3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2015; 12(12):995-999. doi:10.7150/ijms.13193 This issue Cite
Short Research Communication
Chronic Alcohol Consumption Leads to a Tissue Specific Expression of Uncoupling Protein-2
Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany
# Jan A. Graw and Clarissa von Haefen contributed equally to this work
Received 2015-7-8; Accepted 2015-10-11; Published 2015-11-23
Abstract
Uncoupling proteins (UCPs) are anion channels that can decouple the mitochondrial respiratory chain. "Mild uncoupling" of internal respiration reduces free radical production and oxidative cell stress. Chronic alcohol consumption is a potent inducer of oxidative stress in multiple tissues and regulates UCP-2 and -4 expression in the brain. To analyse the impact of chronic alcohol intake on UCP-2 expression in tissues with high endogenous UCP-2 contents, male Wistar rats (n=34) were treated with a 12-week 5% alcohol diet. In the lungs and the spleen of rats with a chronic alcohol diet cytochrome c release from mitochondria was significantly increased. Both organs did not show any altered gene and protein expression of UCP-2. Different to cerebral tissue chronic alcohol consumption has no regulatory effect on UCP-2 gene and protein expression in organs with a high endogenous UCP-2 content. Therefore, chronic alcohol consumption leads to a tissue specific expression of UCP-2.
Keywords: Chronic alcohol consumption, Uncoupling proteins, Cytochrome c, reactive oxygen species
Introduction
Increased oxidative stress and intracellular accumulation of reactive oxygen species (ROS) lead to impaired cell function, cell death, and are associated with a wide variety of systemic diseases [1-3]. With the electron transport chain being the major source for the generation of ROS, controlled “mild uncoupling” of the proton gradient of the inner mitochondrial membrane by uncoupling proteins (UCPs) is thought to ameliorate the burden of oxidative stress [4-7]. Besides others, chronic alcohol consumption is a potent inducer of oxidative stress in multiple tissues [1, 8-11]. Previously we could show that chronic alcohol consumption increased the expression of UCP-2 and -4 in the rat brain [12]. Therefore, UCPs with their potential to regulate ROS production can be interesting targets for the protection from ethanol induced cell stress.
Chronic alcohol consumption leads to an increased susceptibility to infections [13]. UCP-2 is expressed ubiquitously and was shown to have regulatory properties in immunity and inflammatory responses to pathogens [14-16]. In rodents, UCP-2 shows highest expression levels in macrophage-rich organs such as the lungs and the spleen [14, 17]. This led us to analyse the effects of chronic ethanol consumption on UCP-2 expression levels in these organs in a model of chronic alcohol consumption in rats. The results are discussed with regard to the possible functions of UCP-2.
Materials and Methods
Animal model
Animal experiments were approved by the state authorities for animal welfare (G 0259/08, LAGeSo Berlin, Germany) and followed institutional guidelines of the Charité-Universitätsmedizin Berlin, Germany. A rat model of chronic alcohol consumption was used as described previously [12]. Briefly, using the Lieber-DeCarli liquid diet technique, 34 male six-week-old Wistar rats were randomly assigned into a control and an alcohol treated group [18]. The diet contained ethanol 5% (v/v) for the alcohol treated group (n=17) and a corresponding isocaloric diet with maltose-dextrin for the control group (n=17). Blood alcohol levels were checked by regular 200 µl-blood samples taken from the tail vein. Rats were anesthetized with isoflurane and sacrificed by cervical dislocation after 12 weeks. Lungs and spleen were removed, immediately frozen and stored at -80 °C for subsequent processing. The number of animals used in each experiment is shown in the figure legends.
Molecular Studies
Semiquantitative RT-PCR for UCP-2
Total cellular RNA from the rats´ lungs and spleen was isolated from snap-frozen tissue by using the absolutely RNA kit (Stratagene, La Jolla, CA, USA) followed by cDNA synthesis and quantification as described previously [12]. Data represent the mean expression level ± standard deviation and gene expression was normalized to 18S rRNA. The expression of the genes was analysed by RT-PCR using the ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA, USA) according to the 2-ΔΔCt method [19]. RT-PCR was performed using the following primer pairs: UCP-2: 5´-gagagtcaagggctagcgc-3´ (sense), 5´-gcttcgacagtgctctggta-3´ (antisense); 18S rRNA: 5´-aacattaccagctaggaataatggaata-3´ (sense), 5´-tctagcggcgcaatacgaat-3´ (antisense).
Immunoblotting
The rats´ lungs and spleens were homogenized and processed for mitochondrial extraction with subsequent Western blotting as described previously [12]. The purity of the different fractions, representing the cytoplasmic and mitochondrial subcellular compartments was validated by immunoblotting for specific marker proteins: β-actin (cytosol) and voltage-dependent anion channel (VDAC, mitochondria). Monoclonal anti-rat β-actin antibody from Becton Dickinson (#612657, Heidelberg, Germany) was used at a concentration of 1:5000. Polyclonal cytochrome c antibody was purchased from BioVision (#3025-100, Mountain View, CA, USA) and used at a concentration of 1:5000. Polyclonal anti-rat VDAC antibody was purchased from Cell Signaling (#4866, Danvers, MA, USA) and used at a concentration of 1:1000, and polyclonal UCP-2 antibody was purchased from Abcam (ab67241, Cambridge, UK) and used at a concentration of 1:500. Secondary goat anti-rabbit and goat anti-mouse horseradish peroxidase (HRP)-conjugated antibody were purchased from Southern Biotechnology Associates (Birmingham, AL, USA) and used at a concentration of 1:50000. Blots were revealed by SuperSignal® West Pico Chemiluminescent Substrate detection kit (Pierce, Rockford, IL, USA).
Statistical evaluation
Experiments were performed in 10-12 animals per group (n=10-12). Data were analyzed using IBM SPSS Statistics (IBM, Frankfurt, Germany) and presented as mean ± standard error (SEM). Comparisons among groups were made using the Mann-Whitney U test. A p-value <0.05 was considered statistically significant.
Results
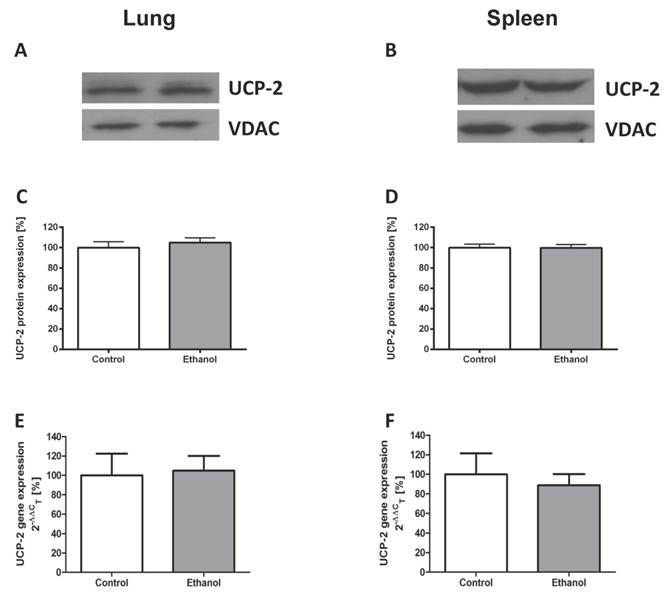
Chronic alcohol consumption does not affect the expression of UCP-2 in the lung or the spleen
To study ethanol affected UCP-2 gene expression, we performed quantitative real-time polymerase chain reactions of whole lungs and spleen total RNA isolates. To assess differences in UCP-2 protein expression between animals with and without a chronic ethanol containing diet, Western blots of mitochondrial and cytosolic isolates of whole lung and spleen tissue were performed. Compared to control animals, rats that were fed with an ethanol containing diet for 12 weeks showed no difference of UCP-2 protein expression (Fig. 1A - D) and UCP-2 mRNA expression levels (Fig. 1E - F) in both organs.
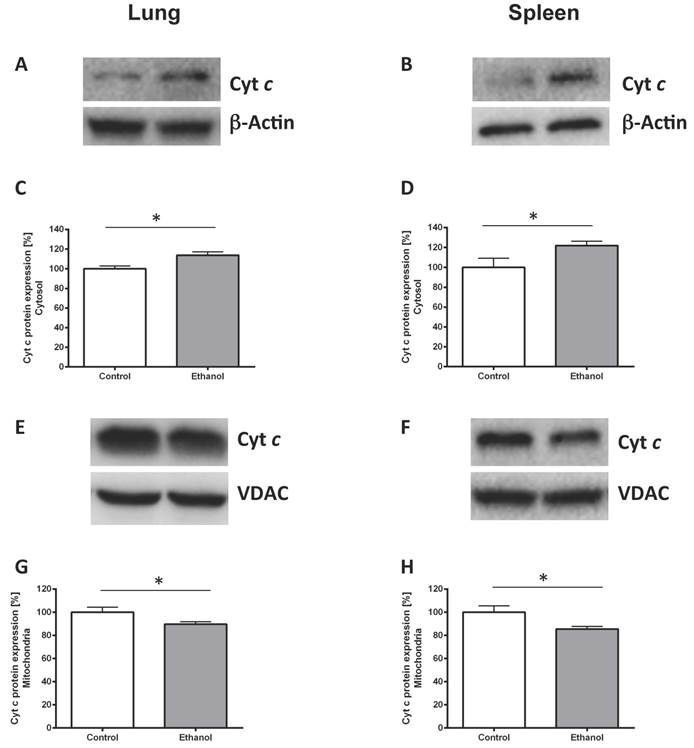
Chronic alcohol consumption leads to mitochondrial cytochrome c release in the lungs and the spleen
To prove ethanol-induced cell stress we performed Western blots against cytochrome c protein in the cytoplasm and in mitochondrial isolates. Chronic ethanol consumption led to a higher cytochrome c release in the rat lung (Fig. 2C; p=0.017). Similarly cytochrome c release was higher in the spleen of rats chronically fed with an alcohol diet (Fig. 2D; p=0.041). Significantly less cytochrome c could be detected in mitochondria of the ethanol group compared with mitochondria of the control group (p=0.046 for lung, Fig. 2G and p=0.028 for spleen, Fig. 2H). Fig. 2A, B and 2E, F show representative Western blots of a pair of rats demonstrating the protein content of lung and spleen cytosolic (Fig. 2A, B) and mitochondrial (Fig. 2E, F) cytochrome c.
Chronic alcohol consumption does not affect the expression of UCP-2 in the lung or the spleen. UCP-2 protein expression was measured by Western blot analysis in lung and spleen mitochondrial extracts of ethanol treated and control rats. UCP-2 gene expression was analysed by quantitative real-time polymerase chain reaction. Representative Western blot of lung (1A) and spleen (1B) tissue of a pair of ethanol treated and control rats. Dose-response curves for the lung (1C) and the spleen (1D). Results are mean ± SEM (n=8-10). Gene expression of UCP-2 in the lung (1E) and the spleen (1F) of a control- and an alcohol-fed group. Results are mean ± SEM (n=8-12). Chronic alcohol consumption resulted in no increased gene and protein expression of UCP-2 in both analysed organs. The experiments were repeated and similar results were obtained. VDAC served as a control for equal protein loading.
Discussion
With this model of chronically alcohol consuming Wistar rats we could demonstrate that chronic alcohol consumption has no regulatory effects on the expression of UCP-2 in the lungs or the spleen.
This differs to findings in brain tissue where an upregulation of UCP-2 and UCP-4 was detected for rats that were subjected to chronic alcohol consumption [12].
Generally, UCP-2 is regulated differently on mRNA and protein level upon oxidative stress [17]. In organs with high UCP-2 mRNA expression the mitochondria do not necessarily contain high amounts of UCP-2 protein [15, 17, 20]. The organs analyzed in this study are tissues with high baseline UCP-2 mRNA expression in rats [14]. Although reports on tissue distribution of UCP-2 protein vary, UCP-2 protein could always be detected in the spleen and the lungs [15-17]. However, neither on mRNA level nor on protein level a change in the expression levels could be noted upon ethanol-induced cell stress.
Chronic alcohol consumption predisposes to bacterial infections predominantly pneumonias [21]. It also leads to an increased susceptibility to systemic bacterial infections and significantly lowers the numbers and alters the biological functions of immune cells in the spleen [22-23]. Chronic alcohol consumption augments production of pro-inflammatory cytokines and impairs the induction of the adaptive immune response by the monocyte/macrophage system [13]. In mice, immune cells are responsible for total basal UCP-2 expression in the spleen and a third of UCP-2 expression in the lungs [24]. At an early phase of infection down-regulation of UCP-2 allows macrophages to produce more ROS associated with secretion of more pro-inflammatory cytokines [16]. However, at the late stage of the immune response after LPS-injection in mice, UCP-2 is upregulated in lungs and spleen probably to protect the cells against an excess of ROS [24]. As chronic alcohol consumption increases oxidative stress but also impairs the functions of alveolar and splenic macrophages, further increase of already high basal UCP-2 expression could lead to a potentially lethal decline of ATP production in the alveolar and splenic macrophages.
The lung, where UCP-2 is not only expressed by immune cells, is equipped with enzymes that can neutralize free radicals, for example enzymes like superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase as well as with non-enzymatic systems like vitamin E or ascorbic acid [25]. Therefore, lung tissue has a stock of antioxidative defence systems ready to cope with increased doses of oxidative stress. Accordingly, reduced pulmonary levels of glutathione are a key element of alcoholic lung disease [26].
Chronic alcohol consumption increases cytochrome c release out of the mitochondria. Cytosolic and mitochondrial extracts of lung and spleen samples were separated by SDS-PAGE, the expression of cytochrome c was detected by immunoblotting. Representative Western blot of cytosolic cytochrome c expression of a pair of ethanol treated and control rats (2A: lung, 2B: spleen). Dose-response curves (2C: lung, 2D: spleen). Results are mean ± SEM (n=8-10). *P < 0.05 represents the difference between chronic alcohol consumption and control groups. Chronic alcohol consumption resulted in increased cytochrome c release out of the mitochondria. Representative Western blot of mitochondrial cytochrome c protein expression of a pair of ethanol treated and control rats (2E: lung, 2F: spleen). Dose response curves (2G: lung, 2H: spleen). Results are mean ± SEM (n=8-10). *P < 0.05 represents the difference between chronic alcohol consumption and control groups. Chronic alcohol consumption resulted in a decreased mitochondrial cytochrome c amount. The experiments were repeated and similar results were obtained. VDAC or β-actin served as a control for equal protein loading.
As chronic alcohol consumption increased pulmonary and splenic cell damage but UCP-2 expression in these organs remained unchanged, it needs to be taken into consideration, that in organs with high ATP demand and a high baseline UCP-2-expression, further induction of UCP-2 can lead to an unnecessary decline of ATP production.
Conclusion
The upregulation of UCP-2 after chronic alcohol ingestion that was seen in brain tissue is not reflected in organs with high mRNA and protein expression levels of UCP-2. Therefore, the regulative effect of chronic alcohol consumption on UCP-2 expression is tissue-specific.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hoek JB, Cahill A, Pastorino JG. Alcohol and Mitochondria: A Dysfunctional Relationship. Gastroenterology. 2002;122:2049-2063
2. Liu J, Li J, Li WJ, Wang CM. The role of uncoupling proteins in diabetes mellitus. J. Diabetes Res. 2013:585897. doi: 10.1155/2013/585897
3. Lonn EM, Yusuf S. Evidence based cardiology: emerging approaches in preventing cardiovascular disease. B.M.J. 1999;318:1337-1341
4. Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707-716
5. Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85-93
6. Diano S, Matthews RT, Patrylo P, Yang L, Beal MF, Barnstable CJ, Horvath TL. Uncoupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for preconditioning. Endocrinology. 2003;144:5014-5021
7. Echtay KS. Mitochondrial uncoupling proteins - What is their physiological role? Free Radic. Biol. Med. 2007;43:1351-1371
8. Bailey SM, Patel VB, Young TA, Asayama K, Cunningham CC. Chronic ethanol consumption alters the glutathione/glutathione peroxidase-1 system and protein status in rat liver. Alcohol Clin. Exp. Res. 2001;25:726-733
9. Cederbaum AI. Introduction-Serial review: alcohol, oxidative stress and cell injury. Free Radic. Biol. Med. 2001;31:1524-1526
10. Mantle D, Preedy VR. Free radicals as mediators of alcohol toxicity. Adverse Drug React. Toxicol. Rev. 1999;18:235-252
11. Nordmann R, Ribiere C, Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic. Biol. Med. 1992;12:219-240
12. Graw JA, von Haefen C, Poyraz D, Möbius N, Sifringer M, Spies CD. Chronic alcohol consumption increases the expression of uncoupling protein-2 and -4 in the brain. Alcohol Clin. Exp. Res. 2013;37:1650-1656
13. Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin. Exp. Res. 2009;33:220-232
14. Alan L, Smolkova K, Kronusova E, Santorova J, Jezek P. Absolute levels of transcripts for mitochondrial uncoupling proteins UCP2, UCP3, UCP4, and UCP5 show different patterns in rat and mice tissues. J. Bioenerg. Biomembr. 2009;41:71-78
15. Rupprecht A, Bräuer AU, Smorodchenko A, Goyn J, Hilse KE, Shabalina IG, Infante-Duarte C, Pohl EE. Quantification of uncoupling protein 2 reveals its main expression in immune cells and selective up-regulation during T-cell proliferation. PLoS One. 2012;7:e41406
16. Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 2000;26:435-439
17. Pecqueur C, Alves-Guerra MC, Gelly C, Levi-Meyrueis C, Couplan E, Collins S, Ricquier D, Bouillaud F, Miroux B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J. Biol. Chem. 2001;276:8705-8712
18. Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol and Alcoholism. 1989;24:197-211
19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408
20. Rupprecht A, Sittner D, Smorodchenko A, Hilse KE, Goyn J, Moldzio R, Seiler AE, Bräuer AU, Pohl EE. Uncoupling protein 2 and 4 expression pattern during stem cell differentiation provides new insights into their putative function. PLoS One. 2014;9:e88474
21. Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc. Am. Thorac. Soc. 2005;2:428-432
22. Chadha KC, Stadler I, Albini B, Nakeeb SM, Thacore HR. Effect of Alcohol on Spleen Cells and Their Functions in C57BL/6 Mice. Alcohol. 1991;8:481-485
23. Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109-1116
24. Alves-Guerra MC, Rousset S, Pecqueur C, Mallat Z, Blanc J, Tedgui A, Bouillaud F, Cassard-Doulcier AM, Ricquier D, Miroux B. Bone marrow transplantation reveals the in vivo expression of the mitochondrial uncoupling protein 2 in immune and nonimmune cells during inflammation. J. Biol. Chem. 2003;24:42307-42312
25. Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic. Biol. Med. 1992;21:669-681
26. Guidot DM, Roman J. Chronic ethanol ingestion increases susceptibility to acute lung injury - a role of oxidative stress and tissue remodeling. Chest. 2002;122:309S-314S
Author contact
![]() Corresponding author: Claudia D. Spies, MD, Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany. Phone +49 30 450 551 001; Fax +49 30 450 551 900; E-Mail claudia.spiesde
Corresponding author: Claudia D. Spies, MD, Department of Anesthesiology and Intensive Care Medicine, Campus Charité Mitte and Campus Virchow-Klinikum, Charité - Universitätsmedizin Berlin, Augustenburger Platz 1, D-13353 Berlin, Germany. Phone +49 30 450 551 001; Fax +49 30 450 551 900; E-Mail claudia.spiesde

 Global reach, higher impact
Global reach, higher impact