Impact Factor
ISSN: 1449-1907
Int J Med Sci 2015; 12(4):306-311. doi:10.7150/ijms.11343 This issue Cite
Research Paper
Using Vascular Closure Devices Following Out-Of-Hospital Cardiac Arrest?
Department of Cardiology and Angiology, Marienhospital Herne, Ruhr - University Bochum, Germany
Received 2014-12-14; Accepted 2015-3-4; Published 2015-3-28
Abstract
Objectives and Background: Despite a generally broad use of vascular closure devices (VCDs), it remains unclear whether they can also be used in victims from out-of-hospital cardiac arrest (OHCA) treated with mild therapeutic hypothermia (MTH).
Methods: All victims from OHCA who received immediate coronary angiography after OHCA between January 1st 2008 and December 31st 2013 were included in this study. The operator decided to either use a VCD (Angio-Seal™) or manual compression for femoral artery puncture. The decision to induce MTH was based on the clinical circumstances.
Results: 76 patients were included in this study, 46 (60.5%) men and 30 (39.5%) women with a mean age of 64.2 ± 12.8 years. VCDs were used in 26 patients (34.2%), and 48 patients (63.2%) were treated with MTH. While there were significantly more overall vascular complications in the group of patients treated with MTH (12.5% versus 0.0%; p=0.05), vascular complications were similar between patients with VCD or manual compression, regardless of whether or not they were treated with MTH.
Conclusion: In our study, the overall rate of vascular complications related to coronary angiography was higher in patients treated with mild therapeutic hypothermia, but was not affected by the application of a vascular closure device. Therefore, our data suggest that the use of VCDs in victims from OHCA might be feasible and safe in patients treated with MTH as well, at least if the decision to use them is individually carefully determined.
Keywords: out-of-hospital cardiac arrest, resuscitation, mild therapeutic hypothermia, coronary angiography, vascular closure device
Introduction
The current Guidelines of the European Resuscitation Council emphasise early coronary angiography in all post-cardiac arrest patients who are suspected of having coronary artery disease [1]. Several studies confirmed that the combination of mild therapeutic hypothermia (MTH) and early coronary angiography - inclusive percutaneous coronary intervention (PCI), if necessary - is feasible and safe in patients following out-of-hospital cardiac arrest (OHCA) [2-6]. However, although the use of vascular closure devices (VCDs) has increased during the past decade, no study has focussed on the safety of VCDs prior to MTH in victims from OHCA.
Material and Methods
Patients
Altogether, 76 patients who received emergency coronary angiography within the first two hours after OHCA between January 1st 2008 and December 31st 2013 were included in this study. Mean age was 64.2 ± 12.8 years.
Patients' data were collected from the patients' health records and anonymously stored on a central database.
Coronary angiography
Catheterisation was performed according to standard techniques with the six French femoral approach. If a recent coronary-artery stenosis was found, coronary angioplasty was attempted, unless the artery was too small or the operator considered the procedure to be technically impossible. Standard resuscitative and stabilisation procedures were continued during the procedure if necessary.
All patients who received percutaneous coronary intervention received 500 mg acetylsalicylic acid and 5.000 IU unfractionated heparin intravenously during PCI, and 600 mg clopidogrel immediately after placement of a nasogastric tube.
Vascular closure device
At the end of the procedure, the operator decided to use either a vascular closure device or manual compression for femoral artery puncture. If a VCD was chosen, an Angio-Seal™ device (St. Jude Medical, Minnetonka, MN, USA) that consists of an intra-arterially deployed polymer anchor, a collagen sponge positioned on the outer artery wall, and a self-tightening suture was used. The whole system is bioabsorbed within 90 days. All patients were followed-up by an experienced emergency physician. Hb-relevant bleeding was defined as a haemoglobin drop of more than 3 g/dl within the first 24 hours following coronary angiography and exclusion of other obvious causes or clinical bleeding signs.
Mild therapeutic hypothermia
The decision to induce MTH was based on the clinical circumstances, taking into account aspects like initial rhythm, duration of resuscitation, and state of consciousness. If necessary, MTH was induced immediately after the emergency coronary procedure with the CoolGard 3000™ system since several studies confirmed the safety and effectiveness of intravascular cooling [7]. The target temperature was 33 °Celsius for 24 hours, the rate of temperature change was maximised to induce MTH and was 0.3 °Celsius/hour to rewarm the patients. The Cooling Catheter with the saline flowing within was placed via the femoral vein in the cardiac catheterisation laboratory at the end of the coronary procedure.
Statistical analysis
Statistical analysis was performed with the Statistical Package of Social Science (SPSS 22.0, IBM, Armonk, NY, USA). Continuous variables are expressed as the mean ± SD, and comparisons of categorical variables among groups were conducted using Chi-square tests or Student's t-test. Data collection and analysis was approved by the local ethical review committee.
Results
All patients
Of the 76 patients were included in our study, 46 (60.5%) were men and 30 (39.5%) were women, and a mean age of 64.2 ± 12.8 years. Altogether, 60 events (78.9%) were witnessed, lay resuscitation was attempted in 43 events (56.6%), and 44 patients (57.9%) presented with an initial shockable rhythm.
Of the 38 (50.0%) patients that presented with myocardial infarction, 27 (35.5%) presented with ST elevation myocardial infarction (STEMI) and 11 (14.5%) presented with non ST elevation myocardial infarction (NSTEMI).
Coronary angiography confirmed coronary artery disease in 63 (82.9%) patients: 13 (17.1%) patients had one vessel disease, 17 (22.4%) patients had two vessel disease, and 33 (43.4%) patients had three vessel disease.
Percutaneous coronary intervention was attempted in 53 (69.7%) patients, with the culprit lesion in the left anterior descendens (LAD) in 21 (27.6%) patients, in the Ramus circumflexus (RCX) in seven (9.2%) patients, in the right coronary artery (RCA) in 14 (18.4%) patients, and in a coronary artery bypass graft (CABG) in one (1.3%) patient, while 10 (13.2%) patients received multi-vessel intervention. If necessary, the duration of the coronary angiography and PCI was 50.0 ± 31.0 minutes. In 17 (22.4%) patients, Eptifibatide was used during intervention.
Angio-Seal™ devices were used in 26 (34.2%) patients, and six (7.9%) showed vascular complications, such as Hb-relevant bleeding (6.6%) or arterial occlusion (1.3%). Forty-one (53.9%) patients who received immediate coronary angiography after hospital admission survived until hospital discharge (Table 1).
Comparison of patients treated with MTH and patients not treated with MTH
48 victims from OHCA (63.2%) were treated with mild therapeutic hypothermia after immediate coronary angiography, 28 patients (36.8%) were not.
In comparison of both groups we observed significant more lay resuscitations in the group of patients not treated with MTH (45.8% vs 75.0%; p=0.01) and significant more overall vascular complications in the group of patients treated with MTH (12.5% vs 0.0%; p=0.05).
No significant differences could be observed with regard on patients` gender, age, witnessed arrests, initial shockable rhythm, myocardial infarction, prevalence of coronary artery disease, PCI attempt, duration of coronary angiography, use of eptifibatide during PCI, use of Angio-Seal™ and survival until hospital discharge (Table 1).
Patient characteristics from all patients (first column), patients treated with mild therapeutic hypothermia (MTH) following out-of-hospital cardiac arrest (OHCA; second column), and OHCA victims who were not treated with MTH (third column)
| All patients n = 76 | Patients treated with MTH n = 48 | Patients not treated with MTH n = 28 | p | |
|---|---|---|---|---|
| Male gender | 46 (60.5%) | 29 (60.4%) | 17 (60.7%) | 0.98 |
| Age (years) [range] | 64.2 ± 12.8 [34-88] | 62.3 ± 13.7 [34-88] | 67.5 ± 11.3 [47-88] | 0.09 |
| Witnessed cardiac arrest | 60 (78.9%) | 38 (79.2%) | 22 (78.6%) | 0.81 |
| Lay resuscitation | 43 (56.6%) | 22 (45.8%) | 21 (75.0 %) | 0.01 |
| Initial shockable rhythm | 44 (57.9%) | 30 (62.5%) | 14 (50.0 %) | 0.37 |
| Myocardial infarction | 38 (50.0%) | 25 (52.1%) | 13 (46.4%) | 0.92 |
| ST elevation myocardial infarction (STEMI) | 27 (35.5%) | 19 (39.6%) | 8 (28.6%) | |
| Non-ST elevation myocardial infarction (NSTEMI) | 11 (14.5%) | 6 (12.5%) | 5 (17.9%) | |
| Coronary artery disease | 63 (82.9%) | 40 (83.3%) | 23 (82.1%) | 0.89 |
| One vessel disease | 13 (17.1%) | 7 (14.6%) | 6 (21.4%) | |
| Two vessel disease | 17 (22.4%) | 12 (25.0%) | 5 (17.9%) | |
| Three vessel disease | 33 (43.4%) | 21 (43.8%) | 12 (42.9%) | |
| Percutaneous coronary intervention (PCI) | 53 (69.7%) | 35 (72.9%) | 18 (64.3%) | 0.43 |
| Left anterior descendens (LAD) | 21 (27.6%) | 16 (33.3%) | 5 (17.9%) | |
| Ramus circumflexus (RCX) | 7 (9.2%) | 4 (8.3%) | 3 (10.7%) | |
| Right coronary artery (RCA) | 14 (18.4%) | 8 (16.7%) | 6 (21.4%) | |
| Multi vessel disease (MV) | 10 (13.2%) 1 (1.3%) | 6 (12.5%) 1 (2.1%) | 4 (14.3%) 0 (0.0%) | |
| Coronary artery bypass graft (CABG) | ||||
| Use of Eptifibatide (Integrilin™) | 17 (22.4%) | 12 (25.0%) | 5 (17.9%) | 0.47 |
| Duration of coronary angiography (min) [range] | 50.0 ± 31.0 [7-186] | 48.3 ± 27.0 [8-132] | 51.0 ± 35.7 [7-186] | 0.71 |
| Vascular closure device (Angio-Seal™) | 26 (34.2%) | 16 (33.3%) | 10 (35.7%) | 0.83 |
| Vascular complication | 6 (7.9%) | 6 (12.5%) | 0 (0.0%) | 0.05 |
| Hb relevant bleeding | 5 (6.6%) | 5 (10.4%) | - | 0.08 |
| conservative therapy | 1 (1.3%) | 1 (2.1%) | - | |
| transfusion | 3 (3.9%) | 3 (6.3%) | - | |
| operation + transfusion | 1 (1.3%) | 1 (2.1%) | - | |
| Arterial occlusion | 1 (1.3%) | 1 (2.1%) | - | 0.44 |
| operation | 1 (1.3%) | 1 (2.1%) | - | |
| Survival until hospital discharge | 41 (53.9%) | 27 (56.3%) | 14 (50.0%) | 0.60 |
Comparison of patients treated with MTH who received Angio-Seal™ and those who did not
Patients who were treated with MTH and received Angio-Seal™ presented less often with myocardial infarction (31.3% versus 62.5%; p=0.03), received less frequent percutaneous coronary intervention (50.0% versus 84.4%; p=0.01), were treated with eptifibatide less frequently (6.25% versus 34.4%; p=0.03), and coronary angiography was shorter (34.4 ± 21.6 min versus 55.3 ± 27.0 min; p=0.01) than in patients who did not receive an Angio-Seal™ device.
No significant differences were observed with regard to the patients' gender, age, witnessed arrests, lay-resuscitation, initial shockable rhythm, prevalence of coronary artery disease, vascular complications, or survival until hospital discharge (Table 2).
Comparison of patients not treated with MTH who received Angio-Seal™ and those who did not
In the victims who suffered from OHCA who were not treated with MTH, no differences could be observed between those patients who received an Angio-Seal™ and those patients who did not (Table 2).
Discussion
Mild therapeutic hypothermia
In victims who suffered OHCA, the combination of MTH and early coronary angiography inclusive PCI, if necessary, has been described as feasible and safe [2-6]. Specifically, bleeding complications have been excluded as relevant clinical problems related to MTH in several previous studies [8-12]. However, bleeding rates varied enormously in the different studies. While Nielsen et al. [2] described an increased risk of bleeding in only 4% of all patients following OHCA if coronary angiography with (6.2%) or without PCI (2.8%) was performed, other studies reported much higher bleeding rates of more than 20% [6, 13, 14] and a tendency towards increased bleeding complications in the MTH-treated group, which we also observed in our data (p=0.08) (table 2) [6, 14]. The underlying mechanism for this observation is unknown. Coronary angiography per se affects coagulation [15], and therapeutic hypothermia may, depending on the depth and duration, induce coagulopathy [16]. Additionally, in an animal model, thrombelastometry at 34°C during hypothermia showed significant differences for clotting time and clot formation [17]. Nevertheless, to our knowledge, there is no study that could further differentiate the influence of each of these individual factors. In addition, patients routinely receive heparin before coronary angiography and platelet inhibitors in association with PCI in a standard dose regardless of their post-resuscitation status. However, although it could be shown that MTH does not augment abciximab-induced inhibition of platelet aggregation [18], there are no reports on the influence of hypothermia on clopidogrel, prasugrel, or ticagrelor concentrations, and a potentially resulting risk of under- or overtreatment with these drugs in OHCA victims treated with MTH.
Comparison of out-of-hospital cardiac arrest (OHCA) victims who received an Angio-Seal™ after coronary angiography and those who did not receive an Angio-Seal™ with special attention to additional treatment with mild therapeutic hypothermia
| Patients treated with MTH | Patients not treated with MTH | |||||
|---|---|---|---|---|---|---|
| Angio-Seal™ n = 16 | No Angio-Seal™ n = 32 | p | Angio-Seal™ n = 10 | No Angio-Seal™ n = 18 | p | |
| Male gender | 8 (50.0%) | 21 (65.6%) | 0.30 | 5 (50.0%) | 12 (66.7%) | 0.39 |
| Age (years) [range] | 66.0 ± 14.6 [37-88] | 60.4 ± 13.0 [34-87] | 0.19 | 66.8 ± 15.4 [47-88] | 67.9 ± 8.7 [53-85] | 0.81 |
| Witnessed cardiac arrest | 13 (81.3%) | 25 (78.1%) | 0.13 | 7 (70.0%) | 15 (83.3%) | 0.14 |
| Lay resuscitation | 6 (37.5%) | 16 (50.0%) | 0.41 | 8 (80.0%) | 13 (72.2%) | 0.73 |
| Initial shockable rhythm | 8 (50.0%) | 22 (68.8%) | 0.21 | 6 (60.0%) | 8 (44.4%) | 0.52 |
| Myocardial infarction | 5 (31.3%) | 20 (62.5%) | 0.03 | 5 (50.0%) | 8 (44.4%) | 0.79 |
| ST elevation myocardial infarction | 5 (31.3%) | 14 (43.8%) | 3 (30.0%) | 5 (27.8%) | ||
| (STEMI) | 0 (0.0%) | 6 (18.8%) | 2 (20.0%) | 3 (16.7%) | ||
| Non-ST elevation myocardial infarction (NSTEMI) | ||||||
| Coronary artery disease | 11 (68.8%) | 29 (90.6%) | 0.06 | 8 (80.0%) | 15 (83.3%) | 0.83 |
| One vessel disease | 2 (12.5%) | 5 (15.6%) | 0 (0.0%) | 6 (33.3%) | ||
| Two vessel disease | 5 (31.3%) | 7 (21.9%) | 3 (30.0%) | 2 (11.1%) | ||
| Three vessel disease | 4 (25.0%) | 17 (53.1%) | 5 (50.0%) | 7 (38.9%) | ||
| Percutaneous coronary intervention (PCI) | 8 (50.0%) | 27 (84.4%) | 0.01 | 7 (70.0%) | 11 (61.1%) | 0.64 |
| Left anterior descendens (LAD) | 4 (25.0%) | 12 (37.5%) | 2 (20.0%) | 3 (16.7%) | ||
| Ramus circumflexus (RCX) | 0 (0.0%) | 4 (12.5%) | 1 (10.0%) | 2 (11.1%) | ||
| Right coronary artery (RCA) | 3 (18.8%) | 5 (15.6%) | 1 (10.0%) | 5 (27.8%) | ||
| Multi vessel disease (MV) | 1 (6.3%) | 5 (15.6%) | 3 (30.0%) | 1 (5.6%) | ||
| Coronary artery bypass graft (CABG) | 0 (0.0%) | 1 (3,1%) | 0 (0.0%) | 0 (0.0%) | ||
| Use of Eptifibatide (Integrilin™) | 1 (6.3%) | 11 (34.4%) | 0.03 | 1 (10.0%) | 4 (22.2%) | 0.42 |
| Duration of coronary angiography (min) [range] | 34.4 ± 21.6 [8-93] | 55.3 ± 27.0 [15-132] | 0.01 | 58.5 ± 54.4 [7-186] | 46.8 ± 20.2 [16-76] | 0.42 |
| Vascular complication | 3 (18.8%) | 3 (9.4%) | 0.36 | 0 (0.0%) | 0 (0.0%) | n.a. |
| Hb relevant bleeding | 3 (18.8%) | 2 (6.3%) | 0.18 | - | - | |
| conservative therapy | 1 (6.3%) | 0 (0.0%) | - | - | ||
| transfusion | 1 (6.3%) | 2 (6.3%) | - | - | ||
| operation | 1 (6.3%) | 0 (0.0%) | - | - | ||
| Arterial occlusion | 0 (0.0%) | 1 (3.1%) | 0.48 | - | - | |
| operation | - | 1 (3.1%) | - | - | ||
| Survival until hospital discharge | 8 (50.0%) | 19 (59.4%) | 0.54 | 6 (60.0%) | 8 (44.4%) | 0.43 |
n.a.: not available
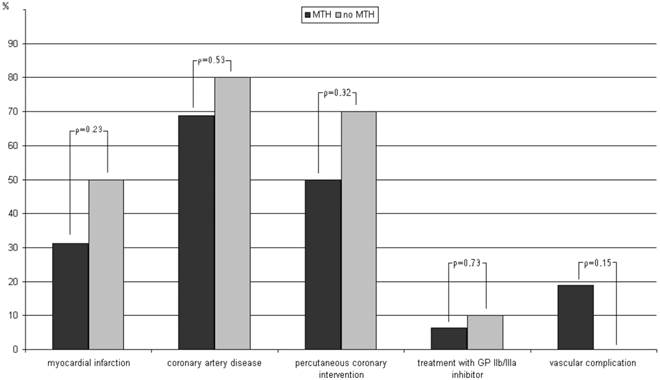
Differences within the group of patients treated with vascular closure devices depending on whether they received mild therapeutic hypothermia (MTH) or not.
We therefore recommend that previous studies should try to verify whether some of these factors combined may increase the risk of bleeding, requiring transfusion when an invasive procedure is performed in resuscitated patients treated with MTH.
Vascular closure devices
Several studies describe the safety of vascular closure devices after routine coronary angiogram and routine PCI [19, 20], as well as following coronary interventions using anticoagulation and GP IIb/IIIa inhibitor therapy [21]. Therefore, the use of VCDs has increased during the last decade, especially since the application of VCDs has been described as independently associated with a reduction in the rate of vascular complications and the post-PCI length of hospital stay [22]. Nevertheless, there are also data that reported an increase in the serious risk for retroperitoneal bleeding in patients treated with VCDs [23]. Additionally, in contrast to elective settings, the risk of access site-related vascular complications was significantly increased after application of the VCD Angio-Seal™ in patients undergoing emergency catheterisations for NSTEMI/STEMI when compared with manual compression [24]. Since victims from OHCA undergo emergency catheterisation, VCDs should be used carefully in this group. However, no previous study focused specifically on this patient collective.
In our study, VCDs were used in about one third (34.2%) of all OHCA victims (Table 1). Further subgroup analysis revealed that, at least in patients treated with MTH, the application of Angio-Seal™ devices was influenced by coronary findings, such as myocardial infarction, percutaneous coronary intervention, or treatment with GP IIb/IIIa inhibitor, with less frequent use of VCDs in more complex procedures (Table 2). However, the use of VCDs was not adapted for further treatment with MTH in general (Table 2, Figure 1). Regardless of whether the OHCA were treated with MTH after coronary angiography or not, we could not observe any difference in the rate of vascular complications or survival rates between patients treated with or without VCD (Table 2).
Limitations
The present study is a single center study and, therefore, our findings should be verified by further investigations.
Conclusions
In our study, the overall rate of vascular complications related to coronary angiography was higher in patients treated with MTH, but was not affected by the application of a VCD. Therefore, our data suggest that the use of VCDs in OHCA victims might be also feasible and safe in patients treated with MTH, at least if the decision to use them is carefully considered and adapted on an individual basis.
Conflict of interest
All authors declare no conflicts of interest.
References
1. Nolan JP, Soar J, Zideman DA. et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 1. Executive summary. Resuscitation. 2010;81:1219-76
2. Nielsen N, Hovdenes J, Nilsson F. et al. Outcome, timing and adverse events in therapeutic hypothermia after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2009;53:926-34
3. Sunde K, Pytte M, Jacobsen D. et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29-39
4. Knafelj R, Radsel P, Ploj T. et al. Primary percutaneous coronary intervention and mild induced hypothermia in comatose survivors of ventricular fibrillation with ST-elevation acute myocardial infarction. Resuscitation. 2007;74:227-34
5. Hovdenes J, Laake JH, Aaberge L. et al. Therapeutic hypothermia after out-of-hospital cardiac arrest: experiences with patients treated with percutaneous coronary intervention and cardiogenic shock. Acta Anaesthesiol Scand. 2007;51:137-42
6. Wolfrum S, Pierau C, Radke PW. et al. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med. 2008;36:1780-6
7. Hinz J, Rosmus M, Popov A. et al. Effectiveness of an intravascular cooling method compared with a conventional cooling technique in neurologic patients. J Neurosurg Anesthesiol. 2007;19:130-5
8. Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549-56
9. Belliard G, Catez E, Charron C. et al. Efficacy of therapeutic hypothermia after out-of-hospital cardiac arrest due to ventricular fibrillation. Resuscitation. 2007;75:252-9
10. Laish-Farkash A, Matetzky S, Kassem S. et al. Therapeutic hypothermia for comatose survivors after cardiac arrest. Isr Med Assoc J. 2007;9:252-6
11. Oddo M, Schaller MD, Feihl F. et al. From evidence to clinical practice: effective implementation of therapeutic hypothermia to improve patient outcome after cardiac arrest. Crit Care Med. 2006;34:1865-73
12. Sunde K, Pytte M, Jacobsen D. et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29-39
13. Kozinski M, Pstragowski K, Kubica JM. et al. ACS network-based implementation of therapeutic hypothermia for the treatment of comatose out-of-hospital cardiac arrest survivors improves clinical outcomes: the first European experience. Scand J Trauma Resusc Emerg Med. 2013;21:22
14. Zimmermann S, Flachskampf FA, Schneider R. et al. Mild therapeutic hypothermia after out-of-hospital cardiac arrest complicating ST-elevation myocardial infarction: long-term results in clinical practice. Clin Cardiol. 2013;36:414-21
15. Böttiger BW, Motsch J, Böhrer H. et al. Activation of blood coagulation after cardiac arrest is not balanced adequately by activation of endogenous fibrinolysis. Circulation. 1995;92:2572-8
16. Mayer S. Therapeutic hypothermia. New York, USA: Marcel Dekker. 2005
17. Mohr J, Ruchholtz S, Hildebrand F. et al. Induced hypothermia does not impair coagulation system in a swine multiple trauma model. J Trauma Acute Care Surg. 2013;74:1014-20
18. Frelinger AL III, Furman MI, Barnard MR. et al. Combined effects of mild hypothermia and glycoprotein IIb/IIIa antagonists on platelet-platelet and leukocyte-platelet aggregation. Am J Cardiol. 2003;92:1099-101
19. Lee SW, Tam CC, Wong KL. et al. Long-term clinical outcomes after deployment of femoral vascular closure devices in coronary angiography and percutaneous coronary intervention: an observational single-centre registry follow-up. BMJ Open. 2014;4:e005126
20. Gregory D, Midodzi W, Pearce N. Complications with Angio-Seal™ vascular closure devices compared with manual compression after diagnostic cardiac catheterization and percutaneous coronary intervention. J Interv Cardiol. 2013;26:630-8
21. Applegate RJ, Grabarczyk MA, Little WC. et al. Vascular closure devices in patients treated with anticoagulation and IIb/IIIa receptor inhibitors during percutaneous revascularization. J Am Coll Cardiol. 2002;40:78-83
22. Kerré S, Kustermans L, Vandendriessche T. et al. Cost-effectiveness of contemporary vascular closure devices for the prevention of vascular complications after percutaneous coronary interventions in an all-comers PCI population. EuroIntervention. 2014;10:191-7
23. Gurm HS, Hosman C, Share D. et al. Comparative safety of vascular closure devices and manual closure among patients having percutaneous coronary intervention. Ann Intern Med. 2013;159:660-6
24. Stegemann E, Hoffmann R, Marso S. et al. The frequency of vascular complications associated with the use of vascular closure devices varies by indication for cardiac catheterization. Clin Res Cardiol. 2011;100:789-95
Author contact
![]() Corresponding author: Dr. med. Martin Christ, Department of Cardiology and Angiology, Marienhospital Herne, Ruhr - University Bochum, Hoelkeskampring 40, 44625 Herne, Germany. Tel: (0049)-2323-499-5620; Fax: (0049)-2323-499-301; Email: martin.christde
Corresponding author: Dr. med. Martin Christ, Department of Cardiology and Angiology, Marienhospital Herne, Ruhr - University Bochum, Hoelkeskampring 40, 44625 Herne, Germany. Tel: (0049)-2323-499-5620; Fax: (0049)-2323-499-301; Email: martin.christde

 Global reach, higher impact
Global reach, higher impact