3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(5):347-356. doi:10.7150/ijms.14393 This issue Cite
Research Paper
The Influence of Acute Hyperglycemia in an Animal Model of Lacunar Stroke That Is Induced by Artificial Particle Embolization
1. Department of Neurology, China Medical University Hospital, Taichung 404, Taiwan
2. School of Medicine, China Medical University, Taichung 404, Taiwan
3. Center for Stem Cell Research, Kaohsiung Medical University, Kaohsiung 807, Taiwan
4. School of Pharmacy, Kaohsiung Medical University, Kaohsiung 807, Taiwan
5. Department of Cell Biology and Anatomy, National Cheng Kung University, Tainan 701, Taiwan
6. Department of Neurology, China Medical University, An-Nan Hospital, Tainan 709, Taiwan
*: Equal contributors
Received 2015-11-11; Accepted 2016-3-31; Published 2016-4-27
Abstract
Animal and clinical studies have revealed that hyperglycemia during ischemic stroke increases the stroke's severity and the infarct size in clinical and animal studies. However, no conclusive evidence demonstrates that acute hyperglycemia worsens post-stroke outcomes and increases infarct size in lacunar stroke. In this study, we developed a rat model of lacunar stroke that was induced via the injection of artificial embolic particles during full consciousness. We then used this model to compare the acute influence of hyperglycemia in lacunar stroke and diffuse infarction, by evaluating neurologic behavior and the rate, size, and location of the infarction. The time course of the neurologic deficits was clearly recorded from immediately after induction to 24 h post-stroke in both types of stroke. We found that acute hyperglycemia aggravated the neurologic deficit in diffuse infarction at 24 h after stroke, and also aggravated the cerebral infarct. Furthermore, the infarct volumes of the basal ganglion, thalamus, hippocampus, and cerebellum but not the cortex were positively correlated with serum glucose levels. In contrast, acute hyperglycemia reduced the infarct volume and neurologic symptoms in lacunar stroke within 4 min after stroke induction, and this effect persisted for up to 24 h post-stroke. In conclusion, acute hyperglycemia aggravated the neurologic outcomes in diffuse infarction, although it significantly reduced the size of the cerebral infarct and improved the neurologic deficits in lacunar stroke.
Keywords: lacunar stroke, animal model, hyperglycemia, embolization, microsphere
1. Introduction
Lacunar infarct is a small isolated infarct that is caused by occluding circulation to the penetrating arteries in the deep brain. Lacunar stroke is one of the most common types of sub-cortical strokes, and accounts for approximately 25% of all ischemic stokes [1]. The pathogenesis of lacunar stroke is different from that of other types of ischemic stroke, and the prognosis after lacunar stroke is better than that after other types of ischemic stroke [2]. However, clinical evidence has revealed that lacunar stroke accounts for approximately half of all transient or non-disabling ischemic strokes [3].
Diabetes is one of the most important risk factors for both ischemic and hemorrhagic stroke. Hyperglycemia is associated with greater mortality rates up to 5 years after stroke [4]. A number of clinical trials have demonstrated that controlling hyperglycemia decreases the risk of ischemic stroke in both primary and secondary prevention [5, 6]. Both diabetes and pre-diabetes were associated with a poor early prognosis after acute ischemic stroke [7]. Unfortunately, up to 50% of patients with acute ischemic stroke have hyperglycemia [8], and many patients have no previous history of diabetes.
The possible pathogenesis of hyperglycemia in acute ischemic stroke is stress response or pre-existing impaired glucose intolerance in patients without history of diabetes [9, 10], although there is no sufficient evidence regarding the management of hyperglycemia in these patients. Furthermore, the studies regarding hyperglycemia in lacunar stroke have reported inconclusive findings, and one meta-analysis of 1375 patients with ischemic stroke from two placebo-controlled trials reported that hyperglycemia did not harm patients with lacunar stroke, and that moderate hyperglycemia (> 8mmol/L) might even be beneficial [11].
Fluctuation of glucose levels are throughout to be correlated with the severity of the stroke throughout the duration of acute stroke. For example, a recent study has demonstrated that patients with ischemic stroke experience more severe symptoms when hyperglycemia is repeatedly detected from admission to 24 h post-admission, compared to detection at admission alone [12]. However, monitoring glucose levels throughout the duration of acute ischemic stroke is wildly inconsistent. In addition, the exact time of the stroke onset is often impossible to accurately recall, as neurologic deficits (due to ischemic stroke) are often not recognized until after awakening. Therefore, this lag in testing glucose levels can create misleading information regarding the relationship between hyperglycemia and the symptom severity. Furthermore, to our best knowledge, no clinical studies have evaluated the duration of hyperglycemia in relation to lacunar stroke outcomes. Thus, the inconclusive reports regarding the effects of hyperglycemia on non-diabetic lacunar stroke may be caused by limited clinical testing of glucose levels, uncertainty regarding the stroke duration, or fluctuating post-stroke hyperglycemia in non-diabetic patients.
We have recently developed a novel rat model of lacunar stroke [13] by injecting well-designed artificial embolic particles into the cerebral circulation, which replicates the clinical characteristics regarding the infarct's relative size, location, and shape. We have also developed a method for closely observing the neurologic deficits immediately after the stroke onset by inducing embolic stroke during full consciousness. This method allows us to evaluate the full ischemic stroke course, and to observe any important early neurologic symptoms that occur immediately after the ischemic stroke induction.
The aim of the present study was to further evaluate the effect of hyperglycemia on lacunar stroke, using our rat model of lacunar stroke and a rat model of diffuse infarction as the active controls. We induced hyperglycemia via a modified method from a previous study [14] which involved injecting streptozocin (60mg/kg intraperitoneally) at 3 days before the stroke. This method induces persistent steady-state hyperglycemia and prevents the fluctuating intra-stroke glucose levels that have influence the outcomes in previous clinical studies. Our finding revealed that hyperglycemia significantly improved the neurologic deficits and reduced the infarct volume in lacunar stroke, compared to the diffuse infraction controls. In contrast, hyperglycemia increased the infarct volume in the diffuse infarction group.
2. Results
2.1 Physiological parameters of the streptozocin-induced hyperglycemic rats and controls before and after stroke
Stroke induction was performed in the rats after 3 days of streptozocin induced hyperglycemia (60 mg/kg intraperitoneally). Table 1 shows the physiological parameters of the streptozocin-induced hyperglycemic rats and the controls (no hyperglycemia) before and after the stroke. No significant differences in the physiological parameters between the two groups were observed, except in the mean pre-stroke oxygen concentrations. However, the mean pre-stroke oxygen concentrations for the stroke in the two groups were both within the normal range. No neurologic deficits were detected, and no significant post-stroke differences were observed; these results suggest the different oxygen concentrations may be part of normal physiological variability.
2.2 A steady state of hyperglycemia during ischemic stroke is achieved via streptozocin injection
Previous studies have reported that obvious blood glucose level variations within 24 h after an ischemic stroke can influence the prognosis [12]. Thus, we sought to create a steady state of hyperglycemia in our experiments.
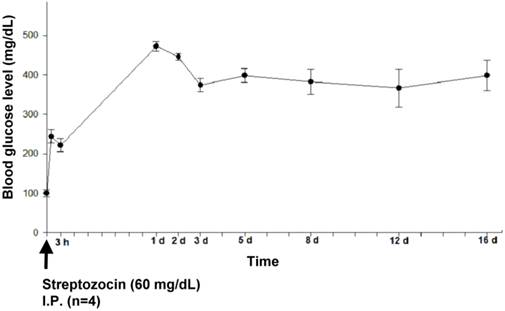
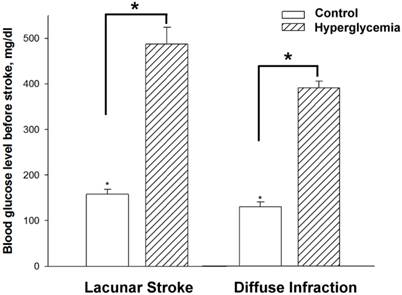
Figure 2 shows the time-line of the changes in glucose levels after the streptozocin injection. After the induction, high glucose levels were observed within the first day, and then remained at 300 mg/dL with minimal fluctuation for 16 days. The blood glucose levels at 3 days after streptozocin injection achieved a steady state, with minimal fluctuation within the 5 final checkpoints (every 2 days). Figure 3 shows the mean blood glucose levels before the stroke induction for the experimental groups and controls. As expected, the pre-stroke blood glucose levels in streptozotocin-induced groups were significantly higher than those in both control groups (lacunar stroke and diffuse infarction) (n=5-9, p < 0.05).
The pre- and post-stroke physiological parameters of the rats with streptozocin- induced hyperglycemia and the control rats. The data are expressed as mean ± standard error for each group (n = 6) (p < 0.05).
| Streptozocin induction diabetic rats (n=6) | Control group | ||||||
|---|---|---|---|---|---|---|---|
| (n=6) | |||||||
| Physiologic parameter | mean | ± | sd | mean | ± | sd | |
| Before stroke | Neurologic score | 0 | ± | 0 | 0 | ± | 0 |
| pH | 7.37 | ± | 0.04 | 7.37 | ± | 0.04 | |
| pCO2 (mmHg) | 36.9 | ± | 4.88 | 41.94 | ± | 3.02 | |
| pO2 (mmHg) | 77.42 | ± | 34.25 | 122.7 | ± | 20.44 | |
| Glucose level (mg/dL) | 427.4 | ± | 47.69 | 146.17 | ± | 105.21 | |
| BP (mmHg) | 95.1 | ± | 6.65 | 99.84 | ± | 19.08 | |
| After stroke | pH | 7.31 | ± | 0.13 | 7.42 | ± | 0.04 |
| (30 mins later) | pCO2 (mmHg) | 35.13 | ± | 6.66 | 33.94 | ± | 4.35 |
| pO2 (mmHg) | 101.8 | ± | 16.86 | 107.11 | ± | 14.85 | |
| Glucose level (mg/dL) | 418 | ± | 56.03 | 119 | ± | 53.57 | |
| BP (mmHg) | 95.28 | ± | 17.05 | 101.23 | ± | 10.78 | |
The 16-day time-course of the blood glucose levels in 4 rats with streptozocin-induced hyperglycemia. The blood glucose levels reached a steady state at 3 days after streptozocin injection, and exhibited minimal fluctuation until day 16.
Plasma glucose levels before lacunar stroke and diffuse infarction induction. The dashed line indicates the hyperglycemic groups and the blank line indicates the normoglycemic controls. The data is expressed as mean ± standard error for each group (n = 5-9). *p < 0.05.
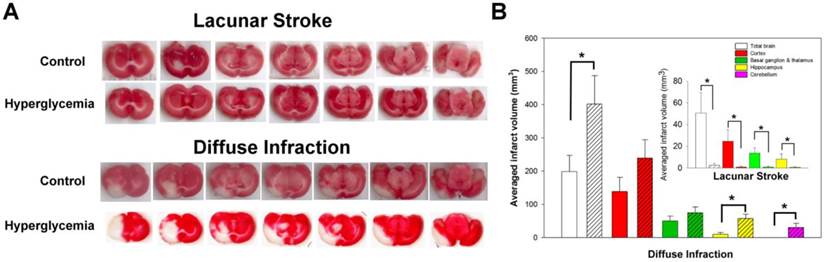
The effects of hyperglycemia on infarct volume in lacunar stroke and diffuse infarction. A) The TTC-stained serial sections revealed different effects of hyperglycemia on infarct volume in lacunar stroke and diffuse infarction. B) Quantitative analysis of hyperglycemia's effects on infarct volume in various brain regions.
2.3 Effect of acute hyperglycemia on cerebral infarct volume
As described in previous studies [13], we induced lacunar stroke or diffuse infraction by injecting different sizes of chitin/ poly-lactic-co-glycolic acid (PLGA)-mixed particles into the rats' brains (75-90 μm diameter for lacunar stroke and 38-45 μm for diffuse infraction). This method creates small isolated infarcts that are typically located in the sub-cortical regions. These infracts have a similar size, location, and shape, compared to human lacunar infarcts or diffuse infarcts that involve the cortex and most of the sub-cortical areas (Fig. 4A).
Acute hyperglycemia influenced the infarct volume in both lacunar stroke and diffuse infarction. As shown in Figure 4A, acute hyperglycemia reduced the sub-cortical infarct volume in lacunar stroke, although acute hyperglycemia aggravated the cortical and sub-cortical infarct volume in diffuse infarction. Compared to controls, acute hyperglycemia significantly reduced the infarct volume in lacunar stroke (n = 9, p < 0.05). In contrast, acute hyperglycemia significantly aggravated the infarct volume in diffuse infarction compared to controls (n = 5-6, p < 0.05).
2.4 The relationship between glucose levels and infarct volume in lacunar stroke and diffuse infarction
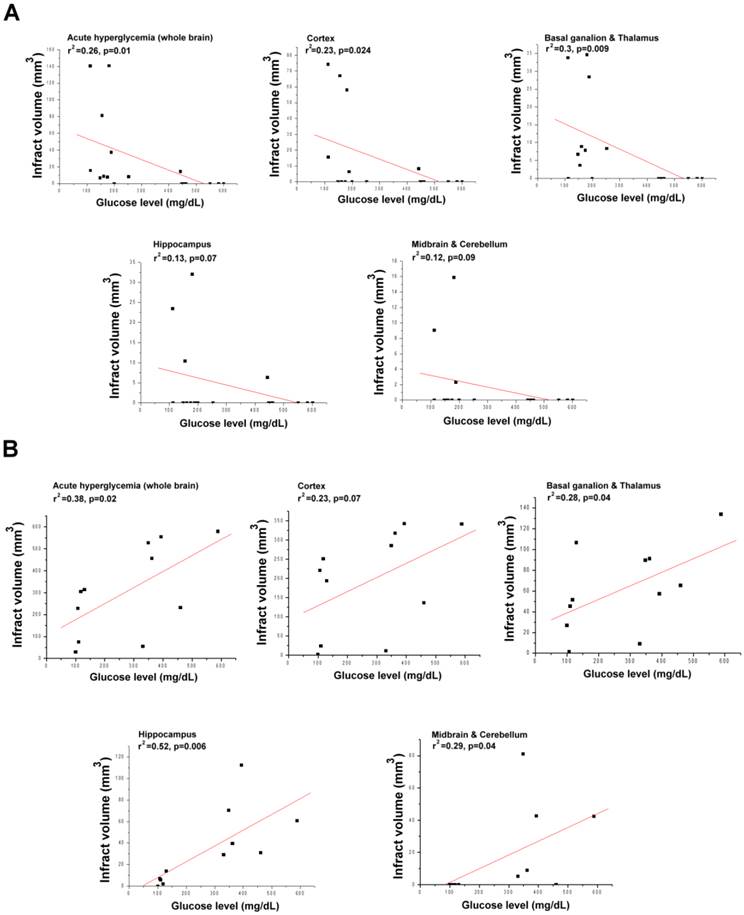
To further investigate how hyperglycemia affects infarct volume in both types of stroke, we evaluated the correlation between glucose levels and infarct volume. As shown in Figure 5A, the glucose levels significantly and negatively correlated with infarct volume in lacunar stroke. In addition, glucose levels correlated with the infarct volumes in the whole brain, cortex, basal ganglion and thalamus, although not with the volumes in the hippocampus, midbrain and cerebellum. However, glucose levels significantly and positively correlated with infarct volume in diffuse infarction (Fig. 5A). In those rats, glucose levels well correlated with the infarct volumes in the whole brain, basal ganglion, thalamus, hippocampus, midbrain and cerebellum, although not with the volume in the cortex.
2.5 Effects of hyperglycemia on neurologic deficits after the onset of lacunar stroke or diffuse infarction
The artificial particles were only injected to induce stroke after the rat achieved fully consciousness. This method allowed us to evaluate the neurologic symptoms immediately after inducing the stroke, including any early minor neurologic deficits that might disappear during reperfusion or other situations immediately after the stroke. Observable neurologic deficits were observed within 1 min in both types of stroke.
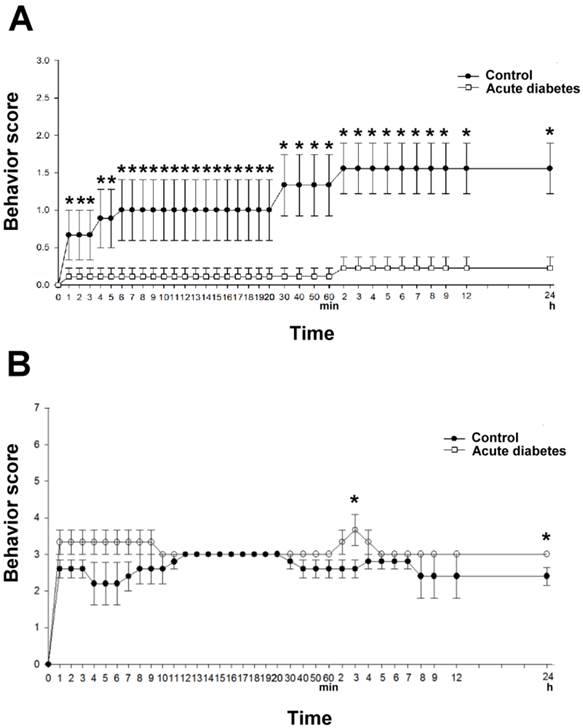
In lacunar stroke, the acute hyperglycemia significantly reduced the neurologic deficits at 4 min (compared to the controls), and this effect persisted for at least 24 h (n = 9 in both the hyperglycemia groups and the controls). The mean neurologic symptoms did not exhibit obvious fluctuation during the 24 h post-stroke period in both the hyperglycemia groups and controls groups (Fig. 6A).
In diffuse infarction, hyperglycemia significantly worsened the neurologic deficits within 10 min after stroke induction, compared to the controls. At 30 min after stroke induction, a mild improvement in the mean neurologic deficit was observed in the controls, although not in the hyperglycemic groups. After 3 h, significantly worsened neurologic symptoms were observed in the hyperglycemic rats, and significantly worsened neurologic deficits were also observed after 24 h, compared to the controls (Fig. 6B).
The relationship between infarct volume in various brain regions and the blood glucose levels in lacunar stroke and diffuse infarction. A significant and positive correlation between total infarct volume and glucose levels is clear in diffuse infarction (r2 = 0.38, p = 0.02). A significant and negative correlation between total infarct volume and glucose levels is clear in lacunar stroke (r2 = 0.26, p = 0.01). A) Lacunar stroke. B) Diffuse infarction. A p-value of <0.05 indicates a significant correlation between the two groups via correlation analysis.
The effects of hyperglycemia on the neurologic deficits immediately after lacunar stroke and diffuse infarction. A) A 24-h time-course of the neurologic scores in lacunar stroke. B) A 24-h time-course of the neurologic scores in diffuse stroke. *Significantly different from the control groups (p < 0.05).
3. Discussion
Many previous animal studies have demonstrated that acute hyperglycemia expanded the infarct volume and aggravated the neurologic deficits [15-18]. Similarly, we found that acute hyperglycemia increased the infarct volumes and aggravated the neurologic deficits in our previously reported rat model of diffuse infarction [13]. In this context, diffuse infarction in the rat brain was induced by injecting chitin/PLGA-mixed particles that were 38-45 μm in diameter into the internal carotid artery. This method causes in diffuse infarction in the cortex and most of the sub-cortical brain, with a success rate of up to 92%. Furthermore, this method is valuable because it allows us monitor any neurologic deficits that occur immediately after the induction of stroke.
In the present study, we found that glucose levels were positively correlated with infarct volume in most brain regions (although not in the cortex) for diffuse infarction. This finding suggests that hyperglycemia may aggravate the infarct volume in diffuse stroke by aggravating the infarct volume in the sub-cortical regions. However, unlike the previous studies, we did not detect any detrimental effects of hyperglycemia in our novel rat model of lacunar stroke. Furthermore, we found that rats with acute hyperglycemia exhibited significantly reduced infarct volumes and improved neurologic deficits in that model, and that the improved neurologic deficits lasted from approximately 4 min to 24 h after stroke induction. Moreover, in the model of lacunar stroke, we found that glucose levels significantly and negatively correlated with infarct volume in the cortex, basal ganglion, and thalamus. Previous clinical studies supported our results. Patients with hyperglycemia did not have larger perfusion deficits in ischaemic stroke [19]. Hyperglycemia was found not to associate with functional outcome in lacunar stroke [20]. Therefore, our finding suggested hyperglycemia can reduce the infarct volume of lacunar stroke in both the cortex and sub-cortical regions.
One of the present study's strengths is that we confirmed that the glucose levels achieved an elevated steady state before and after inducing the stroke. This consideration is important, as one previous study had demonstrated that acute hyperglycemia with obvious blood glucose levels fluctuations within 24 h after the ischemic stroke [12]. Therefore, to avoid any effects related to fluctuating blood glucose levels during the acute hyperglycemia, we used streptozotocin injections to create elevated steady-state glucose levels throughout the entire ischemic stroke. Based on our preliminary testing, we chose to induce stroke at 3 days after the streptozotocin induction, as the rats' mean glucose levels had stabilized at that point in time.
Another strength is that we induced ischemic stroke with the rats in a fully conscious state. This method allowed us evaluate to neurologic deficits immediately after the onset of stroke, which allowed us to observe that acute hyperglycemia improved the neurologic symptoms within a few minutes after lacunar stroke induction (compared to control); this effect persisted for up to 24 h. In contrast, acute hyperglycemia induced progressively worse neurologic deficit within 3 h after diffuse infarction induction, and significantly worse neurologic deficits were noted at 24 h after stroke induction. No previous animal studies have reported this phenomenon, although it may partially explain the diverse effects of hyperglycemia during ischemic stroke that have been reported in previous studies. For example, these variations may have been missed in previous studies because they did not observe the earliest stages of stroke. Nevertheless, the exact cause of this novel phenomenon is not clear, and we plan to evaluate this topic in our next study.
Destruction of the blood-brain barrier may increase the influx of toxic substances that are related to hyperglycemia (e.g. ketone bodies) into the brain, and subsequently result in worsened neurologic outcomes after ischemic stroke [21]. However, the destruction of the blood-brain barrier is only evident in diffuse infarction, and is not observed in lacunar stroke [22]. Thus, the relatively intact blood-brain barrier in lacunar stroke may partially protect the rat brain from any circulating toxic substances that are created during hyperglycemia.
Hyperglycemia can also compromise collateral circulation which may result in a greater infarct volume in the cortical area [23]. However, unlike diffuse infarction, lacunar stroke is predominately located in the sub-cortical regions, which have less cortical involvement. Thus lacunar stroke may be less susceptible to the compromised collateral circulation that is induced by acute hyperglycemia. Moreover, type 2 diabetes did not appear to affect ischemic stroke severity in previous clinical finding [24].
Interestingly, lacunar stroke is predominately located in the white matter, which predominately involves axons and glial cells, although not neurons. One in vitro study has demonstrated that lactate, which increases during uncompensated hyperglycemia is an one major source of energy for axons [25] and glial cells [26]. These laboratory findings may partially explain why acute hyperglycemia exerted the beneficial effect of lacunar stroke in our experiments. However, hyperglycemia- associated with worse clinical outcomes may be individual with coexistence with acute ischemic stroke [24, 27].
In conclusion, our novel model allowed us to accurately evaluate the effects of hyperglycemia from immediately after stroke induction to 24 h post-stroke. Using this model, found that acute hyperglycemia reduced the cerebral infarct size and neurologic deficits in a rat model of lacunar stroke. In contrast, acute hyperglycemia aggravated the cerebral infarct size and neurologic deficits in diffuse infarction.
4. Experimental Procedure
4.1 Materials
The PLGA with a 50/50 ratio of lactide:glycolide (molecular weight: approximately 40,000) was obtained from Sigma-Aldrich (USA). Chitin was obtained from Tokyo Chemical Industry (Japan). Tetrazolium Red (2,3,5-triphenyltetrazolium chloride [TTC]) was obtained from Alfa Aesar Company (USA). All other chemicals and solvents were of analytical grade.
4.2 Preparation of chitin/PLGA 50/50 mixed microparticles
The preparation of the chitin/PGLA microparticles has been reported in our previous study [13, 28, 29]. In briefly, a 1% (W/V) chitin solution was prepared by suspending the chitin powder in a dimethylacetamide (DMAC) solution that contained 5% (W/V) lithium chloride (LiCl). The chitin/DMAC-LiCl mixed suspension was stirred with a mechanical stirrer and refluxed at 130°C to dissolve the chitin powder, until a brown solution was obtained.
The chitin/PLGA mixed solution was prepared by directly dissolving the PLGA powder in the prepared chitin solution. The ratio of chitin: PLGA was 1: 1 in the final solution.
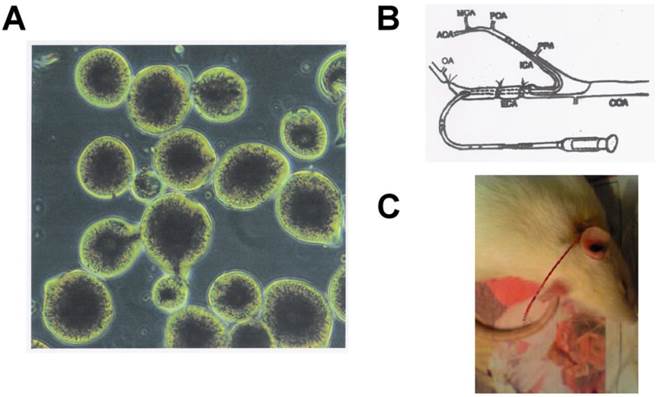
To prepare the microparticles, the chitin/PLGA solution was kept at 70 °C and dropped through a 27-gauge syringe into a 1% sodium lauryl sulfate water bath. The temperature of the water bath was kept at 25°C, which provided a coagulation sink for completely replacing of the DMAC-LiCl solution from the chitin/PLGA droplets. The gelled microparticles were then allowed to harden in the cool water bath (25°C) for 12h. After hardening, the microparticles were filtered, rinsed with deionized water, air dried overnight, and then classified according to their mesh size (40-400 mesh). Before drying, the representative light micrographs revealed the rounded shape of the particles with the PLGA in the middle and the chitin on the outside (Fig. 1A). The particles, were then grouped according to size (38 -45 μm and 75 -90 μm) for use in the embolic stroke models.
4.3 Animal model and preparation
Three-month-old male Wistar rats (300 -350 g) were used for all experiments. The animal experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University. The committee confirmed that the animal experiments followed the guidelines set by the Guide for Laboratory Factlines and Care. The rats were housed under diurnal lighting in a temperature- and light-controlled animal care facility, and were allowed free access to food and water.
To prepare for the microparticle injection, 300 mg/kg of chloral hydrate via intra-peritoneal injection was used to achieve anesthesia. The rat's body temperature was maintained at 37oC using an automated temperature regulation system, and the rats were fixed in the supine position on an operation plate. A midline excision in the ventral neck was used to expose the bifurcation of the right carotid artery, which was then excised.
To induce lacunar stroke, we injected the chitin/PLGA mixed particles (75 -90 μm) into the right internal carotid artery via an indwelling PE-10 tube, with the rats fully conscious. This method for inducing lacunar stroke has been described in our previous study [13], although we modified this method slightly to maintain consciousness (Fig. 1B). In briefly, the PE-10 tube was inserted into the right internal carotid artery at approximately 1.2 cm from the right external carotid artery, in order to reach the middle cerebral artery. The tube was then carefully fixed into the external carotid artery, and additional PE-10 tubing was exposed on the neck skin to facilitate the injection of the microparticles. An appropriate amount of heparin was used to prevent clotting on the PE-10 tube, and surgical wounds were carefully cleaned to prevent infection. After achieving full consciousness after the operation, all rats underwent a neurologic evaluation. If any focal neurologic deficits were found, the rat was excluded from all further experiments. To evaluate neurologic deficits immediately after stroke induction, we injected the chitin/PLGA particles into the right internal carotid artery via the indwelling PE-10 tube with the rats fully conscious.
Embolic stroke was induced by injecting an artificial embolus into the rat brain during full consciousness. (A) The morphological characteristics of the chitin/PLGA microparticles. (B) The PE-10 tube was inserted into right internal carotid artery from the right external carotid artery, in order to reach the middle cerebral artery. (C) The tube was then carefully fixed into the external carotid artery, and residual PE-10 tubing was exposed on the neck skin to facilitate the microparticle injection. An appropriate amount of heparin was used to prevent clotting on the PE-10 tube.
To induce diffuse infarction, we injected slightly smaller chitin/PLGA microparticles (38-45 μm), as described in our previous study [13]. All other procedures followed the same steps and modifications as the lacunar stroke model (Fig. 1B).
4.4 Induction of acute hyperglycemia
Acute hyperglycemia was induced in rats that had fasted overnight via a single intraperitoneal injection of streptozotocin (60 mg/kg in citrate buffer, pH 4.5) at 3 days before stroke induction [14]. Hyperglycemia was confirmed via elevated plasma glucose levels as determined at 24 h and day 3 after the streptozotocin injection. Only rats that achieved blood glucose levels of > 200 mg/dL were used for the experiments.
4.5 Neurologic deficit evaluation
The neurologic deficits in all rats were evaluated via neurologic scoring. The scores were evaluated immediately after stroke induction and up to 24 h post-stroke at the following time points: once per minute (1-20 min); at 20 min, 30 min, 40 min, and 50 min; once per hour (1-9 h); and at 12 and 24 h.
The neurologic deficits were scored as 0 (no neurologic defects), 1 (one paw clumsiness), 2 (tilt), 3 (rounding in only a unilateral circle), 4 (akinesia), 5 (seizure), 6 (absence of any spontaneous movement), and 7 (death). To limit variability in the scoring, all neurologic deficit evaluations were performed at the same time by the same investigator.
4.6 Tissue processing and calculating the infarction volume
We used TTC staining to measure the infarct volume. After deep anesthesia, the rat brain was rapidly removed and positioned on a brain matrix, and the brain was cut into 12 sections (2 mm thick) using the brain matrix. The TTC staining was performed by incubating the brain sections in a saline solution with 0.05% TTC for 30 min at 37°C which was followed by fixation using 4% paraformaldehyde in phosphate-buffered saline. Twenty-four hours later, the TTC staining patterns were recorded on a flat-bed color digitizer that was connected to a computer. The images of the TTC staining were scanned and the infarct areas on each image were evaluated using the imageJ analysis system (NIH, USA). The total infarct volume was calculated as the sum of all images from the same brain, and was, expressed in mm3. Brain edema was calculated via the indirect method and was subtracted from the total infarct volume [30]. We also evaluated the infarct volume in various functional areas in the rat brain, including the cortex, basal ganglia, thalamus, hippocampus, cerebellum and brain stem.
4.7 Statistical analysis
All results were presented as mean ± standard error of the mean, and the Student t test was used to evaluate inter-group differences. The univariate correlations between infarct volume and neurologic scores or plasma glucose levels were assessed using Pearson correlation coefficient. A p-value of <0.05 was considered statistically significant.
Acknowledgements
This work was supported by the Ministry of Science and Technology of Republic of China (MOST104-2320-B-039-044; MOST104-2314-B-037-003), Kaohsiung Medical University “Aim for the Top Universities Grant [KMU-TP104G00], [KMU-TP104G01] & [KMU-TP104G03], and China Medical University—An Nan Hospital (ANHRF103-8).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Norrving B. Long-term prognosis after lacunar infarction. The Lancet Neurology. 2003;2:238-45
2. Bogousslavsky J. The plurality of subcortical infarction. Stroke; a journal of cerebral circulation. 1992;23:629-31
3. Halkes PH, Kappelle LJ, van Gijn J, van Wijk I, Koudstaal PJ, Algra A. Large subcortical infarcts: clinical features, risk factors, and long-term prognosis compared with cortical and small deep infarcts. Stroke; a journal of cerebral circulation. 2006;37:1828-32
4. Kostulas N, Markaki I, Cansu H, Masterman T, Kostulas V. Hyperglycaemia in acute ischaemic stroke is associated with an increased 5-year mortality. Age and ageing. 2009;38:590-4
5. Sappok T, Faulstich A, Stuckert E, Kruck H, Marx P, Koennecke HC. Compliance with secondary prevention of ischemic stroke: a prospective evaluation. Stroke; a journal of cerebral circulation. 2001;32:1884-9
6. Jeerakathil T, Johnson JA, Simpson SH, Majumdar SR. Short-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetes: a population-based cohort study. Stroke; a journal of cerebral circulation. 2007;38:1739-43
7. Tanaka R, Ueno Y, Miyamoto N, Yamashiro K, Tanaka Y, Shimura H. et al. Impact of diabetes and prediabetes on the short-term prognosis in patients with acute ischemic stroke. Journal of the neurological sciences. 2013;332:45-50
8. Kiers L, Davis SM, Larkins R, Hopper J, Tress B, Rossiter SC. et al. Stroke topography and outcome in relation to hyperglycaemia and diabetes. Journal of neurology, neurosurgery, and psychiatry. 1992;55:263-70
9. Counsell C, McDowall M, Dennis M. Hyperglycaemia after acute stroke. Other models find that hyperglycaemia is not independent predictor. Bmj. 1997;315:810 author reply 1
10. Kernan WN, Inzucchi SE, Viscoli CM, Brass LM, Bravata DM, Horwitz RI. Insulin resistance and risk for stroke. Neurology. 2002;59:809-15
11. Uyttenboogaart M, Koch MW, Stewart RE, Vroomen PC, Luijckx GJ, De Keyser J. Moderate hyperglycaemia is associated with favourable outcome in acute lacunar stroke. Brain: a journal of neurology. 2007;130:1626-30
12. Yong M, Kaste M. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke. 2008;39:2749-55
13. Tsai MJ, Tsai YH, Kuo YM. Characterization of the pattern of ischemic stroke induced by artificial particle embolization in the rat brain. Biomaterials. 2011;32:6381-8
14. Murugan P, Pari L. Effect of tetrahydrocurcumin on lipid peroxidation and lipids in streptozotocin-nicotinamide-induced diabetic rats. Basic & clinical pharmacology & toxicology. 2006;99:122-7
15. Siemkowicz E, Hansen AJ. Clinical restitution following cerebral ischemia in hypo-, normo- and hyperglycemic rats. Acta neurologica Scandinavica. 1978;58:1-8
16. Prado R, Ginsberg MD, Dietrich WD, Watson BD, Busto R. Hyperglycemia increases infarct size in collaterally perfused but not end-arterial vascular territories. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1988;8:186-92
17. Huang NC, Wei J, Quast MJ. A comparison of the early development of ischemic brain damage in normoglycemic and hyperglycemic rats using magnetic resonance imaging. Experimental brain research. 1996;109:33-42
18. Quast MJ, Wei J, Huang NC, Brunder DG, Sell SL, Gonzalez JM. et al. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1997;17:553-9
19. Luitse MJ, van Seeters T, Horsch AD, Kool HA, Velthuis BK, Kappelle LJ. et al. Admission hyperglycaemia and cerebral perfusion deficits in acute ischaemic stroke. Cerebrovascular diseases. 2013;35:163-7
20. Fang Y, Zhang S, Wu B, Liu M. Hyperglycaemia in acute lacunar stroke: a Chinese hospital-based study. Diabetes & vascular disease research. 2013;10:216-21
21. Shimamura M, Sato N, Oshima K, Aoki M, Kurinami H, Waguri S. et al. Novel therapeutic strategy to treat brain ischemia: overexpression of hepatocyte growth factor gene reduced ischemic injury without cerebral edema in rat model. Circulation. 2004;109:424-31
22. Bailey EL, McCulloch J, Sudlow C, Wardlaw JM. Potential animal models of lacunar stroke: a systematic review. Stroke; a journal of cerebral circulation. 2009;40:e451-8
23. Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G. et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Annals of neurology. 2002;52:20-8
24. Tziomalos K, Spanou M, Bouziana SD, Papadopoulou M, Giampatzis V, Kostaki S. et al. Type 2 diabetes is associated with a worse functional outcome of ischemic stroke. World journal of diabetes. 2014;5:939-44
25. Brown AM, Tekkok SB, Ransom BR. Glycogen regulation and functional role in mouse white matter. The Journal of physiology. 2003;549:501-12
26. Sanchez-Abarca LI, Tabernero A, Medina JM. Oligodendrocytes use lactate as a source of energy and as a precursor of lipids. Glia. 2001;36:321-9
27. Zhou J, Wu J, Zhang J, Xu T, Zhang H, Zhang Y. et al. Association of Stroke Clinical Outcomes with Coexistence of Hyperglycemia and Biomarkers of Inflammation. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association. 2015
28. Mi FL, Shyu SS, Lin YM, Wu YB, Peng CK, Tsai YH. Chitin/PLGA blend microspheres as a biodegradable drug delivery system: a new delivery system for protein. Biomaterials. 2003;24:5023-36
29. Mi FL, Lin YM, Wu YB, Shyu SS, Tsai YH. Chitin/PLGA blend microspheres as a biodegradable drug-delivery system: phase-separation, degradation and release behavior. Biomaterials. 2002;23:3257-67
30. Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1990;10:290-3
Author contact
![]() Corresponding authors: Yi-Hung Tsai, PhD, School of Pharmacy, Kaohsiung Medical University, 100 Shih-chuan 1st Road, Kaohsiung, Taiwan. Tel:+886-7-3121101 ext 2261; Fax:_886-7-3210683; E-mail: yhtsaiedu.tw, and Yu-Min Kuo, PhD, Department of Cell Biology and Anatomy, National Cheng Kung University. 1 Ta Hsueh Road, Tainan, Taiwan. Tel.:+886-6-2353535 ext. 5294; Fax: +-886-6-2093007; E-mail: kuoymncku.edu.tw
Corresponding authors: Yi-Hung Tsai, PhD, School of Pharmacy, Kaohsiung Medical University, 100 Shih-chuan 1st Road, Kaohsiung, Taiwan. Tel:+886-7-3121101 ext 2261; Fax:_886-7-3210683; E-mail: yhtsaiedu.tw, and Yu-Min Kuo, PhD, Department of Cell Biology and Anatomy, National Cheng Kung University. 1 Ta Hsueh Road, Tainan, Taiwan. Tel.:+886-6-2353535 ext. 5294; Fax: +-886-6-2093007; E-mail: kuoymncku.edu.tw

 Global reach, higher impact
Global reach, higher impact