Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(6):451-456. doi:10.7150/ijms.15389 This issue Cite
Research Paper
The Association of -429T>C and -374T>A Polymorphisms in the RAGE Gene with Polycystic Ovary Syndrome
1. Department of Biomedical Science, CHA University, Bundang CHA Hospital, Gyeonggi-Do 463-400, Republic of Korea.
2. Columbia College of Columbia University, New York, NY 10027, USA.
Received 2016-2-26; Accepted 2016-5-9; Published 2016-6-1
Abstract
Polycystic ovary syndrome (PCOS) is a complex disorder characterized by hyperandrogenism and insulin resistance. In addition, a number of females with PCOS have ovaries with multiple cysts, an irregular or no menstrual cycle, and an imbalance of female hormones compared to those of normal controls. A variety of genetic factors have been involved in the pathogenesis of PCOS. Among these genetic factors, the receptor for advanced glycation end products (RAGE) that is associated with diabetes and involved in the complications of PCOS, was selected. We aimed to assess the relationship between -429T>C and -374T>A single nucleotide polymorphisms (SNPs) of RAGE gene with the susceptibility to PCOS.128 controls and 265 PCOS patients were used for -374T>A polymorphism and 141 controls and 290 PCOS patients were used for -429T>C polymorphism, respectively. Genotyping of two polymorphisms were analyzed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) assay and statistical analysis was performed. P values for both alleles were higher than 0.05. Frequencies of genotype and allele of two polymorphisms in RAGE gene showed no significant differences between controls and PCOS patients. The initial study on the correlation between RAGE gene and PCOS indicates that the two polymorphisms of RAGE are not associated with the pathogenesis of PCOS. However, further studies regarding the association between RAGE gene and PCOS patients in different ethnic groups are required.
Keywords: Receptor for advanced glycation end products, Single nucleotide polymorphism, Polycystic ovary syndrome.
Introduction
Polycystic ovary syndrome (PCOS) is a common and heterogeneous reproductive endocrine disorder that affects 6-8% of premenopausal women [1,2]. Its etiology is unknown and it is diagnosed by the American Society for Reproductive Medicine (ASRM) and the European Society of Human Reproduction and Embryology (ESHRE). According to ASRM/ESHRE, PCOS is defined when PCOS patients belong to two of the three criteria: oligo- or anovulation, hyperandrogenism, and polycystic ovaries [3,4]. Generally, women with PCOS have several metabolic aberrations such as a high risk of impaired glucose tolerance [5], insulin resistance, hyperlipidemia [6,7], diabetes mellitus, and cardiovascular disease [8].
Advanced glycation end-products (AGEs) are protein or lipid products of nonenzymatic glycation that is one of the most important post-translational modifications in cells [9,10]. These compounds can be increased under conditions such as oxidative damage by hyperglycemia in patients with hyperlipidemia and diabetes mellitus [11,12]. AGE affects a variety of cells and tissues in the body through the formation of interaction between molecules in the extracellular matrix by their receptor (RAGE) [13]. AGEs bind several receptors such as oligosaccharyltransferase-4 (AGE-R1/OST-48), AGE-R2/80K-H phosphoprotein, AGE-R3/Galectin-3, CD36, Macrophage scavenger receptor class A (SRA), and CD36 [14]. Most of AGE interacts with a cellular receptor RAGE [15]. These receptors are differently expressed based on cell and tissue types and play a role in degradation and removal of AGE [16].
RAGE is one of transmembrane receptors of the immunoglobulin superfamily. RAGE gene is located in the major histocompatibility complex locus (MHC) class III locus on chromosome 6p21.3 and comprises a 1.7kb 5` flanking region and 11 exons interlaced by 10 introns [17]. RAGE is expressed in a variety of cell types such as endothelial cells, dendritic cells, T-lymphocytes, monocytes, macrophages, and smooth muscles [15,18]. RAGE binds multiple ligands, including HMGB1, amyloid-β-protein, Mac-1, LPS, and AGEs [19]. RAGE has been linked to many different diseases, such as cancer, atherosclerosis, vascular disease, Alzheimer's disease, diabetic retinopathy, and diabetic nephropathy [21, 22]. In particular, patients with diabetes show increased levels of expression and accumulation of RAGE in retina and mesangial cell [22].
In normal homeostasis, RAGE binds and degrades AGE for maintaining decreased levels of AGE. However, in diabetes in particular, levels of AGE are increased. Following high levels of AGE, the high interactions between RAGE and AGE cause the secretions and activations of a variety of cytokines [24]. In PCOS, the expression levels of AGE and RAGE in serum are elevated in women with PCOS compared to those of controls [18].
To date, a few studies about the association between single nucleotide polymorphisms (SNPs) of RAGE gene and PCOS exist. The interaction of AGE-RAGE might lead to the pathogenesis of PCOS. Therefore, the aim of the present study was to investigate the association between polymorphisms of the RAGE gene and PCOS. Among 30 polymorphisms of RAGE, we selected -429T>C and -374T>A polymorphisms of the RAGE because these two polymorphisms are implicated in diabetes associated with PCOS.
Materials and methods
Study subjects
A total of 431 women which were unrelated controls and PCOS patients were recruited from the Fertility Center at CHA General Hospital (Seoul, Korea) between 2008 and 2011. PCOS patients were diagnosed by the criteria defined by the 2003 ASRM/ESHRE Rotterdam consensus. This study was approved by the Institutional Review Boards of Fertility Center of the CHA General Hospital. Written informed consent was obtained from all participants.
Biochemical measurements
Plasma follicular stimulating hormone (FSH), luteinizing hormone (LH), estrogen (E2), prolactin (PRL), thyroid stimulating hormone (TSH), dehydroepiandrosteronesulphate (DHEAS), and testosterone (T) were measured by a chemiluminescent analyzer (Beckman Coulter Inc., Fullerton, CA, USA).
DNA extraction
Blood samples of PCOS patients and controls were collected in tubes containing EDTA as an anti-clotting factor and stored at 4°C. Genomic DNA was extracted from the blood in PCOS patients and controls.
Genetic analysis
To determine genotypes of the -429T>C and -374T>A polymorphisms in exon 1 of RAGE gene in PCOS cases and healthy controls, polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) analysis were carried out. DNA fragments for the -429T>C and -374T>A polymorphisms were amplified by polymerase chain reaction (PCR), using 5'-AAA ACA TGA GAA ACC CCA GA-3' as the forward primer and 5'-CCC CGA TCC TAT TTA TTC CA-3' as the reverse primer in a total volume of 30μl. In the reaction mixture, 100ng genomic DNA was used as a template. Cycling parameters were denaturation at 95°C for 5 minutes, 30 cycles with 95°C for 30 seconds, 58°C for 40 seconds, 72°C for 40 seconds, and 72°C for 10 minutes. After PCR, the PCR products of 222 bp were digested with AluΙ and Mluc I (New England BioLabs, MA, USA) for 2 hours at 37°C. The restricted DNA fragments were electrophoresed on a 2% agarose gel containing ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA) and visualized by DNA Image Visualizer (SeouLin Bioscience Co., Ltd, Seoul, Korea). In RAGE -429T>C polymorphism, three genotypes exist in restricted DNA fragments: a single 222 bp band indicates homozygosity for the T allele. The presence of two fragments, 176 bp and 46 bp, indicates homozygosity for the C allele. The presence of three fragments, 222 bp, 176 bp, and 46 bp bands, indicates heterozygosity for the T allele and the C allele, respectively. In RAGE -374T>A polymorphism, three genotypes exist in restricted DNA fragments: a single 222 bp band indicates homozygosity for the T allele. The presence of two fragments, 121 bp and 101 bp, indicates homozygosity for the A allele. The presence of three fragments, 222 bp, 121 bp, and 101 bp bands, indicates heterozygosity for the T allele and the A allele, respectively.
Statistics analysis
Statistical analysis was performed by Hap Analysis (NGRI, Seoul, Korea; www.hap.ngri.re.kr) and the χ² test to investigate the genotypes frequencies in controls and PCOS cases. A P value less than 0.05 was considered to be statistically significant.
Results
We investigated the two polymorphisms -429T>C and -374T>A in the promoter region of RAGE gene in control and PCOS patient groups. PCOS patients were diagnosed by the 2003 ASRM/ESHRE Rotterdam Consensus. These criteria include two out of the flowing three criteria: oligomenorrhea and/or anovulation, clinical and/or biochemical hyperandrogenism, and polycystic ovaries (Table 1).
The clinical and biochemical characteristics of subjects were listed in Table 2. Normal and PCOS patient groups were described the body mass index (BMI), waist/hip ratio, obesity, levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estrogen (E2), prolactin (PRL), thyroid stimulating hormone (TSH), dehydroepiandrosteronesulphate (DHEAS), and testosterone (T). No significant differences for the levels of these proteins except LH and T between controls and PCOS patients were observed. Levels of LH and T were higher in PCOS patients compared to those of controls (Table 2).
The allelic and genotypic distribution of -374T>A and -429T>C polymorphisms were shown in Figure 1. The alleles of -374T>A and -429T>C polymorphisms were confirmed by RFLP analysis by using Mluc I and Alu I restriction enzymes, respectively. Regarding -374T>A polymorphism, no significant difference between controls and PCOS patients was identified. The TT genotype was shown in 6 controls (4.7%) and 12 PCOS patients (4.5%), TA genotype in 40 controls (31.3%) and 98 PCOS patients (37%), and AA genotype in 82 controls (64%) and 155 PCOS patients (58.5%). The frequencies of T alleles were 52 controls (20.3%) and 122 PCOS patients (23%) and A alleles were 204 controls (79.7%) and 408 PCOS patients (77%) (Table 3).
Comparison of disorders/symptoms between the normal controls and PCOS patients.
| -374T>A | -429T>C | |||
|---|---|---|---|---|
| Characteristics | Controls (n=128) | PCOS patients (n=265) (%) | Controls (n=141) | PCOS patients (n=290) (%) |
| Hyperandrogenism and oligo- or amenorrhea | n=0 | n= 61 (23.02) | n=0 | n=52 (17.93) |
| Hyperandrogenism and polycystic ovaries | n=0 | n=50 (18.87) | n=0 | n=51 (17.59) |
| Oligo- or amenorrhea and polycystic ovaries | n=0 | n=114 (43.02) | n=0 | n=140 (48.28) |
| Hyperandrogenism, oligo- or amenorrhea and polycystic ovaries | n=0 | n=40 (15.09) | n=0 | n=47 (16.20) |
Clinical and biochemical characteristics of normal controls and PCOS patients.
| -374T>A | -429T>C | |||||
|---|---|---|---|---|---|---|
| Characteristics | Controls (n=128) | PCOS patients (n=265) | P value | Controls (n=141) | PCOS patients (n=290) | P value |
| Body Mass Index (kg/m²) | 21.53±2.14 (17.31-31.64) | 22.44±3.58 (17.52-27.81) | NS | 20.68±2.19 (16.1-31.52) | 22.15±3.02 (16.54-29.3) | NS |
| Waist/hip ratio | 0.8±0.06 (0.65-0.94) | 0.79±0.05 (0.62-1.02) | NS | 0.75±0.57 (0.68-0.97) | 0.82±0.03 (0.59-1.15) | NS |
| FSH levels (mIU/ml) | 7.43±2.01 (4.08-19.51) | 6.39±1.76 (2.54-18.75) | NS | 7.35±1.98 (3.92-19.43) | 6.35±1.71 (2.67-18.94) | NS |
| LH levels (mIU/ml) | 3.2±1.58 (0.81-7.56) | 6.71±5.29 (1.15-18.8) | < 0.001 | 3.13±1.46 (0.89-7.44) | 6.58±5.32 (1.2-18.86) | < 0.001 |
| E2 levels (pg/ml) | 30.84±14.25 (5.63-62.79) | 40.64±13.11 (7.98-75.23) | NS | 29.51±13.28 (5.57-62.9) | 38.75±12.18 (7.84-74.3) | NS |
| Prolactin levels (ng/ml) | 11.7±6.36 (3.82-50.92) | 13.17±8.09 (2.4-72.53) | NS | 11.83±5.62 (3.75-55.4) | 12.23±7.49 (2.37-75.93) | NS |
| TSH levels (μIU/ml) | 1.74±0.71 (0.06-4.12) | 2.27±1.32 (0.39-5.3) | NS | 1.84±0.74 (0.05-4.08) | 2.31±1.27 (0.4-5.26) | NS |
| DHEA-S levels (μg/dl) | 150.8±52.13 (64.9-260.96) | 192.46±70.37 (49.42-378.5) | 0.01 | 147.7±53.62 (62.1-264.73) | 188.5±70.29 (49.7-380.2) | 0.01 |
| Testosterone (ng/ml) | 0.3±0.16 (0.03-0.47) | 0.48±0.29 (0.07-0.92) | < 0.001 | 0.25±0.18 (0.02-0.52) | 0.4±0.33 (0.06-0.87) | < 0.001 |
NS: not significant.
Results of PCR-RFLP assay in -374T>A and -429T>C polymorphisms of RAGE gene. (A) The amplified -374T>A polymorphism of RAGE gene was shown three different genotypes. The TT genotype is shown by a single band in 222bp, the TA genotype is shown by three bands in 222, 121, and 101 bp, and the AA genotype is indicated by two bands in 121 and 101bp. (B) The amplified -429T>C polymorphisms of RAGE gene was shown three different genotypes. The TT genotype has one band in 222 bp, the TC genotype has three bands in 222 bp, 176 bp, and 46 bp, and CC genotype has two bands in 176 bp and 46 bp.
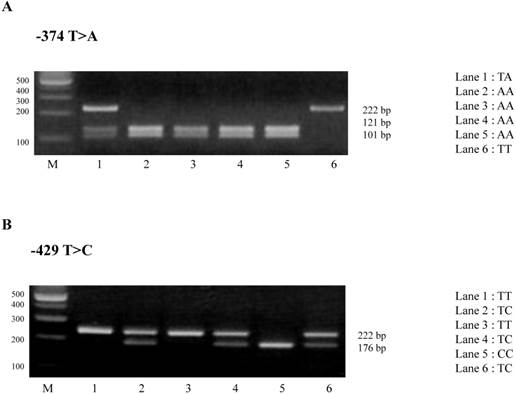
Also, no significant difference between controls and PCOS patients was identified in -429T>C polymorphism (Table 3). The TT genotype was shown in 103 controls (73%) and 214 PCOS patients (73.8%), TC genotype in 37 controls (26.3%) and 73 PCOS patients (25.2%), and CC genotype in 1 control (0.7%) and 3 PCOS patients (1%). The frequencies of T alleles were 243 controls (86.2%) and 501 PCOS patients (86.4%) and C alleles were 39 controls (13.8%) and 79 PCOS patients (13.6%). These results indicate that -374T>A and -429T>C polymorphisms in the promoter region of RAGE gene are not associated with the pathogenesis of PCOS.
Genotypes and allele frequencies of RAGE in controls and PCOS patients.
| -374T>A | Control (n=128) (%) | PCOS (n=265) (%) |
|---|---|---|
| TT | 6 (4.7%) | 12 (4.5%) |
| TA | 40 (31.3%) | 98 (37%) |
| AA | 82 (64%) | 155 (58.5%) |
| Alleles | ||
| T | 52 (20.3%) | 122 (23%) |
| A | 204 (79.7%) | 408 (77%) |
| Allele frequency | OR(95% CI) = 1.173 (0.8139-1.691) | P value = 0.3917 |
| -429T>C | Control (n=140) (%) | PCOS (n=290) (%) |
| TT | 103 (73%) | 214 (73.8%) |
| TC | 37 (26.3%) | 73 (25.2%) |
| CC | 1 (0.7%) | 3 (1%) |
| Alleles | ||
| T | 243 (86.2%) | 501 (86.4%) |
| C | 39 (13.8%) | 79 (13.6%) |
| Allele frequency | OR(95% CI) = 0.9825 (0.6501-1.485) | P value = 0.9332 |
Discussion
AGEs are the products of non-enzymatic glycation and oxidation of target proteins and lipids [19]. AGEs are formed by exogenous and endogenous factors. The exogenous AGEs are significantly produced by environmental factors such as diet and smoking. The endogenous AGEs are relatively slowly accumulated under increased glucose level in blood [20]. AGEs accumulate in various environments such as aging, inflammation, and renal diseases [21]. AGEs are main factors of a variety of disease such as obesity, type 2 diabetes, cardiovascular disease, and inflammation [22]. Levels of AGEs in plasma are associated with adipogenesis that is one of the characteristics for PCOS [23].
AGE binds to RAGE that is expressed in a variety of cells such as monocytes, lymphocytes, and endothelial cells [24]. This AGE-RAGE interaction has a role in the activation of cytokine, expression of adhesion molecules, and proliferation of fibroblast by upregulating diverse signaling pathways [25]. As increased expression levels of AGEs induce the production and accumulation of AGEs, the upregulated AGE-RAGE interaction has been implicated in the pathogenesis of diabetes [26]. High levels of RAGE are shown in diabetes patients compared to those of healthy controls [27].
AGE also elevates the expression of RAGE in PCOS patients with insulin resistance [18]. According to the increased levels of circulating AGEs in serum, AGE-RAGE interaction was elevated in PCOS patients [18]. Communications between AGE and the insulin receptor signaling (IRS) pathway have an effect on the elimination of AGEs [18]. AGEs are eliminated by IRS/PI3K pathway that is up-regulated by insulin [18]. In IRS/PI3K pathway, IRS induces autophosphorylation of tyrosine residues of insulin receptor and PI3K stimulates receptor-mediated endocytosis of AGEs [18]. On the other hand, PCOS patients that have defective IRS/PI3K pathway fail to eliminate AGEs normally [18]. Therefore, the down-regulated endocytosis and high levels of accumulation of AGEs are shown in PCOS women [18].
Interaction between AGE and RAGE also stimulates and activates nuclear NF-κB that is one of the redox sensitive transcription factors [28]. AGE-RAGE interaction with activated NF-κB pathway controls the expression of various inflammatory cytokines such as TNF-α, IL-1, and PAI-1 [28, 29]. Expression levels of these cytokines are increased in PCOS women compared to those of healthy controls. They have an effect on endothelial dysfunction and vascular injury that can implicated in the pathogenesis of PCOS and infertility [30]. AGE localizes in follicular cell layers except for endothelial cells in ovaries of normal controls. However, in PCOS ovaries, AGE expression is observed in follicular cell layers and endothelial cells. Similar expression of RAGE is detected in follicular, endothelial, and stromal cells in normal ovaries and PCOS ovaries [31].
The expression levels of RAGE gene is regulated by -374T>A and -429T>C polymorphisms in the promoter region of RAGE. The -374T>A and -429T>C polymorphisms affect transcriptional effects of RAGE. The -374T>A polymorphism that substitutes from T to A suppresses the expression of RAGE [32]. On the other hand, the -429T>C polymorphism that substitutes from T to C increases the expression of RAGE [33].
In conclusion, the present study found that there is no significant correlation between the polymorphisms of RAGE gene and PCOS women. However, this association study is the first report regarding RAGE polymorphisms and PCOS patients in Korea. In fact, this study has two limitations. First, the number of PCOS cases and unrelated controls was not big enough. Second, only two SNPs in RAGE gene were used for this study. Therefore, further investigations between other SNPs of the RAGE gene and PCOS in different ethnic populations are required. In conclusion, this study indicated that two polymorphisms of RAGE gene was not associated with PCOS patients in Korean women. However, further studies regarding the correlation between RAGE gene and PCOS patients in different ethnic groups are required.
Acknowledgements
We would like to thank the members of the Fertility Center and CHA Stem Cell Institute at CHA University and CHA General Hospital. This work is supported by a grant from the Brain Korea 21 (BK21) PLUS project in Korea.
Abbreviations
AGEs: Advanced glycation end-products; ASRM/ESHRE: American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology; BMI: Body mass index; DHEAS: Dehydroepiandrosteronesulphate; E2: Estrogen; FSH: Follicle stimulating hormone; INS: Insulin receptor signaling; LH: Luteinizing hormone; NFkB: Nuclear factor-kappa B; PCOS: Polycystic ovary syndrome; PCR-RFLP: Polymerase chain reaction-restriction fragment length polymorphism; PRL: Prolactin; RAGE: Receptor for advanced glycation end-products; SNP: Single nucleotide polymorphism; T: Testosterone; TSH: Thyroid stimulating hormone.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745-9
2. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF. Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome:an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237-45
3. Escobar-Morreale HF, Luque-Ramírez M, San Millán JL. The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev. 2005;26:251-82
4. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25
5. Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence of imparied glucose tolerance and diabetes in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929-35
6. Legro RS. Polycystic ovary syndrome and cardiovascular disease: A premature association? Endocr Rev. 2003;24:302-12
7. Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF 2nd, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2562-8
8. Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18:280-5
9. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597-605
10. Inagi R. Inhibitors of advanced glycation and endoplasmic reticulum stress. Methods Enzymol. 2011;491:361-80
11. Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17:436-43
12. Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S, Vassalotti JA, Vlassara H. Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failurepatients. Am J Kidney Dis. 2003;42:532-8
13. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Cirulation. 2006;114:597-605
14. Casselmann C, Reimann A, Friedrich I, Schubert A, Silber RE, Simm A. Age-dependent expression of advanced glycation end product receptor genes in the human heart. Gerontology. 2004;50:127-3
15. Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411-29
16. Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17:436-43
17. Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22:1572-80
18. Chuah YK, Basir R, Talib H, Tie TH, Nordin N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflam. 2013;2013:403460
19. Kierdorf K, Fritz G. RAGE regulation and signaling in inflammation and beyond. J Leukoc Biol. 2013;94:55-68
20. O'Brien J, Morrissey PA. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit Rev Food Sci. 1989;28:211-48
21. Gasser A, Forbes JM. Advanced glycation: implications in tissue damage and disease. Protein Pept Lett. 2008;15:385-91
22. Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Münch G. Advanced glycationendproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging. 2011;32:763-77
23. Jia X, Chang T, Wilson TW, Wu L. Methylglyoxal mediates adipocyte proliferation by increasing phosphorylation of Akt1. PLoS One. 2012;7:e36610
24. Flyvbjerg A, Denner L, Schrijvers BF, Tilton RG, Mogensen TH, Paludan SR, Rasch R. Long-term renal effects of a neutralizing RAGE antibody in obese type 2 diabetic mice. Diabetes. 2004;53:166-72
25. Falcone C, Emanuele E, D'Angelo A, Buzzi MP, Belvito C, Cuccia M, Geroldi D. Plasma levels of soluble receptor for advanced glycation end products and coronary artery disease in nondiabetic men. Arterioscler Thromb Vasc Biol. 2005;25:1032-7
26. Diamanti-Kandarakis E, Piperi C, Kalofoutis A, Creatsas G. Increased levels of serum advanced glycation end-products in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2005;62:37-43
27. Sharp PS, Rainbow S, Mukherjee S. Serum levels of low molecular weight advanced glycation end products in diabetic subjects. Diabet Med. 2003;20:575-9
28. Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15:16R-18R
29. Yamagishi S, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr Diabetes Rev. 2005;1:93-106
30. Herold K, Moser B, Chen Y, Zeng S, Yan SF, Ramasamy R, Emond J, Clynes R, Schmidt AM. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: inflammatory signals gone awry in the primal response to stress. J leukoc Biol. 2007;82:204-12
31. Wada R, Yagihashi S. Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann NY Acad Sci. 2005;1043:598-604
32. Piwowar A, Knapik-Kredecka M, Szczecinska J, Warwas M. Plasma glycooxidation protein products in type 2 diabetic patients with nephropathy. Diabetes Metab Res Rev. 2008;24:549-53
33. Tripathi AK, Chawla D, Bansal S, Banerjee BD, Madhu SV, Kalra OP. Association of RAGE gene polymorphisms with vascular complications in Indian type 2 diabetes mellitus patients. Diabetes Res Clin Pract. 2014;103:474-81
Author contact
![]() Corresponding author: Kwang-Hyun Baek, Department of Biomedical Science, CHA University, Bundang CHA General Hospital, 335 Pangyo-Ro, Bundang-Gu, Seongnam-Si, 463-400, Republic of Korea. Tel: 031 - 881- 7134 Fax: 031 - 881 - 7249 E-mail: baekac.kr.
Corresponding author: Kwang-Hyun Baek, Department of Biomedical Science, CHA University, Bundang CHA General Hospital, 335 Pangyo-Ro, Bundang-Gu, Seongnam-Si, 463-400, Republic of Korea. Tel: 031 - 881- 7134 Fax: 031 - 881 - 7249 E-mail: baekac.kr.

 Global reach, higher impact
Global reach, higher impact