Impact Factor
ISSN: 1449-1907
Int J Med Sci 2016; 13(4):310-315. doi:10.7150/ijms.14953 This issue Cite
Research Paper
Sustained Virologic Response at 24 Weeks after the End of Treatment Is a Better Predictor for Treatment Outcome in Real-World HCV-Infected Patients Treated by HCV NS3/4A Protease Inhibitors with Peginterferon plus Ribavirin
1. Department of Gastroenterology and Nephrology, Chiba University Graduate School of Medicine, Chiba, Japan;
2. Department of Molecular Virology, Chiba University Graduate School of Medicine, Chiba, Japan;
3. Kikkoman General Hospital, Noda, Chiba, Japan;
4. Safety and Health Organization, Chiba University, Chiba, Japan
Received 2016-1-13; Accepted 2016-3-15; Published 2016-4-10
Abstract
Background. Direct-acting antiviral agents against HCV with or without peginterferon plus ribavirin result in higher eradication rates of HCV and shorter treatment duration. We examined which is better for predicting persistent virologic response, the assessment of serum HCV RNA at 12 or 24 weeks after the end of treatment for predicting sustained virologic response (SVR12 or SVR24, respectively) in patients treated by HCV NS3/4A protease inhibitors with peginterferon plus ribavirin.
Methods. In all, 149 Japanese patients infected with HCV genotype 1b treated by peginterferon plus ribavirin with telaprevir or simeprevir were retrospectively analyzed: 59 and 90 patients were treated with telaprevir- and simeprevir-including regimens, respectively. HCV RNA was measured by TaqMan HCV Test, version 2.0, real-time PCR assay. SVR12 or SVR24, respectively, was defined as HCV RNA negativity at 12 or 24 weeks after ending treatment.
Results. Total SVR rates were 78.0% and 66.7% in the telaprevir and simeprevir groups, respectively. In the telaprevir group, all 46 patients with SVR12 finally achieved SVR24. In the simeprevir group, 60 (93.8%) of the total 64 patients with SVR12 achieved SVR24, with the other 4 patients all being previous-treatment relapsers.
Conclusions. SVR12 was suitable for predicting persistent virologic response in almost all cases. In simeprevir-including regimens, SVR12 could not always predict persistent virologic response. Clinicians should use SVR24 for predicting treatment outcome in the use of HCV NS3/4A protease inhibitors with peginterferon plus ribavirin for any group of real-world patients chronically infected with HCV.
Keywords: direct-acting antivirals, HCV RNA, hepatitis C, sustained virologic response
Introduction
Hepatitis C virus (HCV) infection causes acute and chronic hepatitis and results in cirrhosis and hepatocellular carcinoma (HCC) [1,2]. Sustained virologic response (SVR) after antiviral treatment could reduce the progression rates of cirrhosis and the incidence rates of HCC [3,4]. Thus, SVR is one of the most important factors for predicting a better prognosis after antiviral treatments against chronic HCV infection [5].
SVR in patients infected with HCV treated with peginterferon plus ribavirin is defined as undetectable serum HCV-RNA at 24 weeks after the end of treatment (SVR24) [6]. Although there are contrary opinions [7], assessment of serum HCV RNA at 12 weeks after the end of treatment (SVR12) is as relevant as SVR24 for predicting SVR, and SVR12 is often used in clinical trials of direct-acting antivirals (DAAs) against chronic HCV infection [8].
DAAs against HCV with or without peginterferon plus ribavirin result in higher rates of eradication of this virus and shorter treatment duration [9-11]. In the present study, we sought to determine which is better for predicting persistent virologic response, the assessment of SVR12 or SVR24 in real-world Japanese patients treated by HCV NS3/4A protease inhibitors with peginterferon plus ribavirin.
Methods
Patients
Between December 2011 and July 2015, 149 consecutive Japanese patients were enrolled at Chiba University Hospital and Kikkoman General Hospital, an affiliated hospital of Chiba University located in Chiba Prefecture, adjacent to Tokyo. This study team retrospectively began to research the effectiveness and safety of telaprevir-based and simeprevir-based triple therapies in a group of real-world Japanese patients with chronic HCV infection. Patients were eligible for enrollment if they met the following criteria: (1) HCV genotype 1b infection; (2) patients were aged 20 years or older; and (3) patients could participate regardless of whether they had received prior interferon-based therapy. Exclusion criteria included positivity for antibody to human immunodeficiency virus, clinical or biochemical signs of hepatic decompensation, and any serious medical condition of other organs or liver diseases such as autoimmune hepatitis, primary biliary cirrhosis, hemochromatosis, Wilson disease, or alcoholic liver disease. Written informed consent was obtained from all patients, and this study conformed to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Chiba University, School of Medicine (No.523, No.1462 and No. 2153). Participation in the study was posted at our institutions.
Clinical and Laboratory Assessment
Hematological and biochemical tests were performed at least at 4 week-intervals after commencement of treatment, and after stoppage of the treatment. These parameters were measured by standard laboratory techniques at central laboratories, Chiba University Hospital [12]. Transient elastography (Fibroscan, Echosens, Paris) was used to measure liver stiffness according to the methods previously described [13].
Measurement of HCV RNA and Definition of Treatment Response
HCV RNA was measured by TaqMan HCV Test, version 2.0, real-time PCR assay (Roche Diagnostics, Tokyo, Japan), with a lower limit of qualification of 15 IU/mL, and with a range of quantitation of 1.2-7.8 log10 IU/mL [12]. Rapid virologic response (RVR) is defined as undetectable HCV RNA after 4 weeks of therapy [6]. SVR12 and SVR24 were defined as HCV RNA negativity at 12 weeks and 24 weeks after the end of treatment, respectively.
Prior treatment response was as follows: relapse, reappearance of HCV RNA after the end of treatment despite achievement of end-of-treatment response (EOTR), which was defined as undetectable HCV RNA at the end of treatment; virologic breakthrough (VBT), reappearance of HCV RNA at any time during treatment after virologic response; partial response, a greater than 2 log10 IU/mL decrease in the HCV RNA level from baseline until week 12 but detectable HCV RNA at week 12; and null response, a decrease in the HCV RNA level of less than 2 log10 IU/mL at week 12 [6].
IL28B Genotyping
Genomic DNA was extracted from blood sample with DNA Extract All Reagent Kits (Applied Biosystems, Foster City, CA, USA). Genotyping of interleukin-28B (IL28B) rs8099917 was performed by TaqMan SNP assay (Applied Biosystems) [14]. Primers were purchased from Applied Biosystems. Thermal cycling was performed with the ABI Step One real-time PCR system as previously described [14]. We analyzed IL28B rs8099917 TT as major genotype and TG and GG as minor genotypes in the present study.
Antiviral Treatment
In the telaprevir group, all patients received combination therapy with peginterferon α-2b (1.0-1.5 μg/kg) weekly (MSD, Tokyo, Japan), ribavirin (MSD) and telaprevir (1,500 mg or 2,250 mg daily) (Tanabe-Mitsubishi, Tokyo, Japan) for 12 weeks, followed by 12 weeks of peginterferon α-2b and ribavirin. In the simeprevir group, patients received a combination treatment of simeprevir (100 mg daily) (Janssen Pharmaceutical K.K., Tokyo, Japan), peginterferonα-2a (180 μg) (Chugai, Tokyo, Japan) or peginterferon α-2b (1.0-1.5 μg/kg) weekly and ribavirin (MSD or Chugai) for 12 weeks, followed by 12 weeks of peginterferon α-2a or peginterferon α-2b and ribavirin. Ribavirin was given orally at a daily dose of 400-1,000 mg based on body weight.
Statistical Analysis
Data were expressed as mean ± standard deviation (SD). Statistical analysis was performed using Student's t-test or Chi-square test with the Excel statistics program for Windows, version 7 (SSRI, Tokyo, Japan). P values of less than 0.05 were considered statistically significant. Variables with P values of less than 0.05 at univariate analysis were retained for multivariate logistic-regression analysis.
Results
Patient Characteristics
Clinical characteristics of patients in the present study are shown in Table 1. Of the total 149 patients, 59 and 90 patients received telaprevir- and simeprevir-based therapies, respectively. Among the 59 patients receiving telaprevir-based therapy, 39 were included in a previous study [15]. Male patients were more prevalent in the telaprevir group (71.2%) than in the simeprevir group (45.6%) (Table 1). Among the simeprevir-group patients, 1 was a relapser of telaprevir-based therapy, and 4 experienced VBT during the telaprevir-based therapy. Treatment-naïve patients and relapsers were dominant in the telaprevir group (Table 1). Concerning the TT/TG/GG genotypes of IL28B rs8099917, in the telaprevir and the simeprevir groups showed 40/19/0 and 58/30/2, respectively (Table 1).
Efficacy of Telaprevir- and Simeprevir-Based Therapy
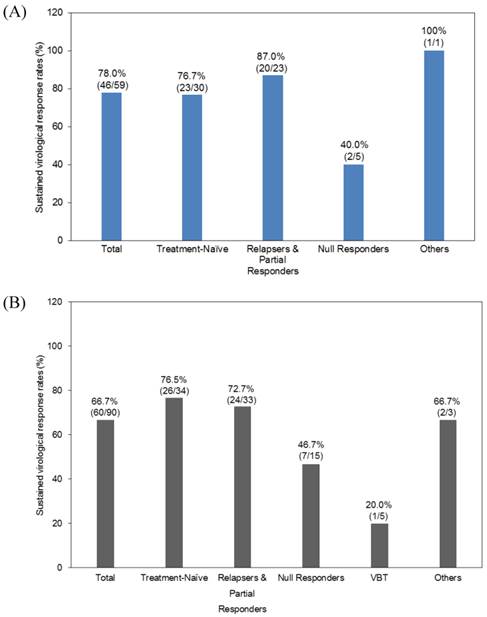
The total SVR24 rates were 78.0% and 66.7% in the telaprevir and simeprevir groups, respectively (Figure 1). In the telaprevir group, the SVR rates of treatment-naïve, previous-treatment relapsers and partial responders, and null responders were 76.7%, 87.0%, and 40.0%, respectively (Figure 1A). In the simeprevir group, the SVR rates of treatment-naïve, previous-treatment relapsers and partial responders, null responders and patients having experienced VBT were 76.5%, 72.7%, 46.7% and 20.0%, respectively (Figure 1B).
Efficacy of telaprevir and simeprevir-based therapy. Sustained virologic response of telaprevir-based therapy (A) and simeprevir-based therapy (B).
Baseline characteristics
| Parameters | Telaprevir group (N=59) | Simeprevir group (N=90) | P-values |
|---|---|---|---|
| Age (years) | 57.6±8.8 | 60.6±10.3 | 0.0678 |
| Gender (male/female) | 42/17 | 41/49 | 0.00359 |
| Previous treatments (naïve/relapse/VBT/null response/unknown) | 30/23/0/5/1 | 34/33/5/15/3 | 0.0350* |
| IL28B rs8099917 (Major/Minor) | 40/19 | 58/32 | 0.806 |
| HCV RNA (Log10 IU/mL) | 6.6±0.7 | 6.4±1.1 | 0.217 |
| Liver stiffness (kPa) | 12.1±7.8 | 11.7±8.0 | 0.764 |
| AST (IU/L) | 55.3±41.7 | 50.5±29.5 | 0.412 |
| ALT (IU/L) | 69.8±60.9 | 57.6±38.2 | 0.135 |
| γ-GTP (IU/L) | 59.6±55.9 | 42.1±51.5 | 0.0518 |
| Hemoglobin (g/dL) | 14.5±1.5 | 15.1±10.5 | 0.664 |
| Platelets (x104/μL) | 16.1±4.8 | 15.3±5.8 | 0.380 |
| AFP (ng/mL) | 8.9±11.2 | 11.0±19.7 | 0.458 |
| Peginterferon-α-2a/2b | 0/59 | 28/62 | 0.00000563 |
*Naïve plus relapse vs. others; VBT, virologic breakthrough. Data are expressed as mean ± standard deviation (SD).
Predictors of SVR
To clarify the predictors of SVR of the telaprevir group, we compared the pretreatment and treatment factors between SVR and non-SVR groups (Table 2A). Univariate analysis showed that liver stiffness (P = 0.0188), AFP (P = 0.00696), and completion of treatment for 12 weeks (P = 0.0000000115) in the telaprevir-treated patients contributed to achievement of SVR (Table 2A). SVR was attained independently of completion of treatment for 12 weeks in telaprevir-treated patients (Table 3A).
To clarify the predictors of SVR of the simeprevir group, we compared the pretreatment and treatment factors between SVR and non-SVR groups (Table 2B). Univariate analysis showed that previous treatment (P = 0.00180), IL28B rs8099917 (P = 0.000423), liver stiffness (P = 0.00866), AST (P = 0.0391), AFP (P = 0.0015), and completion of treatment for 12 weeks (P = 0.0369) in the simeprevir-treated patients contributed to achievement of SVR (Table 2B). SVR was attained independently of IL28B rs8099917 major type in the simeprevir-treated patients (Table 3B).
Retreatment with Simeprevir-Based Therapy of 5 Patients with Previous Telaprevir Failure
One patient with IL28B rs8099917 major genotype, who stopped telaprevir-based therapy at 3 weeks and was a relapser of telaprevir-based therapy, achieved RVR and SVR24 by peginterferon α-2b and ribavirin with simeprevir. Among 4 patients experienced VBT during telaprevir-based therapy, only one patient with IL28B rs8099917 minor genotype, who experienced VBT at 5 months after commencement of telaprevir-based triple therapy, achieved SVR24 by peginterferon α-2b and ribavirin with simeprevir. Among the 3 other patients, simeprevir-based therapy led to relapse in 2 patients with IL28B rs8099917 minor genotype and RVR and to VBT in one patient with IL28B rs8099917 major genotype without RVR. Thus, 2 of 5 patients with telaprevir-based therapies finally achieved SVR24 by simeprevir-based therapy.
Comparison of SVR24 and non-SVR24 patients by univariate analysis. (A) Telaprevir group. (B) Simeprevir group.
| Parameters | SVR | Non-SVR | P-values |
|---|---|---|---|
| A. Telaprevir group (N=59) | (N=46) | (N=13) | |
| Age (years) | 56.9±7.5 | 59.8±5.6 | 0.2013 |
| Gender (male/female) | 34/12 | 8/5 | 0.601 |
| Previous treatments (naïve/relapse/VBT/null response/unknown) | 23/20/0/2/1 | 7/3/0/3/0 | 0.221* |
| IL28B rs8099917 (Major/Minor) | 34/12 | 6/7 | 0.120 |
| HCV RNA (Log10 IU/mL) | 6.48±0.74 | 6.85±0.60 | 0.104 |
| Liver stiffness (kPa) | 11.3±2.6 | 15.9±12.2 | 0.0188 |
| AST (IU/L) | 55.6±32.1 | 54.0±24.9 | 0.869 |
| ALT (IU/L) | 69.9±44.7 | 69.3±45.5 | 0.966 |
| γ-GTP (IU/L) | 60.9±39.3 | 54.8±43.0 | 0.630 |
| Hemoglobin (g/dL) | 14.4±1.6 | 14.9±1.4 | 0.312 |
| Platelets (x104/μL) | 16.6±3.8 | 14.7±4.2 | 0.125 |
| AFP (ng/mL) | 7.2±2.5 | 14.8±18.2 | 0.00696 |
| Completion of treatment for 12 weeks** (yes/no) | 45/1 | 3/10 | 0.0000000115 |
| B. Simeprevir group (N=90) | (N=64) | (N=26) | |
| Age (years) | 59.6±11.4 | 63.2±6.1 | 0.131 |
| Gender (male/female) | 26/38 | 15/11 | 0.215 |
| Previous treatments (naïve/relapse/VBT/null response/unknown) | 26/28/1/7/2 | 8/5/4/8/1 | 0.00180* |
| IL28B rs8099917 (Major/Minor) | 49/15 | 9/17 | 0.000423 |
| HCV RNA (Log10 IU/mL) | 6.28±1.21 | 6.57±0.57 | 0.246 |
| Liver stiffness (kPa) | 10.4±6.5 | 15.3±10.5 | 0.00866 |
| AST (IU/L) | 46.5±28.3 | 60.6±30.5 | 0.0391 |
| ALT (IU/L) | 55.6±40.3 | 62.4±32.5 | 0.446 |
| γ-GTP (IU/L) | 41.8±59.7 | 42.7±21.5 | 0.941 |
| Hemoglobin (g/dL) | 15.6±12.4 | 13.8±1.7 | 0.464 |
| Platelets (x104/μL) | 16.0±5.8 | 13.6±5.5 | 0.0744 |
| AFP (ng/mL) | 6.8±9.8 | 21.0±31.3 | 0.0015 |
| Completion of treatment for 12 weeks** (yes/no) | 63/1 | 22/4 | 0.0369 |
*Naïve plus relapse vs. others; ** Patients finished treatment at least by 12 weeks after the commencement of treatment; SVR, sustained virologic response; VBT, virologic breakthrough. Data are expressed as mean ± standard deviation (SD).
Factors associated with SVR24 among telaprevir group (A) or among simeprevir group (B) by multivariate analysis.
| Factor | Category | Odds ratio | 95% CI | P-values |
|---|---|---|---|---|
| A. Telaprevir group | ||||
| Completion of treatment for 12 weeks | (+/-) | 49.0832 | 3.9008-617.6013 | 0.0026 |
| B. Simeprevir group | ||||
| IL28B rs8099917 Major type | (+/-) | 2.813 | 2.285-16.666 | 0.000331 |
SVR12 and SVR24
In the telaprevir group, all 46 patients with SVR12 finally achieved SVR24. In the simeprevir group, 60 (93.8%) of the total 64 patients with SVR12 achieved SVR24, and the remaining 4 patients with SVR12 but not SVR24 were all previous-treatment relapsers. These 4 patients achieved RVR. Of interest, there was data mismatching between SVR12 and SVR24 in the patients treated with simeprevir-based triple therapy.
Safety
In the present study, we tried to obtain information about the discontinuation of treatment from medical records. In patients treated with telaprevir, the reasons were 2 mental disorders, 1 acute myocardial infarction, 2 anemia, 1 neutropenia, 1 nausea, 1 rash, 1 renal dysfunction, 1 thrombocytopenia and 1 breakthrough. In patients treated with simeprevir, the reasons for treatment stoppage were 1 elevation of ALT [16], 1 upper gastro-intestinal tract bleeding, 1 jaundice, 1 mental disorder, and 8 VBT.
Discussion
We retrospectively examined the treatment outcome of telaprevir or simeprevir in combination with peginterferon and ribavirin in HCV genotype 1b patients. The former standard-of-care, the dual combination of peginterferon and ribavirin could only attain ~50% SVR in HCV genotype 1b patients [5,12]. In the present study, telaprevir or simeprevir in combination with peginterferon and ribavirin, respectively, could result in 78.0% or 66.7% SVR in HCV genotype 1b patients (Figure 1), strongly suggesting that these treatments could bring higher SVR rates and shorter duration of therapy than those of the former standard-of-care treatment. Of note, 11 of 59 patients (18.6%) discontinued telaprevir-based therapy, and 12 of 90 (13.3%) discontinued simeprevir-based therapy, mainly due to adverse events caused by interferon plus ribavirin.
In the peginterferon and ribavirin era, we pointed out that the assessment of serum HCV RNA 24 weeks after EOT using TaqMan PCR was more relevant than 12 weeks for the prediction of SVR [7]. As the development of antivirals against HCV including DAAs have been undergoing very rapid progress, SVR12 now seems suitable for the evaluation of these drugs. There is data mismatching between SVR12 and SVR24 even in the clinical trials of interferon-free regimens [18], although this may be caused by the “lost to follow-up” patients.
However, it is really important for any group of real-world patients chronically infected with HCV to know whether HCV is eradicated or not. The present study revealed that it is better to use SVR24 for predicting SVR in HCV-infected patients treated by HCV NS3/4A protease inhibitors with peginterferon plus ribavirin, and especially with simeprevir-based regimens. In the near future, if greater-sensitivity assays are developed to replace the present TaqMan assay, these situations may change.
We observed the discrepancy between the SVR12 and SVR24 in 4 patients of the simeprevir group. Although the treatment duration was 24 weeks in all 4 patients, the dose of ribavirin was reduced in 2 of 4 patients. Of IL28B rs8099917 genotype, 2 and 2 had major and minor genotypes, respectively. Liver stiffness indicated cirrhosis in only one patient. Only interferon-based regimens were used in the present study. Of interest, all 46 patients in the telaprevir group with SVR12 finally achieved SVR24. Further studies will be needed in interferon-free regimens against HCV-infected patients.
Ogawa et al. [19] reported that the treatment outcome of simeprevir-based triple therapy for HCV genotype 1b patients with telaprevir failure depended on the prior response to peginterferon-α and ribavirin. We also found that HCV genotype 1b patients with breakthrough during telaprevir-based therapy had relatively poor response to simeprevir-based triple therapy; the SVR rate of those patients, although a small sample size, was 25% (1 of 4 patients). In future, these patients will be treated with interferon-free combination with DAAs [9-11, 17, 20].
Chen et al. [21] reported that approximately 2% of patients who achieved an SVR12 did not achieve an SVR24 in phase II and III trials. In conclusion, SVR12 was suitable for predicting persistent virologic response in almost all cases. In simeprevir-including regimens, SVR12 could not always predict persistent virologic response. Clinicians are urged to use SVR24 for predicting persistent virologic response in the use of DAA treatment for real-world patients chronically infected with HCV, although the present study was interferon-including regimens and standard of care is now interferon-free regimens [5, 20].
Acknowledgements
The authors thank the medical staffs of the liver units of Chiba University Hospital and Kikkoman General Hospital who cared for the patients described herein.
Funding
This work was supported by Research Grants for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Competing Interests
Tatsuo Kanda reports receiving grant support from Chugai Pharmaceutical and MSD. Osamu Yokosuka reports receiving grant support from Chugai Pharmaceutical, Bayer, MSD, Daiichi-Sankyo, Tanabe-Mitsubishi, Bristol-Myers Squibb, Taiho Pharmaceutical, and Gilead Sciences. The other authors had no conflicts of interest to declare.
References
1. Saito I, Miyamura T, Ohbayashi A. et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990;87:6547-9
2. Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26(3 Suppl 1):34S-8S
3. Ueno Y, Sollano JD, Farrell GC. Prevention of hepatocellular carcinoma complicating chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:531-6
4. George SL, Bacon BR, Brunt EM. et al. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729-38
5. Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548-61
6. Omata M, Kanda T, Yu ML. et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409-35
7. Kanda T, Imazeki F, Wu S. et al. The assessment of serum hepatitis C virus RNA 12 weeks after the end of treatment using TaqMan polymerase chain reaction is less relevant than after 24 weeks for predicting sustained virological response. Hepatology. 2011;54:1482
8. Martinot-Peignoux M, Stern C, Maylin S. et al. Twelve weeks posttreatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis C virus receiving pegylated interferon and ribavirin. Hepatology. 2010;51:1122-6
9. Jacobson IM, Gordon SC, Kowdley KV. et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867-77
10. Kowdley KV, Gordon SC, Reddy KR. et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879-88
11. Ferenci P, Bernstein D, Lalezari J. et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983-92
12. Kanda T, Imazeki F, Yonemitsu Y. et al. Quantification of hepatitis C virus in patients treated with peginterferon-alfa 2a plus ribavirin treatment by COBAS TaqMan HCV test. J Viral Hepat. 2011;18:e292-7
13. Masuzaki R, Tateishi R, Yoshida H. et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49:1954-61
14. Miyamura T, Kanda T, Nakamoto S. et al. Hepatic STAT1-nuclear translocation and interleukin 28B polymorphisms predict treatment outcomes in hepatitis C virus genotype 1-infected patients. PLoS One. 2011;6:e28617
15. Miyamura T, Kanda T, Nakamoto S. et al. IFNL4 ss469415590 Variant Is Associated with Treatment Response in Japanese HCV Genotype 1 Infected Individuals Treated with IFN-Including Regimens. Int J Hepatol. 2014;2014:723868
16. Kanda T, Nakamura M, Sasaki R. et al. Sustained Virological Response after 8-Week Treatment of Simeprevir with Peginterferon alpha-2a plus Ribavirin in a Japanese Female with Hepatitis C Virus Genotype 1b and IL28B Minor Genotype. Case Rep Gastroenterol. 2015;9:215-20
17. Mizokami M, Yokosuka O, Takehara T. et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645-53
18. Buti M, Gordon SC, Zuckerman E. et al. Grazoprevir, Elbasvir, and Ribavirin for Chronic Hepatitis C Virus Genotype 1 Infection After Failure of Pegylated Interferon and Ribavirin With an Earlier-Generation Protease Inhibitor: Final 24-Week Results From C-SALVAGE. Clin Infect Dis. 2016;62:32-6
19. Ogawa E, Furusyo N, Dohmen K. et al. Effectiveness of triple therapy with simeprevir for chronic hepatitis C genotype 1b patients with prior telaprevir failure. J Viral Hepat. 2015;22:992-1001
20. Hepatitis C guidance. AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932-54
21. Chen J, Florian J, Carter W. et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology. 2013;144:1450-5
Author contact
![]() Corresponding author: Tatsuo Kanda, M.D., Ph.D., Associate Professor, Department of Gastroenterology and Nephrology, Chiba University, Graduate School of Medicine, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan. Tel.: +81-43-226-2086; Fax: +81-43-226-2088; E-mail: kandat-cibac.jp
Corresponding author: Tatsuo Kanda, M.D., Ph.D., Associate Professor, Department of Gastroenterology and Nephrology, Chiba University, Graduate School of Medicine, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan. Tel.: +81-43-226-2086; Fax: +81-43-226-2088; E-mail: kandat-cibac.jp

 Global reach, higher impact
Global reach, higher impact