Evidences indicate that the suppression of MSTN cause to increase the regenerative potential of injured soleus muscle via the increase in the population of muscle satellite cells regardless of unloading conditions.
Keywords: skeletal muscle, muscle regeneration, unloading, myostatin, muscle satellite cell.
Introduction
Regenerative potential of injured skeletal muscle is mostly attributed to skeletal muscle-specific stem cells, so-called muscle satellite cells, which are located between the basal lamina and the sarcolemma of mature myofibers [1]. Muscle satellite cells, which have crucial roles in skeletal muscle regeneration, express the paired box transcription factor 7 (Pax7) in their nuclei [2,3]. Skeletal muscle regeneration after injury occurs via activation of muscle satellite cells which are normally quiescent [4,5]. After damage to skeletal muscle, activated muscle satellite cells proliferate and undergo differentiation into myoblasts. Subsequently, the myoblasts from satellite cells differentiate and fuse to form new regenerated myofibers [6].
Loading is well known as a regulatory factor for skeletal muscle size. Atrophy of skeletal muscle, especially in anti-gravitational soleus, is induced by unloading [7-10]. Loading also plays an important role in the regulation of regenerative potential of injured skeletal muscle. Functional overloading stimulates the regenerative potential of injured mouse soleus muscle with the increase in the number of muscle satellite cells [11]. On the contrary, hindlimb unloading suppresses injury-associated increase in muscle satellite cells [7] and inhibits the regeneration of injured soleus muscle in mice [7,12]. Therefore, the regenerative potential of injured skeletal muscle may be highly sensitive to loading.
Myostatin (MSTN) is a member of the transforming growth factor-β (TGF-β) superfamily and acts as a negative regulator of skeletal muscle mass in mice, cattle, and humans [13-15], as well as other mammals via the regulation of both proliferation and differentiation of muscle satellite cells [16]. Presence of MSTN maintains the quiescent state of satellite cells but absence of MSTN leads to proliferation of active satellite cells [16,17]. Deficiency of MSTN gene exhibits an increase in skeletal muscle mass that is attributed to a combination of muscle cell hyperplasia and hypertrophy [13]. On the contrary, overexpression of MSTN causes severe muscle atrophy [18,19]. Furthermore, the regeneration of injured tibialis anterior muscle in MSTN-null mice was facilitated via the activation of muscle satellite cells [17,20]. However, it is still unclear whether MSTN-associated regulation of regenerative potential in injured skeletal muscle exhibits in the unloading condition. In the present study, therefore, we investigated the effects of MSTN on the regeneration of injured skeletal muscle under unloading condition by using mutant mice expressing dominant-negative form of MSTN (MSTN-DN).
Materials and Methods
Animals
All experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Bethesda, MD) and were approved by National Institute for Longevity Sciences. MSTN-DN mice (n = 9) were prepared as described previously [21] and used in this experiment. In addition, wild-type (WT) mice (C57BL/6J, n = 20) were also used. All mice were housed in a clean room controlled at approximately 23°C with a 12/12 hours light-dark cycle. Solid diet and water were provided ad libitum.
Experimental protocol
In the present study, we investigated the regenerative potential of injured skeletal muscle in MSTN-DN mice under unloading condition, compared with that under weight-bearing condition. Both kinds of mice were randomly divided into 1) unloading (WT: n=10; MSTN-DN: n=5) and 2) weight-bearing (WT: n=10; MSTN-DN: n=4) groups. WT and MSTN-DN mice in the unloading group were subjected to continuous hindlimb suspension (HS) for 6 weeks as following the methods described previously [7,22]. Briefly, tails of the mice were cleaned, and were loosely surrounded by adhesive tapes cross-sectionally, fixing a string at the dorsal side of the tail, to maintain the blood flow intact. The string was fastened to the roof of the cage at a height allowing the forelimbs to support the weight, yet preventing the hindlimbs from touching the floor and the sides of the cage. The mice could reach food and water freely by using their forelimbs.
To induce muscle injury followed by regeneration, 0.1 mL cardiotoxin (CTX, 10 μmol/L in physiological saline, Sigma-Aldrich, St. Louis, MO, USA) of Naja naja atra venom was injected into left soleus muscle of WT (unloading: n=5; weight-bearing: n=5) and MSTN-DN (unloading: n=5; weight-bearing: n=4) mice 2 weeks after initiation of HS. Injection of CTX was performed using a 27-gauge needle under anesthesia with i.p. injection of sodium pentobarbital (50 mg/kg) [7,23]. This procedure for the initiation of necrosis-regeneration was performed carefully to avoid the damage to the nerves and blood vessels, as was suggested elsewhere [24,25]. The left soleus muscle of uninjected WT mice (unloading: n=5; weight-bearing: n=5) and the right soleus muscle of MSTN-DN mice were assigned as the control, respectively.
Sampling
Six weeks after initiation of HS (4 weeks after CTX-injection), all mice were sacrificed by cervical dislocation under anesthesia with i.p. injection of sodium pentobarbital (50 mg/kg). Immediately after the scarification, the left soleus muscle of WT mice and both soleus muscles of MSTN-DN mice were excised from each hindlimb. Dissected soleus muscles were rapidly weighed and frozen in isopentane cooled by liquid nitrogen. The muscle samples were stored at -80°C until analyses.
Immunohistochemical analyses
Frozen soleus muscles were cut cross-sectionally into halves. Serial transverse cryosections (8-μm thick) of the proximal portion of soleus muscles were cut at -20°C and mounted on the slide glasses. The sections were air-dried and stained to analyze the cross-sectional area (CSA) of muscle fibers by hematoxylin and eosin (H&E), and the profiles of Pax7-positive nuclei by the standard immunohistochemical technique, respectively [7,26]. Monoclonal anti-Pax7 antibody (undiluted tissue culture supernatant of hybridoma cells obtained from the Developmental Studies Hybridoma Bank, Iowa, IA, USA) was used for the detection of muscle satellite cells [2]. Cross sections were fixed with 4% paraformaldehyde, and then were post-fixed in ice-cold methanol. After blocking by using a reagent (1% Roche blocking reagent, Roche Diagnostic, Penzberg, Germany), samples were incubated with the primary antibodies for Pax7 and rabbit polyclonal anti-laminin (Z0097, DakoCytomation, Glostrup, Denmark). Sections were also incubated with the secondary antibodies for Cy3-conjugated anti-mouse IgG (dilution 1:100; Jackson Immuno Research, West Grove, PA, USA) and with fluorescein isothiocyanate-conjugated anti-rabbit IgG (dilution 1:200; Sigma-Aldrich). Then nuclei were stained in a solution of 4',6-diamidino-2-phenylindole dihydrochloride (DAPI, 1 μg/ml; Sigma-Aldrich).
The images of muscle sections were incorporated into a personal computer (DP-BSW version 02.02, Olympus, Tokyo, Japan) using a microscope (IX81 with DP70, Olympus). In H&E staining, the CSAs of approximately 200 fibers from each muscle were analyzed using the National Institutes of Health Image J 1.38X (NIH, Bethesda, MD, USA) software for Windows. In immunohistochemical staining, the percentage of Pax7-positive nuclei located within the laminin-positive basal membrane relative to the total number of DAPI-positive nuclei in ~200 muscle fibers from each muscle was calculated.
Statistical analysis
All values were expressed as means ± SEM. Significant levels in each loading condition were analyzed using a two-way (mouse and injection) analysis of variance (ANOVA) for multiple comparisons followed by Tukey-Kramer test. When a significant interaction between two effects (mice and injection) was observed, one-way ANOVA followed by Tukey-Kramer test was performed. The significance level was accepted at p<0.05.
Results
Unloading condition
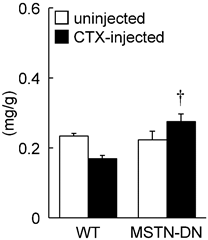
Under unloading condition, relative soleus muscle wet weight was decreased in both WT (39%) and MTSN-DN (32%) mice, compared with that under weight-bearing condition. Furthermore, CTX-injection induced ~28% decrease in the relative weight in WT mice, but not in MSTN-DN mice. The weight of CTX-injected soleus muscle in MSTN-DN mice was significantly higher than that in WT mice (Figure 1, p<0.05). Mean fiber CSA in MSTN-DN mice was significantly higher than that in WT mice (Figure 2B, p<0.05). There were many regenerating fibers having central nuclei in CTX-injected muscle of MSTN-DN mice, compared to WT mice (Figure 2A).
Effects of cardiotoxin-injection on the soleus muscle weight relative to body weight in WT and MSTN-DN mice under unloading condition. WT: wild-type mice; MSTN-DN: transgenic mice expressing the dominant negative form of myostatin; uninjected: uninjected muscle; CTX-injected: cardiotoxin (CTX)-injected muscle. Values are means ± SEM. n = 5 in each group. †: Significant different from CTX-injected muscle of WT, p<0.05.
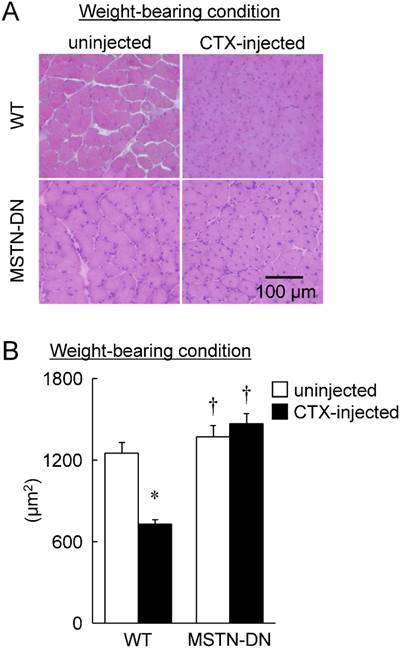
Typical images of transverse cryosections of the midbelly region of mouse soleus muscle stained with hematoxylin and eosin under unloading condition (A). Scale bar = 100 μm. (B) Effects of CTX-injection on the mean cross-sectional area of soleus muscle fibers in WT and MSTN-DN mice under unloading condition. See Figure 1 for other abbreviations, statistics, and symbols. Values are means ± SEM. n = 5 in each group. *: Significant different from uninjected muscle of WT, p<0.05.
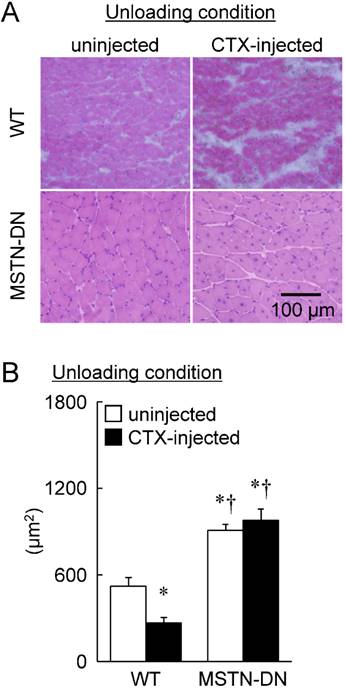
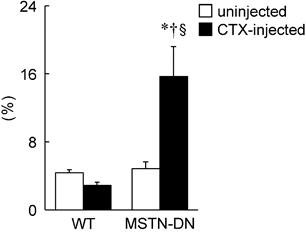
Furthermore, a significant reduction of fiber CSA was induced by CTX-injection in WT mice (p<0.05, Figure 2B). However, there was no significant effect of CTX-injection on fiber CSA in MSTN-DN mice. There was a significant difference in mean fiber CSA of CTX-injected soleus muscle between two types of mice (p<0.05). The relative percentage of Pax7-positive nuclei in MSTN-DN mice was significantly increased by CTX-injection (p<0.05, Figure 3), but not WT mice.
Weight-bearing condition
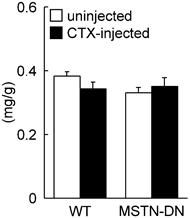
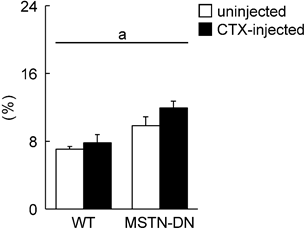
In the present study, we also investigated the regenerative potential of injured soleus muscle in MSTN-DN and WT mice under weight-bearing condition. There was no significant difference in relative muscle weight to body weight between WT and MSTN-DN mice (Figure 4). CTX-injection had no effect on the relative muscle weights of two types of mice (Figure 4). However, CTX-injected muscle in both WT and MSTN-DN mice had many damaged and regenerating fibers having central nuclei (Figure 5A). Mean fiber CSA in WT mice was significantly decreased by CTX-injection (p<0.05), but not in MSTN-DN mice (Figure 5B). The fiber CSA of CTX-injected muscle in MSTN-DN mice was significantly higher than that in WT mice (p<0.05). There was a significant difference in the relative percentage of Pax7-positive nuclei between WT and MSTN-DN mice (p<0.05, Figure 6). The relative percentage of Pax7-positive nuclei in MSTN-DN mice was significantly higher compared with that in WT mice (Figure 6). In two types of mice, however, there was no significant effects of CTX-injection on the relative percentage of Pax7-positive nuclei.
Effects of CTX-injection on the percentage of Pax7-positive nuclei relative to total myonuclei in soleus muscle of WT and MSTN-DN mice under unloading condition. See Figures 1 and 2 for other abbreviations, statistics, and symbols. Values are means ± SEM. n = 5 in each group. §: Significant different from uninjected muscle of MSTN-DN, p<0.05.
Effects of cardiotoxin-injection on the soleus muscle weight relative to body weight in WT and MSTN-DN mice under weight-bearing condition. See Figure 1 for other abbreviations. Values are means ± SEM. n = 4-5 in each group.
Typical images of transverse cryosections of the midbelly region of mouse soleus muscle stained with hematoxylin and eosin under weight-bearing condition (A). Scale bar = 100 μm. (B) Effects of CTX-injection on the mean cross-sectional area of soleus muscle fibers in WT and MSTN-DN mice under weight-bearing condition. See Figures 1 and 2 for other abbreviations, statistics, and symbols. Values are means ± SEM. n = 4-5 in each group.
Effects of CTX-injection on the percentage of Pax7-positive nuclei relative to total myonuclei in soleus muscle of WT and MSTN-DN mice under weight-bearing condition. See Figure 1 for other abbreviations. Values are means ± SEM. n = 4-5 in each group. a: Significant different between WT and MSTN-DN, p<0.05.
Discussion
The present study investigated that the muscle weight, mean fiber CSA, and the population of Pax7-positive nuclei of CTX-induced regenerating soleus muscle in MSTN-DN and WT mice under unloading and weight-bearing conditions. Under unloading and weight-bearing conditions, mean fiber CSA in WT mice was decreased by CTX-injection, but not in MSTN-DN mice. The population of Pax7-positive nuclei in MSTN-DN mice was higher than that in WT mice regardless of whether CTX was injected or not. Under unloading condition, the population of Pax7-positive nuclei in MSTN-DN mice was increased by CTX-injection, but not in WT mice.
Regeneration of injured soleus muscle under unloading condition
In the present study, CTX-injection cause a reduction of mean fiber CSA of soleus muscle mass in WT mice under unloading condition. This result is consistent with previously reported data in WT mice [7]. Since CTX-injected muscle under unloading condition had many atrophied fibers with small diameter, it was suggested that unloading suppresses the regeneration of injured skeletal muscle.
It is well known that muscle satellite cells have a crucial role [3] and proliferate in the regenerative process of injured skeletal muscle [7,11,23,27]. Newly regenerated myofibers are attributed to muscle satellite cells that is activated and proliferated by muscle injury [6]. It has been reported that unloading inhibits the increase in numbers of muscle satellite cells in WT mice [7]. In the present study, however, Pax7-positive satellite cells of injured muscle in unloaded MSTN-DN mice, were significantly increased by 3.2 fold compared with uninjured muscle. Since the absence of MSTN leads to activation and proliferation of satellite cells [16,17], larger diameter of regenerating fiber in unloaded MSTN-DN mice may be attributed to the increase in satellite cells. Although the absence of MSTN-associated facilitation of injured skeletal muscles has been well reported [17,20], this is the first report showing that the suppression of MSTN stimulates the regeneration of injured skeletal muscle under unloading condition.
Regeneration of injured soleus muscle under weight-bearing condition
In the present study, soleus muscle weight recovered to the uninjected control level in both WT and MSTN-DN mice 4 weeks after CTX-injection. On the other hand, mean fiber CSA was decreased by CTX-injection in WT mice. These results in the present study were supported by previous studies using wild-type C57BL/6J mice showed that CTX-injection-associated decrease in soleus muscle weight was observed until 2 weeks after the injection [27] and there were atrophied fibers with small diameter until 6 weeks after the injection [7,11]. It is known that CTX-injection reduce the mass of skeletal muscle and this decline of muscle mass gradually recover through the cycle of necrosis and regeneration in a certain period [7,11,27].
On the contrary, mean fiber CSA in MSTN-DN mice 4 weeks after CTX-injection was significantly higher than that in WT mice. There is no report showing the effects of CTX-injection on soleus muscle in MSTN-DN mice. It has been reported that the regeneration of injured tibialis anterior muscle in MSTN-null mice was facilitated via the activation of muscle satellite cells [17,20]. In the present study, the population of Pax7-positive satellite cells of soleus muscle in MSTN-DN mice was significantly higher than that in WT mice (Figure 6). Accelerated recovery of fiber CSA in MSTN-DN mice may be attributed to the larger population of satellite cells, compared with WT mice.
In conclusion, the suppression of MSTN stimulates regenerative potential of injured soleus muscle via the increase in the population of muscle satellite cells even under unloading condition.
Acknowledgements
This study was supported, in part, by Grants-in-Aid for challenging Exploratory Research (16K12942, Y. Ohno; 16K13022, K.G.), and Grants-in-Aid for Scientific Research (C, 26350818, T.Y.) from the Japan Society for the Promotion of Science, the Uehara Memorial Foundation (K.G.), the Naito Foundation (K.G.), and Graduate School of Health Sciences, Toyohashi SOZO University (K.G.).
Authors thank Dr. L. L. Tang of Department of Physiology, Graduate School of Health Sciences, Toyohashi SOZO University for his technical assistance.
Conflict of interest
The authors have declared that no conflicts of interest exist.
References
1. Grounds MD, Yablonka-Reuveni Z. Molecular and cell biology of skeletal muscle regeneration. Mol Cell Biol Hum Dis Ser. 1993;3:210-56
2. Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777-86
3. Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845-56
4. Huard J, Li Y, Fu FH. Muscle injuries and repair: current trends in research. J Bone Joint Surg Am. 2002:84 -A: 822-32
5. Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345-R353
6. Bischoff R. Analysis of muscle regeneration using single myofibers in culture. Med Sci Sports Exerc. 1989;21:S164-S172
7. Matsuba Y, Goto K, Morioka S, Naito T, Akema T, Hashimoto N, Sugiura T, Ohira Y, Beppu M, Yoshioka T. Gravitational unloading inhibits the regenerative potential of atrophied soleus muscle in mice. Acta Physiol. 2009;196:329-39
8. Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol. 2000;88:359-63
9. Yasuhara K, Ohno Y, Kojima A, Uehara K, Beppu M, Sugiura T, Fujimoto M, Nakai A, Ohira Y, Yoshioka T, Goto K. Absence of heat shock transcription factor 1 retards the regrowth of atrophied soleus muscle in mice. J Appl Physiol. 2011;111:1142-9
10. Goto A, Ohno Y, Ikuta A, Suzuki M, Ohira T, Egawa T, Sugiura T, Yoshioka T, Ohira Y, Goto K. Up-regulation of adiponectin expression in antigravitational soleus muscle in response to unloading followed by reloading, and functional overloading in mice. PLoS One. 2013;8:e81929
11. Morioka S, Goto K, Kojima A, Naito T, Matsuba Y, Akema T, Fujiya H, Sugiura T, Ohira Y, Beppu M, Aoki H, Yoshioka T. Functional overloading facilitates the regeneration of injured soleus muscles in mice. J Physiol Sci. 2008;58:397-404
12. Kohno S, Yamashita Y, Abe T, Hirasaka K, Oarada M, Ohno A, Teshima-Kondo S, Higashibata A, Choi I, Mills EM, Okumura Y, Terao J, Nikawa T. Unloading stress disturbs muscle regeneration through perturbed recruitment and function of macrophages. J Appl Physiol. 2012;112:1773-82
13. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83-90
14. McPherron AC. Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457-61
15. Schuelke M, Wagner KR, Stolz LE, Hübner C, Riebel T, Kömen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682-8
16. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135-47
17. Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA. 2005;102:2519-24
18. Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486-8
19. Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285:E876-E888
20. McCroskery S, Thomas M, Platt L, Hennebry A, Nishimura T, McLeay L, Sharma M, Kambadur R. Improved muscle healing through enhanced regeneration and reduced fibrosis in myostatin-null mice. J Cell Sci. 2005;118:3531-41
21. Nishi M, Yasue A, Nishimatu S, Nohno T, Yamaoka T, Itakura M, Moriyama K, Ohuchi H, Noji S. A missense mutant myostatin causes hyperplasia without hypertrophy in the mouse muscle. Biochem Biophys Res Commun. 2002;293:247-51
22. Ohno Y, Sugiura T, Ohira Y, Yoshioka T, Goto K. Loading-associated expression of TRIM72 and caveolin-3 in antigravitational soleus muscle in mice. Physiol Rep. 2014;2:e12259
23. Nishizawa S, Koya T, Ohno Y, Goto A, Ikuita A, Suzuki M, Ohira T, Egawa T, Nakai A, Sugiura T, Ohira Y, Yoshioka T, Beppu M, Goto K. Regeneration of injured skeletal muscle in heat shock transcription factor 1-null mice. Physiol Rep. 2013;1:e00071
24. Couteaux R, Mira JC, d'Albis A. Regeneration of muscles after cardiotoxin injury. I. Cytological aspects. Biol Cell. 1988;62:171-82
25. Fletcher JE. Jiang MS. Possible mechanisms of action of cobra snake venom cardiotoxins and bee venom melittin. Toxicon. 1993;31:669-95
26. Ohno Y, Egawa T, Yokoyama S, Nakai A, Sugiura T, Ohira Y, Yoshioka T, Goto K. Deficiency of heat shock transcription factor 1 suppresses heat stress-associated increase in slow soleus muscle mass of mice. Acta Physiol. 2015;215:191-203
27. Naito T, Goto K, Morioka S, Matsuba Y, Akema T, Sugiura T, Ohira Y, Beppu M, Yoshioka T. Administration of granulocyte colony-stimulating factor facilitates the regenerative process of injured mice skeletal muscle via the activation of Akt/GSK3alphabeta signals. Eur J Appl Physiol. 2009;105:643-51
Author contact
![]() Corresponding author: Katsumasa Goto, Ph.D. Department of Physiology, Graduate School of Health Sciences, Toyohashi SOZO University, 20-1 Matsushita, Ushikawa, Toyohashi, Aichi 440-8511, Japan. TEL: +81 50 2017 2272, FAX: +81 532 55 0803, E-mail: gotokocn.ne.jp.
Corresponding author: Katsumasa Goto, Ph.D. Department of Physiology, Graduate School of Health Sciences, Toyohashi SOZO University, 20-1 Matsushita, Ushikawa, Toyohashi, Aichi 440-8511, Japan. TEL: +81 50 2017 2272, FAX: +81 532 55 0803, E-mail: gotokocn.ne.jp.

 Global reach, higher impact
Global reach, higher impact