3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2015; 12(6):517-523. doi:10.7150/ijms.11376 This issue Cite
Research Paper
Comparison of Drug Concentrations in Human Aqueous Humor after the Administration of 0.3% Gatifloxacin Ophthalmic Gel, 0.3% Gatifloxacin and 0.5% Levofloxacin Ophthalmic Solutions
1. From the Eye Center, Affiliated Second Hospital, College of Medicine, Zhejiang University, Hangzhou, China
2. Analysis Centre of Agrobiology and Environmental Sciences, Zhejiang University, Hangzhou, China
Received 2014-12-17; Accepted 2015-5-25; Published 2015-6-10
Abstract
Purpose: To investigate the penetration of 0.3% gatifloxacin ophthalmic gel, 0.3% gatifloxacin ophthalmic solution and 0.5% levofloxacin ophthalmic solution into aqueous humor after topical application.
Materials and Methods: Age-related cataract patients (150 eyes in 150 cases) receiving phacoemulsification were randomly divided into three groups: a 0.3% gatifloxacin gel group (n=50), a 0.3% gatifloxacin solution group (n=50), and a 0.5% levofloxacin solution group (n=50). Each group was administered one drop of gel or solution every 15 minutes for four doses. Aqueous samples were collected at different time points after the last drop. High pressure liquid chromatography (HPLC) was applied to determine the concentrations. The one-way ANOVA analysis was performed.
Results: Our data indicated that the concentration of the gatifloxacin gel group was higher than that of the gatifloxacin solution group at all time points (P <0.05); moreover, the gatifloxacin gel group exhibited higher levels than the levofloxacin solution group at 120.0 min and 180.0 min (P<0.05). Furthermore, the gatifloxacin gel produced the highest concentration at 120.0 min, and the gatifloxacin and levofloxacin solutions reached their peak values at 60.0 min.
Conclusions: 0.3% gatifloxacin ophthalmic gel application produced highest aqueous humor drug concentration, maintained the longest time, had the best penetration and bioavailability.
Keywords: aqueous humor, levofloxacin, gatifloxacin, ophthalmic gel, ophthalmic solution
Introduction
Postoperative endophthalmitis is an inflammatory condition of the eye, presumed to be due to an infectious process from bacteria, fungi or, on rare occasions, parasites that enter the eye during the perioperative period [1]. The incidence of endophthalmitis after cataract surgery has been reported to be approximately 0.06%-0.20% [2]. It is one of the most serious complications that can cause visual loss and debilitation. Preoperative skin and conjunctival disinfection with povidone-iodine can decrease the bacterial colonization of the ocular surface and reduce the relative risk of postoperative endophthalmitis [3-5]. The intracameral injection of cefuroxime at the end of surgery is another effective prophylaxis to reduce the occurrence of endophthalmitis [6].
The perioperative usage of topical antibiotic drops has been controversial. Some reports demonstrated the application of topical antibiotic drops preoperatively and/or postoperatively didn't show a lower endophthalmitis rate [7-9]. While the possibility of different fluoroquinolone antibiotics may affect the endophthalmitis incidence was also reported [10]. Currently, most countries still preoperatively apply topical antibiotic drops. In Europe, the topical usage prior to surgery are widespread used because some clinicians believe they have a role [1]. Also in Asian countries such as China and Japan, the topical application is suggested in guidelines [11] and the fluoroquinolone drops are the most common perioperative agents used in clinic [12].
Fluoroquinolone drops are favored agents in some areas due to their broad-spectrum, highly efficient, minimally toxic, ability to penetrate the corneal epithelium, and commercial availability [1]. The third-generation fluoroquinolone levofloxacin and the fourth-generation fluoroquinolone gatifloxacin are common drugs that are applied before ophthalmologic operations [13]. Gatifloxacin has a broader antimicrobial spectrum [14-16], stronger antibacterial activity[13], lower resistance [16, 17], less anaphylaxis[18] and fewer toxic side effects[18] than does levofloxacin, which confers great advantages to the clinical application of gatifloxacin. However, a study confirmed that 0.5% levofloxacin ophthalmic solution reaches higher drug concentrations in the human aqueous humor than does 0.3% gatifloxacin ophthalmic solution [19]. Therefore, the increased gatifloxacin concentration and bioavailability in the human anterior chamber remains an issue that should be urgently addressed. The appearance of ophthalmic gels has provided a novel method to address this issue. Compared to the ophthalmic solutions, ophthalmic gels have lower drug wastage rates and longer residences and action times on the ocular surface. Therefore, the bioavailability of gatifloxacin has been greatly improved [20, 21]. Research has demonstrated that 0.3% gatifloxacin ophthalmic gel can attain significantly greater drug concentrations in the human aqueous humor than can 0.3% gatifloxacin ophthalmic solution [22]. Thus far, no articles comparing the intraocular bioavailabilities of gatifloxacin ophthalmic gel and levofloxacin ophthalmic solution at different time points in animal model or clinic research have been published. Therefore, our research focused on multiple time point comparisons of the drug concentrations of the three different fluoroquinolone antibiotics in the human aqueous humor to identify the penetration of topical antibiotic ophthalmic agents and provide data for clinical use.
Materials and Methods
Patients
One hundred fifty cases (150 eyes) were selected from among the patients with upcoming phacoemulsification procedures from June 2010 to September 2011 in the Eye Center of the Second Hospital Affiliated to the College of Medicine, Zhejiang University (Hangzhou, Zhejiang Province, China); The following patients were included in the study: (1) xanthoderm Han Chinese people; (2) 50 years and older; (3) patients who were suffering from age-related cataracts. (4) patients who were for phacoemulsification procedures. The following patients were excluded from the study: (1) patients who were suffering blepharitis, dry eye, ocular trauma, uveitis, high myopia or any other ocular diseases in the study eye that might interfere in the results; (2) patients who had any corneal refractive surgery, drainage surgery, intraocular surgery or any other ocular surgery in the study eye that might interfere in the results; (3) patients who were suffering from diabetes or any other systemic diseases that might confound the results; (4) patients who had the history of allergies to gatifloxacin, levofloxacin or any other fluoroquinolones; (5) patients who had received topical or systemic drugs or treatment that might influence the study within 1 month, such as local or systemic antibiotics usage.
Trial drugs
The 0.3% gatifloxacin ophthalmic gel was obtained from Shenyang Xinqi Pharmaceutical Co., Ltd., China, and it contained gatifloxacin, macromolecular hydrophilic polymers of carbomer, hydroxypropyl methyl cellulose and sodium hyaluronate [23], with preservative (ethylparaben).The 0.3% gatifloxacin ophthalmic solution was obtained from Chuxiong Laoboyuntang Pharmaceutical Co., Ltd., China, and it included gatifloxacin and preservative (benzalkonium bromide)[24]. The 0.5% levofloxacin ophthalmic solution was obtained from Santen Pharmaceutical Co., Ltd., Japan, and it contained levofloxacin hydrate, without preservative.
Methods
The study was approved by Ethics Committee of The Second Affiliated Hospital of Zhejiang University School of Medicine, China (2010,No.18), and consent was obtained from all participants.
Groups
The patients were divided into three groups based on a random number table: a 0.3% gatifloxacin ophthalmic gel group (24 females, 26 males; age: 76.24±7.11 years), a 0.3% gatifloxacin ophthalmic solution group (24 females, 26 males; age: 72.64±10.52 years), and a 0.5% levofloxacin ophthalmic solution group (21 females, 29 males; age: 74.24±8.74 years). The differences between groups were analyzed with one-way ANOVAs, and no significant differences were found between the groups (P=0.132). Every group was divided into five subgroups based on a random number table, and each subgroup contained 10 cases (Table 1).
The subgroups of the cataract patients at different time points
| Group | Subgroups | ||||
|---|---|---|---|---|---|
| 15min | 30min | 60min | 120min | 180min | |
| 0.3% gatifloxacin ophthalmic gel | 10 cases | 10 cases | 10 cases | 10 cases | 10 cases |
| 0.3% gatifloxacin ophthalmic solution | 10 cases | 10 cases | 10 cases | 10 cases | 10 cases |
| 0.5% levofloxacin ophthalmic solution | 10 cases | 10 cases | 10 cases | 10 cases | 10 cases |
Drug administration and Sample collection
Phacoemulsifications and aqueous humor extractions for all patients were performed by an experienced surgeon (Yao, K.). Each group was administered one drop of 0.3% gatifloxacin ophthalmic gel, 0.3% gatifloxacin ophthalmic solution or 0.5% levofloxacin ophthalmic solution every 15 minutes for a total of four doses. The first dose was 60, 75, 105, 165 or 225 minutes before the sample collection depending on the subgroup. Before the main incisions of phacoemulsification, volumes of aqueous humor greater than 100 µl were extracted by 1 ml syringes with needles. The sample extractions were at 15, 30, 60, 120 or 180 minutes after the last dose according to the subgroup. The anterior chambers were refilled with sodium hyaluronate gel and the phacoemulsification proceeded as usual. One hundred microliters of each aqueous humor sample were immediately transferred to 0.6 ml sterile eppendorf tubes and stored in -80℃ until analysis. All samples were collected in the same way.
Assay of the drug concentrations
Chromatographic conditions
The samples were analyzed with high performance liquid chromatograph (Agilent 1100LC, contained, for instance, Agilent 1100 binary infusion pump, fluorescence detector, and automatic sampler). The specifications of the chromatographic column were as follows: Agilent Zorbax XDB-C18 (4.6×250 mm, 5 µm); mobile phase: acetonitrile-phosphate buffer (containing 0.1% phosphoric acid and 0.15% triethylamine), v/v=15/85; flow rate:1.0 mL/min; fluorescence detection: λ Ex295 nm, λ Em495 nm; PMT gain: 10; column temperature: 30℃; and sample volume: 20 µl. The gatifloxacin and levofloxacin reference substances were purchased from the National Institute for the Control of Pharmaceutical and Biological Products, China.
Sample processing
The internal standard method was applied in the study. The gatifloxacin and levofloxacin reference substances were used to performe linear regression. The within-day precision, between-day precision, recovery rate and extraction rate were tested to assess the stability of the method. 150 samples were measured in two consecutive days. Each day, the aqueous humor samples were compared to the standard curve to ensure the stability and the samples were tested separately. The 100 µl samples of aqueous humor were pipetted into 1.5 ml centrifuge tubes, and 10 µL of internal standard solution (10.6 mg/L) and 100 µL methyl alcohol were then added. The samples were oscillated for 30 s and centrifuged at high speed for 20 min (20000 r/min). Next 20 µL of the supernatant was extracted for high-pressure liquid chromatography.
Statistical Analyses
This study was randomized. The drug administrations, surgeries, pharmaceutical tests and statistical analyses were completed by different researchers. One-way ANOVA tests (SPSS 20.0) were used to statistically analyze the differences in the drug concentration in the aqueous humor between the subgroups at the different time points. The differences were considered significant when P <0.05.
Results
Chromatographic specificities and linearities of gatifloxacin and levofloxacin
In our chromatographic conditions, the chromatographic specificities of gatifloxacin and levofloxacin indicated that endogenous substances and other impurities present in the aqueous humor would not interfere with the isolation of the samples, and the resolutions (Rs) of gatifloxacin and levofloxacin were >1.5. The matching of the peak area of samples and standards with the drug concentrations was performed by linear regression. Gatifloxacin in the aqueous humor exhibited good linear relationship from 0.0216 to 5.40 mg/L, the regression equation was y=0.8423x+0.0016 (r=0.9999), and the minimal concentration was 0.0108 mg/L. Levofloxacin in the aqueous humor exhibited an excellent linear correlation from 0.0212 to 5.30 mg/L, the regression equation was y=1.0293x-0.0051 (r=0.9999), and the minimal value was 0.0106 mg/L.
HPLC precision and recovery
The HPLC precision and recovery are described in (Table 2 and Table 3).
Drug concentrations in the aqueous humor at different time points
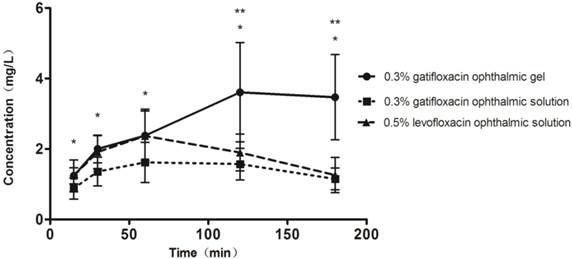
The drug concentrations in the aqueous humor from each subgroup after the final doses at different time points are described in (Table 4). Our study suggested that the concentrations of the gatifloxacin ophthalmic gel group were significantly higher than those of the gatifloxacin ophthalmic solution group at all time points (P<0.05). The gatifloxacin ophthalmic gel group also achieved markedly higher concentrations than did the levofloxacin ophthalmic solution group at 120 and 180 min after the last dose (P<0.05). Furthermore, the gatifloxacin ophthalmic gel reached the maximum concentration of 3.61±1.41 mg/L at 120 min after the last administration, while the other antibiotic peaked at 60 min after last drop at 1.62±0.57 mg/L and 2.37±0.76 mg/L, respectively. The areas under the curve (AUCs) for the bioavailabilities of the drugs[25] were determined by calculating trapezoidal areas (Figure 1). The AUC for the 0.3% gatifloxacin ophthalmic gel group was 482.1 mg·min·L-1 versus 238.8 mg·min·L-1 for the 0.3% gatifloxacin ophthalmic solution group; thus, the gel exhibited a bioavailability that was approximately 2-fold greater than that of the solution. Thus, with this mode of administration, bioavailability was increased by 1-fold via the use of the gel preparation. The area of the 0.5% levofloxacin ophthalmic solution group was 311.0 mg·min·L-1, and gatifloxacin ophthalmic gel was 1.55-fold of the levofloxacin ophthalmic solution group. Therefore, the bioavailability of gatifloxacin was 55% higher than that of levofloxacin.
The precision and recovery of gatifloxacin in the aqueous humor (n=5)
| concentration | within-day precision | between-day precision | ||
| (mg/L) | (X±SD) | RSD(%) | (X±SD) | RSD(%) |
| 0.0216 | 0.0203±0.000646 | 3.19 | 0.0207±0.000728 | 3.52 |
| 1.08 | 1.10±0.00274 | 0.25 | 1.10±0.0186 | 1.69 |
| 5.40 | 5.41±0.0219 | 0.41 | 5.47±0.0420 | 0.77 |
| concentration | recovery rate (%) | extraction rate (%) | ||
| (mg/L) | (X±SD) | RSD(%) | (X±SD) | RSD(%) |
| 0.0216 | 93.90±2.99 | 3.19 | 96.28±2.08 | 2.16 |
| 1.08 | 102.0±0.25 | 0.25 | 97.88±0.12 | 0.12 |
| 5.40 | 100.2±0.41 | 0.41 | 97.08±0.45 | 0.46 |
(RSD: relative standard deviation)
The precision and recovery of levofloxacin in the aqueous humor (n=5)
| concentration | within-day precision | between-day | ||
| (mg/L) | (X±SD) | RSD(%) | (X±SD) | RSD(%) |
| 0.0212 | 0.0214±0.000444 | 2.08 | 0.0224±0.0006 | 2.68 |
| 1.06 | 1.07±0.00455 | 0.43 | 1.09±0.0390 | 3.59 |
| 5.30 | 5.26±0.0144 | 0.27 | 5.25±0.0159 | 0.30 |
| concentration | recovery rate (%) | extraction rate(%) | ||
| (mg/L) | (X±SD) | RSD(%) | (X±SD) | RSD(%) |
| 0.0212 | 100.8±2.10 | 2.08 | 96.74±2.50 | 2.58 |
| 1.06 | 100.8±0.43 | 0.43 | 94.21±0.37 | 0.39 |
| 5.30 | 99.19±0.27 | 0.27 | 99.22±0.35 | 0.35 |
(RSD: relative standard deviation)
Drug concentrations in the aqueous humor from the three groups of cataract patients at different time points after administration(mg/L,x±s)
| Group | Drug concentrations at different time points(min) | ||||
|---|---|---|---|---|---|
| 15 | 30 | 60 | 120 | 180 | |
| 0.3% gatifloxacin ophthalmic gel | 1.24±0.23 | 2.00±0.39 | 2.38±0.70 | 3.61±1.41 | 3.47±1.21 |
| 0.3% gatifloxacin ophthalmic solution | 0.88±0.30a | 1.36±0.41a | 1.62±0.57a | 1.57±0.45a | 1.15±0.31a |
| 0.5% levofloxacin ophthalmic solution | 1.24±0.45 | 1.91±0.47 | 2.37±0.76 | 1.90±0.52b | 1.26±0.50b |
a: The difference between the gatifloxacin ophthalmic gel subgroup and the gatifloxacin ophthalmic solution subgroup was significant at P<0.05.
b: The difference between the gatifloxacin ophthalmic gel subgroup and the levofloxacin ophthalmic solution subgroup was significant at P<0.05.
Drug concentrations in the human aqueous humor at different time points after the final administration. *0.3% gatifloxacin ophthalmic gel and 0.5% levofloxacin ophthalmic solution both compared to 0.3% gatifloxacin ophthalmic solution, P<0.05, the difference was statistically significant. ** 0.3% gatifloxacin ophthalmic gel compared to 0.5% levofloxacin ophthalmic solution, P<0.05, the difference was statistically significant.
Discussion
Endophthalmitis causes severe damage to the eyesight and is one of the most serious complications that can occur following cataract surgery. The most common pathogens are gram-positive Staphylococcus epidermidis (approximately 30-80%) and Staphylococcus aureus (approximately 10-20%). The other causative pathogens include Streptococci (i.e., ß-haemolytic streptococci, S pneumoniae, a-haemolytic streptococci, including S mitis and S salivarius, which comprise approximately 10-35%), Enterococci (<5%), Gram-negative bacteria (rarely including Pseudomonas aeruginosa, approximately 5-20%), fungi (Candida sp, Aspergillus sp, Fusarium sp, up to 8%), and polymicrobial cultures (<5%) [26, 27].
Currently, topical antibiotics are applied perioperatively in most countries [1, 11, 12, 28, 29]. Fluoroquinolones possess the advantages of broad antibiotic spectra, high efficiency, low toxicity and high corneal penetration, and they exert antimicrobial effects by affecting the activities of DNA gyrase and topoisomerase IV [30, 31]. The third-generation fluoroquinolone levofloxacin has a broad spectrum, is the levorotatory isomer of ofloxacin and has better antibiotic properties against gram-positive and gram-negative bacteria; its antibacterial activity is 2-fold greater than that of ofloxacin [32, 33]. The fourth-generation fluoroquinolone gatifloxacin retains the superior antimicrobial activity of levofloxacin against Gram-negative bacteria and exhibits an enhanced antimicrobial activity against Gram-positive bacteria particularly streptococcus. The bactericidal action of gatifloxacin against atypical pathogens, such as mycobacterium tuberculosis, legionella, mycoplasma and chlamydia pneumoniae, and anaerobes, such as Bacteriodes fragilis, Fusobacterium, Peptostreptococcus and Clostridium, are also improved [34]. A study confirmed that, compared to the third generation of quinolones, the fourth can reduce the incidence of bacterial endophthalmitis following cataract surgery from 0.197% to 0.056% [10]. Moreover, gatifloxacin exhibits reduced less anaphylaxis [18] and resistance [16, 17] and is thus widely used in clinics. However, the drug concentration of clinically applied gatifloxacin ophthalmic solution is 0.3%, while that of levofloxacin ophthalmic solution is 0.5%. A previous study reported concentrations of 0.5% levofloxacin ophthalmic solution in the human aqueous humor following topical administration that were higher than those of 0.3% gatifloxacin ophthalmic solution at all time points [19]. Therefore, increasing the bioavailability of gatifloxacin by increasing the drug concentration has become the current focus of the attention of researchers in this field.
In our study, the operations were based on the reported experimental methods of Koch [35]. Each group was administered one drop of drug every 15 minutes for a total of 4 doses, the aqueous humor was extracted at different time points after last dose up to 180 min, and the concentrations of drugs in the aqueous humor were dynamically observed. Our results revealed that the concentration of the 0.3% gatifloxacin ophthalmic gel was significantly higher in the human aqueous humor than that of the 0.3% gatifloxacin ophthalmic solution at all time points; these findings are similar to those of Liu X [22]. The concentration of the 0.3% gatifloxacin ophthalmic gel subgroup took longer to reach its peak value than did that of the 0.3% gatifloxacin ophthalmic solution subgroup. This result demonstrates that ophthalmic gel agents can effectively increase drug concentrations in the aqueous humor and improve bioavailability.
Ophthalmic solutions are easily diluted by tears and quickly eliminated through the lacrimal duct; thus, frequent administration is required to maintain their bioavailability. To prolong the action time, enhance the efficacy, reduce the frequency of administration and decrease drug side effects, abundant research in to sustained-release ophthalmic agents has been performed around the world [36, 37]. Currently, 0.3% gatifloxacin ophthalmic gel is one of the sustained-release agents that are used in China. This gel contains macromolecular hydrophilic polymers (carbomer, hydroxypropyl methyl cellulose, and sodium hyaluronate) as the drug carrier to prolong drug residence time on the ocular surface and reduce drug wastage. Resultantly, the sustained drug release effect is superior to that of water-based agents and other viscous solutions and effectively increases the concentration and bioavailability of gatifloxacin in the aqueous humor. Moreover, the time to reach the peak concentration is prolonged, which aids in reducing the frequency of drug administration, which increases the acceptability of the treatment for patients [23, 38]. Additionally, gatifloxacin cannot achieve perfect corneal penetration to due to its more acidic pH and its lower lipophilicity compared to human tears [19]. However, the inclusion of sodium hyaluronate in the gel can regulate the surface tension and the refractive index such that they are close to those of normal tears, which counteracts the shortfalls of gatifloxacin. Ophthalmic gels also overcome the shortcoming of 'blurred vision' and can be comfortably applied and thus are more acceptable for patients [19, 23, 38]
In contrast to previous results [19], our results first suggested that the concentrations of 0.3% gatifloxacin ophthalmic gel in the human aqueous humor were higher than those of 0.5% levofloxacin ophthalmic solution at 120 min and 180 min after administration (P<0.05), and these differences were 1.9- and 2.75-fold, respectively. Gatifloxacin ophthalmic gel exhibited an extended action time and better bioavailability compared to the levofloxacin ophthalmic solution.
Additionally, another important aspect of choosing the appropriate antibiotic is whether the concentration in the aqueous humor can reach the minimum inhibitory concentration (MIC) required to inhibit common pathogens. The MIC90 is the minimal concentration of an antibiotic that will inhibit 90% of the pathogens' activities [39]. A previous study reported the MIC90s of common bacterial pathogens responsible for infectious endophthalmitis [40] (Table 5). In our study, the concentrations of gatifloxacin and levofloxacin both exceeded the MIC90s of common bacterial pathogens, and the maximum values were several, or even dozens, of times of higher than the MIC90s. Consequently, the concentrations of both gatifloxacin and levofloxacin in the aqueous humor reached the level to inhibit common pathogenic bacteria.
In conclusion, this study demonstrated that gatifloxacin ophthalmic gel achieved the highest concentration, exhibited the longest action time and had the best bioavailability in human aqueous humor compared to 0.3% gatifloxacin and 0.5% levofloxacin ophthalmic solutions. We believe that our results will provide scientific data for the penetration of the three antimicrobial agents.
Comparisons of the MICs of common bacterial pathogens that cause infectious endophthalmitis [40]
| bacterial pathogens | drug | MIC90(mg/L) |
|---|---|---|
| Staphylococcus epidermidis | gatifloxacin | 0.25 |
| levofloxacin | 0.50 | |
| Staphylococcus aureus | gatifloxacin | 0.13 |
| levofloxacin | 0.25 | |
| Streptococcus pneumoniae | gatifloxacin | 0.50 |
| levofloxacin | 2.00 | |
| Streptococcus pyogenes | gatifloxacin | 0.50 |
| levofloxacin | 1.00 | |
| Bacillus cereus | gatifloxacin | 0.25 |
| levofloxacin | -- | |
| Enterococcus faecalis | gatifloxacin | 2.00 |
| levofloxacin | 2.00 | |
| Proteus mirabilis | gatifloxacin | 0.25 |
| levofloxacin | 0.25 | |
| Haemophilus influenzae | gatifloxacin | 0.016 |
| levofloxacin | 0.06 | |
| Escherichia coli | gatifloxacin | 0.008 |
| levofloxacin | 0.03 | |
| Klebsiella pneumoniae | levofloxacin | 0.13 |
| levofloxacin | 0.13 | |
| Neisseria gonorrhoeae | gatifloxacin | 0.016 |
| levofloxacin | 0.016 | |
| Bacteroides fragilis | gatifloxacin | 1.00 |
| levofloxacin | 2.00 | |
| Propionibacterium acnes | gatifloxacin | 0.50 |
| levofloxacin | 0.75 |
Acknowledgements
This study was support by Key Program of National Natural Science Foundation of China( No. 81130018 ), National 'Twelfth Five-Year' Plan for Science & Technology Support of China( No. 2012BAI08B01), Project of National Clinical Key Discipline of Chinese Ministry of Health, National Natural Science Foundation of China(Grant No. 81100640), Zhejiang Provincial Natural Science Foundation of China(LY14H120001) and Specialized Research Fund for the Doctoral Program of Higher Education (Grant No. 20110101120126), China.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Peter B, Luis C, Susanne G. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract Surgery: Data, Dilemmas and Conclusions. Co Dublin, Ireland: The European Society for Cataract & Refractive Surgeons. 2013
2. Du DT, Wagoner A, Barone SB, Zinderman CE, Kelman JA, Macurdy TE. et al. Incidence of endophthalmitis after corneal transplant or cataract surgery in a medicare population. Ophthalmology. 2014;121:290-8 doi:10.1016/j.ophtha.2013.07.016
3. Nentwich MM, Ta CN, Kreutzer TC, Li B, Schwarzbach F, Yactayo-Miranda YM. et al. Incidence of postoperative endophthalmitis from 1990 to 2009 using povidone-iodine but no intracameral antibiotics at a single academic institution. J Cataract Refract Surg. 2015;41:58-66 doi:10.1016/j.jcrs.2014.04.040
4. Wu PC, Li M, Chang SJ, Teng MC, Yow SG, Shin SJ. et al. Risk of endophthalmitis after cataract surgery using different protocols for povidone- iodine preoperative disinfection. J Ocul Pharmacol Ther. 2006;22:54-61 doi:10.1089/jop.2006.22.54
5. Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: an evidence-based update. Ophthalmology. 2002;109:13-24
6. Prophylaxis of postoperative endophthalmitis following cataract surgery. results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33:978-88 doi:10.1016/j.jcrs.2007.02.032
7. Barry P, Seal DV, Gettinby G, Lees F, Peterson M, Revie CW. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study. J Cataract Refract Surg. 2006;32:407-10 doi:10.1016/j.jcrs.2006.02.021
8. Friling E, Lundstrom M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Refract Surg. 2013;39:15-21 doi:10.1016/j.jcrs.2012.10.037
9. Kessel L, Flesner P, Andresen J, Erngaard D, Tendal B, Hjortdal J. Antibiotic prevention of postcataract endophthalmitis: a systematic review and meta-analysis. Acta ophthalmologica. 2015 doi:10.1111/aos.12684
10. Jensen MK, Fiscella RG, Moshirfar M, Mooney B. Third- and fourth-generation fluoroquinolones: retrospective comparison of endophthalmitis after cataract surgery performed over 10 years. J Cataract Refract Surg. 2008;34:1460-7 doi:10.1016/j.jcrs.2008.05.045
11. Chinese Ophthalmological Society. http://d.g.wanfangdata.com.cn/Periodical_zhyk201301020.aspx
12. Inoue Y, Usui M, Ohashi Y, Shiota H, Yamazaki T. Preoperative disinfection of the conjunctival sac with antibiotics and iodine compounds: a prospective randomized multicenter study. Japanese journal of ophthalmology. 2008;52:151-61 doi:10.1007/s10384-008-0517-y
13. Mather R, Karenchak LM, Romanowski EG, Kowalski RP. Fourth generation fluoroquinolones: new weapons in the arsenal of ophthalmic antibiotics. Am J Ophthalmol. 2002;133:463-6
14. Chai Y, Liu ML, Lv K, Feng LS, Li SJ, Sun LY. et al. Synthesis and in vitro antibacterial activity of a series of novel gatifloxacin derivatives. Eur J Med Chem. 2011;46:4267-73 doi:10.1016/j.ejmech.2011.06.032
15. Blondeau JM, Laskowski R, Bjarnason J, Stewart C. Comparative in vitro activity of gatifloxacin, grepafloxacin, levofloxacin, moxifloxacin and trovafloxacin against 4151 Gram-negative and Gram-positive organisms. Int J Antimicrob Agents. 2000;14:45-50
16. Gong L, Sun XH, Qiu XD, Zhang YQ, Qu J, Yuan ZL. et al. [Comparative research of the efficacy of the gatifloxacin and levofloxacin for bacterial conjunctivitis in human eyes]. Zhonghua Yan Ke Za Zhi. 2010;46:525-31
17. Sun ST, Chen ZJ, Xu J, Tian XL. [The concentrations of fluoroquinolones in prevention of Staphylococcus epidermidis from mutant in ocular surface]. Zhonghua Yan Ke Za Zhi. 2006;42:989-91
18. Johannes CB, Ziyadeh N, Seeger JD, Tucker E, Reiter C, Faich G. Incidence of allergic reactions associated with antibacterial use in a large, managed care organisation. Drug Saf. 2007;30:705-13
19. Huang XD, Yao K, Chen WJ, Zhang Z, Yuan JQ. [Human aqueous humor levels of levofloxacin 0.5%, gatifloxacin 0.3% and levofloxacin 0.3% ophthalmic solution after topical dosing]. Zhonghua Yan Ke Za Zhi. 2009;45:987-91
20. Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006;315:12-7 doi:10.1016/j.ijpharm.2006.01.029
21. Liu Z, Yang XG, Li X, Pan W, Li J. Study on the ocular pharmacokinetics of ion-activated in situ gelling ophthalmic delivery system for gatifloxacin by microdialysis. Drug Dev Ind Pharm. 2007;33:1327-31 doi:10.1080/03639040701397241
22. Liu X, Wang NL, Wang YL, Ma C, Ma L, Gao LX. et al. Determination of drug concentration in aqueous humor of cataract patients administered gatifloxacin ophthalmic gel. Chin Med J (Engl). 2010;123:2105-10
23. Liu JD, Tang H, Yang YC, Zhan XL. http://so.5ipatent.com/PatentDetails.aspx?patentID=b8f3e3d4-0ad5-4c5c-8245-28c2790d498c&patentClass=1
24. Zhou ZH. http://so.5ipatent.com/PatentDetails.aspx?patentID=04f23cb0-8837-45e2-a483-667528e8313f&patentClass=1
25. Ohrvik VE, Buttner BE, Rychlik M, Lundin E, Witthoft CM. Folate bioavailability from breads and a meal assessed with a human stable-isotope area under the curve and ileostomy model. Am J Clin Nutr. 2010;92:532-8 doi:10.3945/ajcn.2009.29031
26. Behndig A, Cochener B, Guell JL, Kodjikian L, Mencucci R, Nuijts RM. et al. Endophthalmitis prophylaxis in cataract surgery: overview of current practice patterns in 9 European countries. J Cataract Refract Surg. 2013;39:1421-31 doi:10.1016/j.jcrs.2013.06.014
27. Fisch A, Salvanet A, Prazuck T, Forestier F, Gerbaud L, Coscas G. et al. Epidemiology of infective endophthalmitis in France. The French Collaborative Study Group on Endophthalmitis. Lancet. 1991;338:1373-6
28. Han DC, Chee SP. Survey of practice preference pattern in antibiotic prophylaxis against endophthalmitis after cataract surgery in Singapore. International ophthalmology. 2012;32:127-34 doi:10.1007/s10792-012-9537-1
29. Gordon-Bennett P, Karas A, Flanagan D, Stephenson C, Hingorani M. A survey of measures used for the prevention of postoperative endophthalmitis after cataract surgery in the United Kingdom. Eye (Lond). 2008;22:620-7 doi:10.1038/sj.eye.6702675
30. Mustaev A, Malik M, Zhao X, Kurepina N, Luan G, Oppegard LM. et al. Fluoroquinolone-gyrase-DNA complexes: two modes of drug binding. J Biol Chem. 2014;289:12300-12 doi:10.1074/jbc.M113.529164
31. Ball P. The quinolones: history and overview. In: (ed.) Andriole VT. The Quinolones, 3rd ed. San Diego: Academic Press. 2000:2-24
32. Ernst ME, Ernst EJ, Klepser ME. Levofloxacin and trovafloxacin: the next generation of fluoroquinolones? Am J Health Syst Pharm. 1997;54:2569-84
33. Wimer SM, Schoonover L, Garrison MW. Levofloxacin: a therapeutic review. Clin Ther. 1998;20:1049-70
34. Fung-Tomc J, Minassian B, Kolek B, Washo T, Huczko E, Bonner D. In vitro antibacterial spectrum of a new broad-spectrum 8-methoxy fluoroquinolone, gatifloxacin. J Antimicrob Chemother. 2000;45:437-46
35. Koch HR, Kulus SC, Roessler M, Ropo A, Geldsetzer K. Corneal penetration of fluoroquinolones: aqueous humor concentrations after topical application of levofloxacin 0.5% and ofloxacin 0.3% eyedrops. J Cataract Refract Surg. 2005;31:1377-85 doi:10.1016/j.jcrs.2004.12.063
36. Agrawal AK, Das M, Jain S. In situ gel systems as 'smart' carriers for sustained ocular drug delivery. Expert Opin Drug Deliv. 2012;9:383-402 doi:10.1517/17425247.2012.665367
37. Khan N, Aqil M, Imam SS, Ali A. Development and evaluation of a novel in situ gel of sparfloxacin for sustained ocular drug delivery: in vitro and ex vivo characterization. Pharm Dev Technol. 2014 doi:10.3109/10837450.2014.910807
38. Liu JD, Yang YC, Tang H. http://worldwide.espacenet.com/publicationDetails/biblio?DB=worldwide.espacenet.com&II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20130214&CC=US&NR=2013040960A1&KC=A1
39. Darouiche R, Perkins B, Musher D, Hamill R, Tsai S. Levels of rifampin and ciprofloxacin in nasal secretions: correlation with MIC90 and eradication of nasopharyngeal carriage of bacteria. J Infect Dis. 1990;162:1124-7
40. Hariprasad SM, Mieler WF, Holz ER. Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch Ophthalmol. 2003;121:345-50
Author contact
![]() Corresponding author: Ke Yao, MD, Eye Center, Affiliated Second Hospital, College of Medicine, Zhejiang University, Hangzhou, 310009, China. E-mail: xlrenedu.cn.
Corresponding author: Ke Yao, MD, Eye Center, Affiliated Second Hospital, College of Medicine, Zhejiang University, Hangzhou, 310009, China. E-mail: xlrenedu.cn.

 Global reach, higher impact
Global reach, higher impact