Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(11):1116-1128. doi:10.7150/ijms.9611 This issue Cite
Research Paper
Cultured Human Periosteal-Derived Cells Have Inducible Adipogenic Activity and Can Also Differentiate Into Osteoblasts in a Perioxisome Proliferator-Activated Receptor-Mediated Fashion
1. Clinical Research Institute of Gyeongsang National University Hospital, Jinju, Republic of Korea;
2. Department of Oral and Maxillofacial Surgery, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Republic of Korea;
3. Department of Orthopaedic Surgery, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Republic of Korea;
4. Department of Thoracic and Cardiovascular Surgery, Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Republic of Korea;
5. Department of Oral and Maxillofacial Surgery, College of Medicine, Ulsan University, Ulsan, Republic of Korea;
6. OBS/Theriogenology and Biotechnology, College of Veterinary Medicine, Gyeongsang National University, Jinju, Republic of Korea;
7. College of Pharmacy and Research Institute of Pharmaceutical Sciences, Gyeongsang National University, Jinju, Republic of Korea.
* These authors contributed equally to this work.
Received 2014-5-9; Accepted 2014-8-6; Published 2014-8-16
Abstract
We investigated the adipogenic activity of cultured human periosteal-derived cells and studied perioxisome proliferator-activated receptor (PPAR) ligand-mediated differentiation of cultured human periosteal-derived cells into osteoblasts. Periosteal-derived cells expressed adipogenic markers, including CCAAT/enhancer binding protein α (C/EBP- α), C/EBP-δ, aP2, leptin, LPL, and PPARγ. Lipid vesicles were formed in the cytoplasm of periosteal-derived cells. Thus, periosteal-derived cells have potential adipogenic activity. The PPARα and PPARγ agonists, WY14643 and pioglitazone, respectively, did not modulate alkaline phosphatase (ALP) activity in periosteal-derived cells during induced osteoblastic differentiation, however, the PPARα and PPARγ antagonists, GW6471 and T0070907, respectively, both decreased ALP activity in these cells. WY14643 did not affect, whereas pioglitazone enhanced, alizarin red-positive mineralization and calcium content in the periosteal-derived cells. GW6471 and T0070907 both decreased mineralization and calcium content. By RT-PCR, pioglitazone significantly increased ALP expression in periosteal-derived cells between culture day 3 and 2 weeks. Pioglitazone increased Runx2 expression after 3 days, which declined thereafter, but did not alter osteocalcin expression. Both of GW6471 and T0070907 decreased ALP mRNA expression. These results suggest that pioglitazone enhances osteoblastic differentiation of periosteal-derived cells by increasing Runx2 and ALP mRNA expression, and increasing mineralization. GW6471 and T0070907 inhibit osteoblastic differentiation of the periosteal-derived cells by decreasing ALP expression and mineralization in the periosteal-derived cells.
In conclusion, although further study will be needed to clarify the mechanisms of PPAR-regulated osteogenesis, our results suggest that PPARγ agonist stimulates osteoblastic differentiation of cultured human periosteal-derived cells and PPARα and PPARγ antagonists inhibit osteoblastic differentiation in these cells.
Keywords: Periosteal-derived cells, Adipogenic activity, Osteoblastic differentiation, PPAR ligands.
Introduction
Tissue engineering procedures for restoring bony defects offer significant advantages over autologous bone grafting, because bone grafting procedure could entail a complicated surgical procedure as well as potential morbidity at the donor site. Different cells have been used in tissue-engineered bone formation, including bone marrow-derived mesenchymal stem cells, periosteal-derived cells, and adipose-derived cells. Previously, we reported that the periosteal-derived cells differentiate into active osteoblastic cells that are involved in matrix mineralization [1-3]. Similar to bone marrow-derived mesenchymal stem cells, periosteal-derived cells contain multipotent cells with the potential to differentiate into osteoblasts and chondrocytes. The main advantage of using the periosteum for bone tissue engineering in a clinical setting is the relative ease of tissue harvesting through an intraoral procedure such as the surgical extraction of impacted third molar. Although several studies have reported osteogenic potential of periosteal-derived cells, adipogenic differentiation of cultured human periosteal-derived cells has not yet been fully investigated.
Osteoblasts, adipocytes, and various other mesenchymal lineage cells originate from multipotent mesenchymal stem cells, which display differentiation plasticity. Molecular regulators govern cell fate determination and switching, and in normal bone continuous osteoblastogenesis is maintained while adipogenesis appears to be suppressed. A precursor cell type differentiating along a specific cell lineage can be switched by genetic reprogramming into another cell type of a different lineage. Such fate decisions are regulated in part by transcription factors [4-7].
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the superfamily of nuclear hormone receptors and play important roles in cellular differentiation, development, and metabolism. Three PPARs are currently known, PPARα, PPARβ/δ, and PPARγ. All PPARs share the same molecular mode of action by forming heterodimers with the nuclear retinoid X receptor, followed by binding to peroxisome proliferator response elements on target genes. Expression of PPARα and PPARβ/δ is found ubiquitously, whereas PPARγ is mainly expressed in adipose tissue, macrophages, and colon. PPARγ is very specific to adipogenic differentiation and is induced before transcriptional activation of most adipocyte genes. PPARγ is activated by natural ligands such as polyunsaturated fatty acids and prostaglandin metabolites, and by synthetic ligands such as the anti-diabetic thiazolidinedione group, whose members include rosiglitazone, pioglitazone, and troglitazone. Ectopic PPARγ expression and activation induce adipocyte differentiation in many cell types that are not normally destined for this lineage [8-10]. Several studies have suggested possible adverse effects of PPARγ on bone metabolism. Akune et al. [11] demonstrated that heterozygous PPARγ-deficient (PPARγ +/-) mice exhibit increased bone mass by stimulating osteoblastogenesis from bone marrow progenitors. Endogenous and synthetic PPARγ agonists promote adipogenesis and inhibit osteoblastogenesis in primary bone marrow mesenchymal stem cell cultures. Treatment of mice with synthetic PPARγ agonists decrease bone mineral density by suppressing the dominant osteoblastogenic transcriptional factors: Runx2, osterix, Dlx5, and α1(I)collagen [8,12-14]. However, the effects of PPAR ligands on cultured osteoprogenitor cells remain controversial [9,15]. This study examined the adipogenic activity of cultured human periosteal-derived cells. In addition, the effects of PPAR ligands on osteoblastic differentiation of cultured human periosteal-derived cells were also examined.
Materials and Methods
Culture and Differentiation of Periosteal-Derived Cells
Patients provided informed consent for collection of periosteal tissues, as required by the Ethics Committee of Gyeongsang National University Hospital. Periosteal explants (5×20 mm) were harvested from mandibles during surgical extraction of impacted lower third molars. Periosteal pieces were cultured in 100 mm culture dishes in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU/mL penicillin, and 100 μg/mL streptomycin, at 37°C in 95% humidified air and 5% CO2. Upon reaching 90% confluence, adherent cells passaged by gentle trypsinization and reseeding in fresh medium.
Adipogenic differentiation was induced in confluent periosteal-derived cells by incubation in high-glucose DMEM containing l-glutamine, 10% FBS, 1 IU/mL penicillin, 100 μg/mL streptomycin, 1 μM dexamethasone, 100 μM indomethacin, 500 μM 3-isobutyl-1-methylxanthine, and 1 μg/ml insulin at a density of 3 × 105 cells/well in a six-well plate for 3 weeks. Media were changed every 3 days during adipogenic differentiation.
Osteoblastic differentiation was induced by culture of passage three periosteal cells in osteogenic induction medium composed of DMEM supplemented with 10% FBS, 50 μg/ml L-ascorbic acid 2-phosphate, 10 nM dexamethasone, and 10 mM β-glycerophosphate at a density of 3 × 104 cells/well in 24-well plates. Cells were differentiated for 21 days, with media changed every 3 days.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analyses
Total RNA was extracted from differentiating periosteal cells (cultured for various times in either adipogenic or osteoblastic induction media, as indicated) using Trizol® reagent (Invitrogen/Life Technologies Corp., CA, USA). Five μg of total RNA was reverse transcribed by incubation with 5 pM oligo(dT), 1 mM of each dNTP, and 200 U of Superscript™ III reverse transcriptase (Invitrogen, CA, USA) in a total volume of 20 μl at 50°C for 60 min followed by further incubated at 70°C for 15 min and rapid cooling on ice. Targeted genes were amplified by PCR with the primer sets listed in (Table 1), and products were electrophoresed on a 1.5% agarose gels containing ethidium bromide.
Immunoblotting of aP2
For detecting aP2 protein, periosteal cells that had been cultured in adipogenic media were lysed in NP-40 lysis buffer (20 mM Tris, pH 7.5, 140 mM NaCl, 1 mM EDTA, 1% (v/v) Nonidet P-40, 5 μM AEBSF, 1.5 nM aprotinin, 10 nM E-64, and 10 nM leupeptin) for 30 min, sonicated and centrifuged, and supernatants were treated in 20% trichloroacetic acid for 20 min at 4°C, followed by centrifugation.at 31,000 × g for 20 min. Pellets were washed with -20°C acetone, centrifuged at 31,000 × g for 30 min, air dried, and resuspended in NP-40 lysis buffer. Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membrane, which were probed with anti-aP2 antibody (Cat No.: sc-271529, Santa Cruz Biotechnology, CA, USA). Detection of aP2 protein expression was performed after 7, 14, and 21 days of culture in adipogenic induction medium.
Cytoplasmic Lipid Vesicles in Periosteal-Derived Cells
Oil Red O staining was used to identify cytoplasmic lipids in periosteal cells after 14 days of culture. Formaldehyde-fixed cells were stained for 1 h at room temperature with 0.3% Oil Red O (w/v) in 60% isopropanol, washed with distilled water, and visualized under light microscopy.
Treatment of Periosteal-Derived Osteoblastic Cells with PPARα, PPARγ Agonists and Antagonists
Periosteal cells that had been cultured in osteogenic induction medium were treated with 0.1 to 25 µM PPARα agonist WY14643, 0.1 to 10 µM PPARγ agonist pioglitazone, 0.1 to 10 µM PPARα antagonist GW6471, or 0.1 to 10 µM PPARγ antagonist T0070907 (all from R&D Systems, Minneapolis, USA). Media were changed every 3 days and the ligands were also added at each change of the medium.
Histochemical Staining of Alkaline Phosphatase (ALP), Alizarin Red S Staining and Calcium Quantification in Periosteal-Derived Osteoblasts
ALP expression and mineralized nodule formation are the key factors to determine osteoblast differentiation. ALP and Runt-related transcription factor 2 (Runx2) are early markers for osteoblast differentiation, whereas, osteocalcin (OC) secretion and matrix mineralization are associated with the endpoint of full maturation of the osteoblast phenotype [1-3].
Primers for RT-PCR.
| Gene | Sequence (5′→3′) | Size (bp) | Cycle | Annealing temp. |
|---|---|---|---|---|
| C/EBP-α | (S) cggtggacaagaacagcaac | 365 | 30 | 58 °C |
| (AS) cggaatctcctagtcctggc | ||||
| C/EBP-β | (S) cacagcgacgactgcaagatcc | 188 | 30 | 58 °C |
| (AS) cttgaacaagttccgcagggtg | ||||
| C/EBP-δ | (S) agcgcaacaacatcgccgtg | 267 | 30 | 58 °C |
| (AS) gtcgggtctgaggtatgggtc | ||||
| PPAR-α | (S) ccagtatttaggacgctgtcc | 492 | 30 | 58 °C |
| (AS) aagttcttcaagtaggccagc | ||||
| PPAR-β/δ | (S) aactgcagatgggctgtaac | 484 | 30 | 58 °C |
| (AS) gtctcgatgtcgtggatcac | ||||
| PPARγ | (S) tgtctcataatgccatcaggtttg | 250 | 30 | 58 °C |
| (AS) gataacgaatggtgatttgtctgtt | ||||
| aP2 | (S) accaggaaagtggctggcat. | 331 | 30 | 58 °C |
| (AS) caggtcaacgtcccttggct | ||||
| Leptin | (S) tgccttccagaaacgtgatcc | 164 | 35 | 58 °C |
| (AS) ctctgtggagtagcctgaagc | ||||
| LPL | (S) gagatttctctgtatggcacc | 276 | 30 | 58 °C |
| (AS) ctgcaaatgagacactttctc | ||||
| GAPDH | (S) aatgcatcctgcaccaccaa | 515 | 30 | 58 °C |
| (AS) gtagccatattcattgtcat |
For ALP histochemical staining, formaldehyde/ethanol-fixed periosteal-derived osteoblastic cells were stained with fast 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (BCIP/NBT) ALP substrate (Amresco LLC, OH, USA), and viewed by light microscopy. Staining for ALP was performed after 3, 7, and 14 days of culture in osteoblast induction medium.
The alizarin red S technique was used to assess mineralized nodule formation and quantify calcium contents of deposits. Formaldehyde-fixed cells were treated with a 2% alizarin red S solution for 5 minutes and washed with distilled water to remove residual stain. Alizarin red S-stained cultures were incubated with 100 mM cetylpyridinium chloride (Sigma-Aldrich, MO, USA) for 2 hours to release calcium-bound alizarin, which was measured at 570 nm with a microplate reader. Alizarin red S staining was evaluated at day 21 of culture.
Periosteal-derived osteoblastic cells were decalcified with 0.6 N HCl for 24 h, and the calcium content of supernatants was determined by spectrophotometry using the o-cresolphthalein method (Calcium C-test Wako, Wako Pure Chemical Industries, Osaka, Japan). After decalcification, the total protein content in the supernatants was measured using a BCA protein assay kit (Pierce Chemical Co, IL, USA). Cellular calcium content was normalized to total protein content. Calcium content was examined at day 21 of culture.
Quantitative RT-PCR Analysis
We performed quantitative RT-PCR for ALP, OC, and Runx2 with total RNA extracted from differentiating periosteal-derived osteoblastic cells at indicated times. First-strand cDNA was generated using random hexamer primers provided in the first-strand cDNA synthesis kit (Applied Biosystems Inc., MA, USA). All primers and probes (GAPDH Cat # Hs02758991-g1; OC Cat # Hs00609452-g1; Runx2 Cat # Hs00231692-m1; ALP #Hs01029144-m1) were obtained commercially (TaqMan® Gene Expression Assay, Applied Biosystems Inc.) and amplified using a kit and following manufacturer instructions (TaqMan® Gene Expression Master Mix, Applied Biosytems). Amplification conditions were: 50 °C, 2 min; 95 °C, 10 min; followed by 40 cycles of 94 °C, 15 s and 60 °C, 1 min in 96-well plates using the ViiA™ 7 Real-Time PCR System (Applied Biosystems Inc.). GAPDH was used as an internal control. All experiments were performed in triplicate.
Periosteal-derived osteoblastic cells were treated with 5 and 25 µM PPARα agonists, 5 and 10 µM PPARγ agonists, 5 and 10 µM PPARα antagonists, and 5 and 10 µM PPARγ antagonists, respectively.
Statistical Analysis
All experiments were performed using triplicate cultures, with the results expressed in each experiment as the mean±standard deviation (SD). Statistical analyses were conducted using the GraphPad Prism software (GraphPad Software, CA, USA). Data were evaluated by one-way ANOVA with Tukey's multiple comparison tests. Differences with a p-value <0.05 were considered statistically significant.
Results
Adipogenic Differentiation of Cultured Human Periosteal-derived Cells
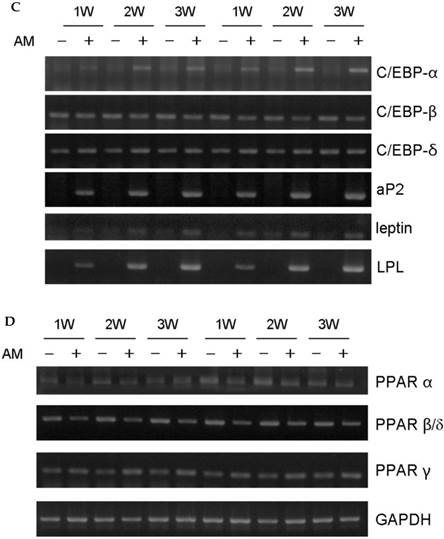
Periosteal-derived cells showed a fibroblastic morphology in the primary cell cultures. Culture in adipogenic differentiation media converted cells to a spherical morphology with accumulated lipid droplets. These periosteal-derived cells were positive for several adipogenic marker genes. By western blotting, aP2 protein expression was induced in a time dependent fashion between weeks 1 and 3. By RT-PCR, gene expression of CCAAT/enhancer binding protein α (C/EBP-α), C/EBP-δ, aP2, leptin, LPL, and PPARγ were increased in these cells (Fig. 1). These results suggest that cultured human periosteal-derived cells differentiated into active adipogenic cells that synthesize cytoplasmic lipid vesicles.
Expression of PPAR Subtypes during Osteoblastic Differentiation of Cultured Human Periosteal-derived Cells
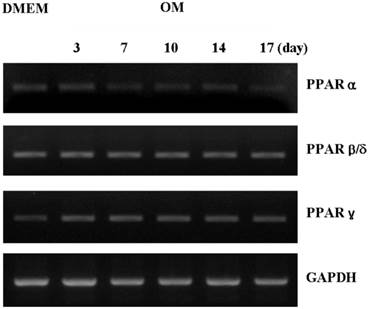
PPARα and PPARγ mRNAs were more highly expressed in osteogenic induction DMEM than in control DMEM at day 3. PPARα expression in the periosteal-derived osteoblastic cells first appeared at day 3, and decreased during the remaining culture period. PPARγ mRNA was clearly upregulated after 7 days culture in osteogenic induction media, after which its expression decreased in periosteal-derived osteoblastic cells. However, PPARβ/δ expression was constant in the periosteal-derived osteoblastic cells throughout the 17 day observation period, in both control DMEM and osteogenic DMEM cultures (Fig. 2).
Adipogenic differentiation of cultured human periosteal-derived cells. A: Oil Red O staining of periosteal-derived cells showed accumulation of lipids in cytoplasmic vesicles (arrows) after 2 weeks (2W) of culture in adipogenic differentiation medium. B: In immunoblotting analysis, aP2 expression first appeared at 1 week (1W) followed by increased expression during the subsequent 2 week culture period. C&D: By RT-PCR, gene expression of CCAAT/enhancer binding protein-α (C/EBP-α), C/EBP-δ, aP2, leptin, LPL, and PPARγ were clearly increased in these periosteal-derived cells. AM; adipogenic differentiation medium.

Expression of PPAR subtypes during osteoblastic differentiation of cultured human periosteal-derived cells. Expression of PPARα and PPARγ in periosteal-derived cells differed between culture in osteogenic induction DMEM (OM) versus control DMEM, whereas expression of PPARβ/δ was constant. OM; osteogenic induction DMEM medium.
ALP Activity in the Periosteal-derived Osteoblastic Cells treated with PPARα or PPARγ Ligands
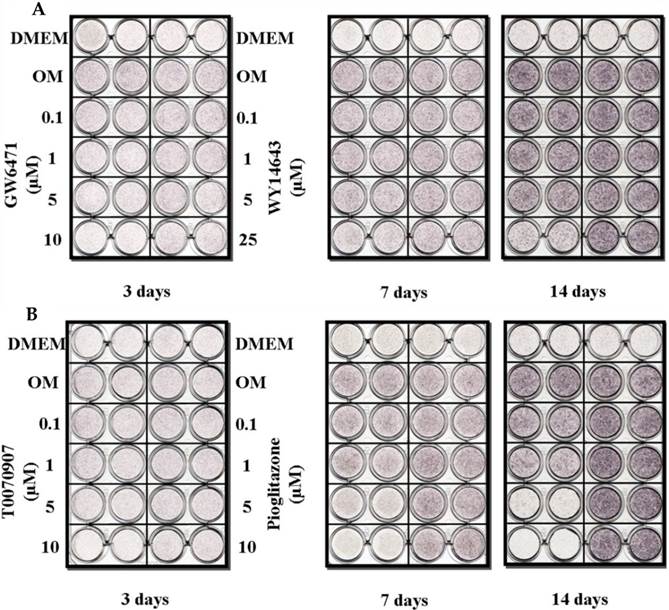
Treatment with either the PPARα agonist (WY14643) or the PPARγ agonist (pioglitazone) did not affect ALP histochemical activity in the periosteal-derived osteoblastic cells. However, both PPARα antagonists (GW6471) and PPARγ antagonist (T0070907) clearly decreased ALP activity in a concentration-dependent manner during 14 days in culture. PPARγ antagonist with T0070907 reduced ALP activity to a greater extent that did PPARα antagonist with GW6471 in periosteal-derived cells that were cultured in osteoblastic differentiation medium (Fig. 3).
Histochemical staining of ALP activity in periosteal-derived cells differentiated in osteoblastic induction media and treated with PPARα or PPARγ ligands. A: The PPARα agonist WY14643 did not affect ALP activity; however, the PPARα antagonist GW6471 decreased ALP activity in a concentration- and time-dependent manner during the 2 week culture period. B: Similar to the effects of PPARα ligands, treatment with the PPARγ agonist pioglitazone did not affect ALP activity in the periosteal-derived cells during osteoblastic differentiation, while, PPARγ antagonist with T0070907 definitely decreased ALP activity in a concentration-dependent manner. OM; osteogenic induction DMEM medium.
Effects of PPARα or PPARγ Ligands on Mineralization
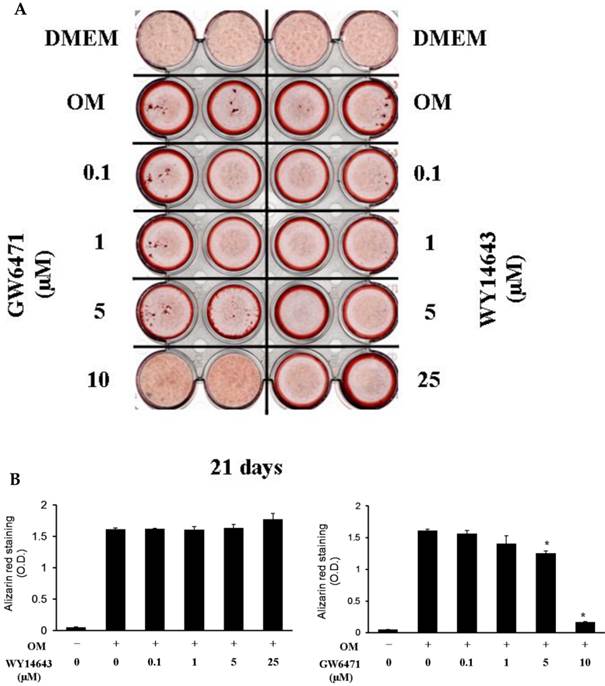
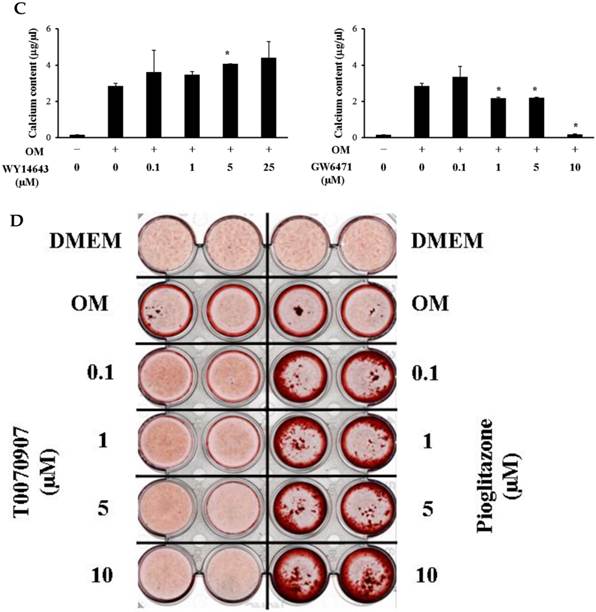
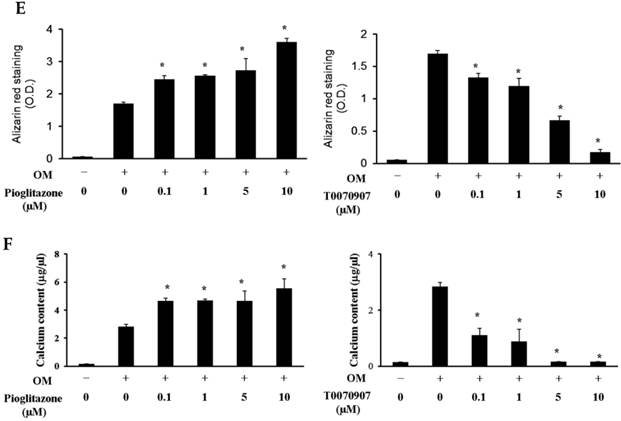
Although 5 µM WY14643 significantly increased calcium contents in periosteal-derived osteoblastic cells, the PPARα agonist WY14643 did not alter alizarin red-positive mineralization and calcium content in the periosteal-derived cells. Conversely, however, the PPARα antagonist GW6471 decreased both alizarin red-positive mineralization and calcium content in periosteal-derived osteoblastic cells. The PPARγ agonist pioglitazone clearly enhanced both mineralization and calcium content in a concentration-dependent manner in osteoblast-differentiating periosteal-derived cells, whereas the PPARγ antagonist T0070907 concentration-dependently decreased mineralization and calcium content in these cells (Fig. 4).
Quantitative RT-PCR Analysis
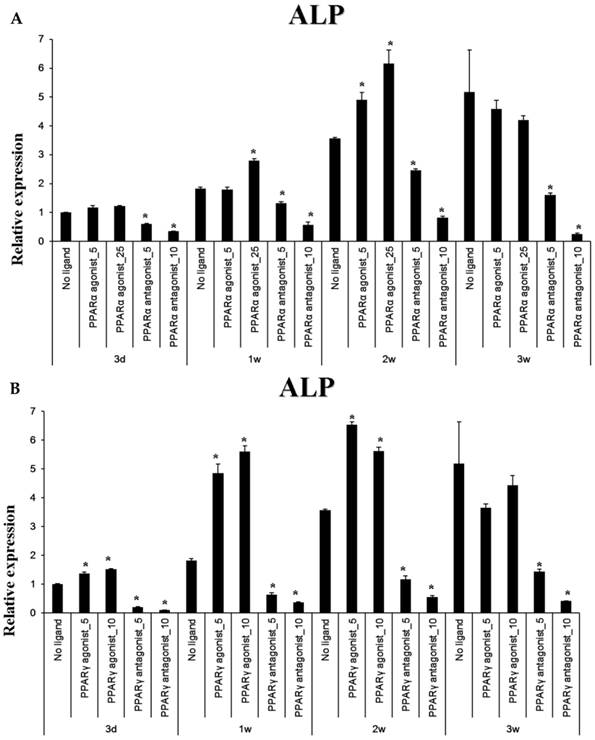
Baseline ALP mRNA expression was time-dependently increased (through 3 weeks) by culture in osteogenic-induction medium. Treatment with the PPARα agonist WY14643 significantly increased ALP expression above these control levels at 1 and 2 weeks of culture, but had no effect beyond osteogenic medium-induced ALP mRNA at 3 weeks. Treatment of these cells with the PPARα antagonist GW6471 caused a concentration- and time-dependent inhibition of ALP mRNA expression in these cells. Pioglitazone, the PPARγ-agonistic ligand, significantly increased osteogenic induction medium-induced ALP mRNA expression through 2 weeks of exposure, but had this effect disappeared at 3 weeks. Conversely, the PPARγ-antagonistic ligand T0070507 decreased osteogenic differentiation medium-induced ALP upregulation throughout the 3 week experimental course.
Effects of PPARα or PPARγ ligands on mineralization and calcium content of periosteal-derived osteoblastic cells. A, B&C: Mineralization of periosteal-derived osteoblastic cells was unaffected by PPARα agonist WY14643 treatment after 21 days of treatment. However, WY14643 tended to modestly increase calcium content, which reached statistical significance at 5 µM WY14643. The PPARα antagonist GW6471, however, significantly decreased alizarin red-positive mineralization and calcium content in periosteal-derived osteoblastic cells, particularly at high concentrations (i.e., ≥5 µM for mineralization and ≥1µM for calcium content). D, E&F: The PPARγ agonist pioglitazone clearly enhanced mineralization and calcium content in periosteal-derived osteoblastic cells. The PPARγ antagonist T0070907 decreased mineralization and calcium content in periosteal-derived cells cultured in osteogenic-differentiation medium, in a concentration dependent fashion. *p<0.05 versus values observed in cells cultured in osteogenic induction DMEM media (OM+) without PPAR ligand treatment.
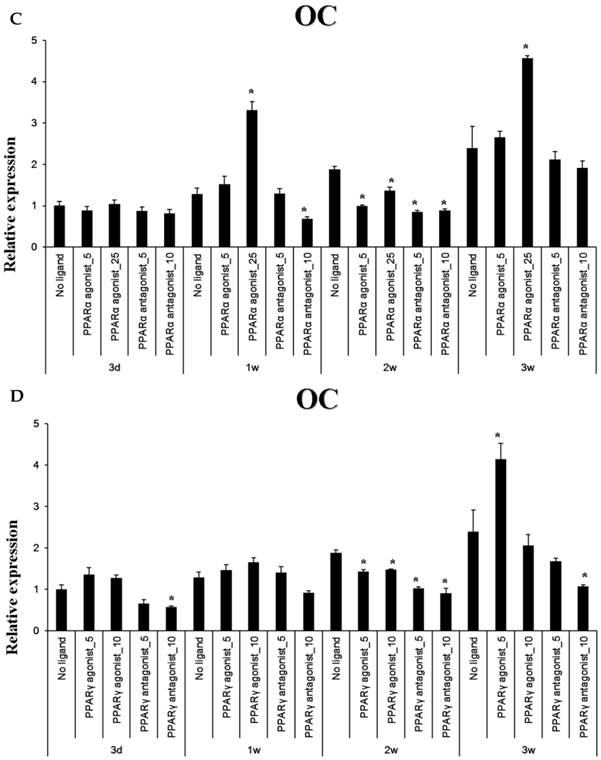
Baseline OC expression in cultured periosteal-derived osteoblastic cells increased modestly between through 3 weeks in culture. After 1 week in osteogenic differentiation medium, the PPARα agonist WY14643 significantly but transiently increased OC expression, with levels declining to or below control levels at weeks 2 and then increasing again above control levels at week 3. The PPARα antagonist GW6471 decreased OC mRNA levels at 1 and 2 weeks in culture, but OC mRNA levels returned to control levels after 3 weeks of stimulation. Pioglitazone, the PPARγ agonist, significantly increased OC expression in these cells after 3 weeks in culture, whereas the PPARγ antagonist T0070507 decreased OC mRNA expression relative to osteogenic induction media-only controls, beginning at 2 weeks of stimulation.
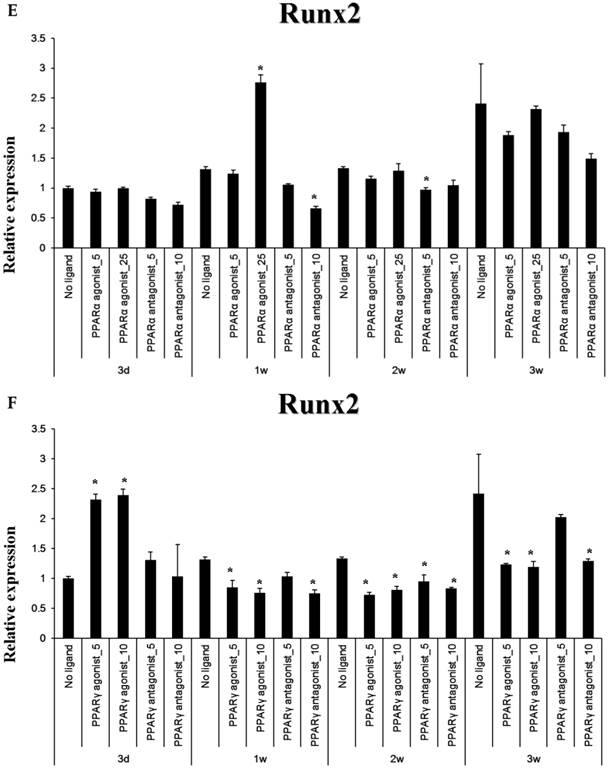
The PPARα agonist WY14643 transiently increased Runx2 mRNA expression at 1 week of culture, with mRNA levels no different from controls thereafter. The PPARα antagonist GW6471 caused a modest decrease in Runx2 mRNA expression after 1-2 weeks of stimulation, which was not apparent at 3 weeks. The PPARγ-agonistic ligand pioglitazone significantly increased Runx2 mRNA expression in osteoblast-induced periosteal-derived cells after 3 days in culture. However, pioglitazone markedly decreased Runx2 expression in these cells thereafter through at least 3 weeks of culture. The PPARγ antagonist T0070907 decreased Runx2 mRNA expression in these periosteal-derived osteoblastic cells, beginning at 1 week of treatment and persisting for at least 3 weeks (Fig. 5).
Quantitative RT-PCR analysis. A&B: ALP mRNA expression in the periosteal-derived osteoblastic cells treated with PPARα or PPARγ ligands. C&D: OC mRNA expression in the periosteal-derived osteoblastic cells treated with PPARα or PPARγ ligands. E&F: Runx2 mRNA expression in the periosteal-derived osteoblastic cells treated with PPARα or PPARγ ligands. PPARα agonist_5; 5 µM PPARα agonist, PPARα agonist_25; 25 µM PPARα agonist, PPARα antagonist_5; 5 µM PPARα antagonist, PPARα antagonist_10; 10 µM PPARα antagonist, PPARγ agonist_5; 5 µM PPARγ agonist, PPARγ agonist_10; 10 µM PPARγ agonist, PPARγ antagonist_5; 5 µM PPARγ antagonist, PPARγ antagonist_10; 10 µM PPARγ antagonist.
Discussion
Osteoblasts and adipocytes and other mesenchymal lineage cells originate from multipotent mesenchymal progenitor cells. Cultured human periosteal-derived cells can be differentiated into adipogenic lineage cells. In the present study, we examined the adipogenic differentiation of cultured human periosteal-derived cells. Adipocyte differentiation is characterized by the expression of particular genes that determine the specific adipocyte phenotype. Two transcriptional factors, C/EBP-α and PPARγ have been shown to transactivate adipocyte-specific genes that are involved in the growth arrest required for adipocyte differentiation. In addition, C/EBP-β, C/EBP-δ, aP2, leptin, and LPL are well-known adipogenic markers. During the terminal phase of differentiation, adipocytes in culture markedly increase de novo lipogenesis and cytoplasmic lipid vesicle formation is a late marker of adipogenesis [5,16]. In the present study, human periosteal-derived cells that were cultured in adipogenic induction medium became positive for these adipogenic markers and accumulated cytoplasmic lipid droplets. These results suggest that the cultured human periosteal-derived cells have good adipogenic activity and are a good candidate application requiring tissue-engineered fat.
Mesenchymal stem cells are a multipotent cell type that can give rise not only to osteoblasts, but also to a range of other cell types including adipocytes, chondrocytes, and myoblasts. The differentiation fate of mesenchymal stem cells is determined in large measure by a complex interplay of extracellular signaling molecules such as growth factors, hormones, and nutrients that affect the expression and activation of lineage-specific transcription factors. The key transcription factors Runx2 and PPARγ act as molecular switches to direct differentiation of precursor cells into osteoblasts or adipocytes, respectively. Recent studies have shown that PPARγ activity directly inhibits osteogenesis by diverting mesenchymal stem cells from the osteogenic phenotype to the adipogenic lineage. This shift in mesenchymal stem cell differentiation to favor the adipocyte lineage over the osteoblast lineage directly contributes to imbalances in bone formation and resorption, and ultimately leads to bone loss [8,10,17,18].
In this study, we have examined the effects of PPARα and PPARγ ligands on in vitro osteoblastic differentiation of cultured human periosteal-derived cells. The expression of PPARβ/δ was constant in the periosteal-derived cells cultured with or without osteogenic induction medium, so we did not examine effects of PPARβ/δ ligands on osteoblastic differentiation of these cells.
Expression of the PPARα is highest in tissues with active fatty acid catabolism, including liver, heart, small and large intestine, and skeletal muscle. The role of PPARα in these tissues is to regulate fatty acid catabolism. Although the role of PPARα ligands in bone metabolism remains poorly elucidated, several studies demonstrated that PPARα agonists suppress osteoclast differentiation by inhibiting nuclear factor kappa B (NF-κB) signaling [19-21]. In a study examining the effects of PPARα and PPARγ agonists on bone in intact female rats, Syversen et al [22] demonstrated that PPARα agonist caused significantly increased femoral bone mineral density and lower medullary volume. Stunes et al [23] also examined the positive effect of PPARα agonists on bone in a study using ovariectomized rats. Takano et al [10] suggested that that PPARα agonist, but not PPARγ agonist, upregulates the dominant osteoblastogenic transcriptional factors, Runx2, osteocalcin, and collagen type-I induced by bone morphogenic protein-4 in the mouse myoblastic cell line C2C12.
PPARγ is well established as a prime regulator that stimulates adipogenesis in multipotent mesenchymal stem cells. Treatment of primary bone marrow mesenchymal stem cells and mesenchymal stem cell lines with PPARγ agonists promotes adipogenesis. In relation to bone homeostasis, many studies reported that PPARγ agonist inhibits osteoblastogenesis in animals and humans. Natural and synthetic PPARγ agonists inhibit osteoblastogenesis in murine marrow-derived UAMS-33 cells. PPARγ haplo-insufficient mice showed increased trabecular bone volume associated with a loss of adipose tissue volume [8,14,24-27]. In human, administration of PPARγ agonist results in progressive bone loss and diminished levels of circulating bone formation markers in older women. Additionally, PPARγ agonist increases the rate of fracture in diabetic human subjects [28-30]. Therefore, PPARγ could serve as a useful target for drugs intended to enhance bone mass. However, the effects of PPAR ligands on the differentiation of cultured osteoprecursor cells are still controversial. Jackson et al [8] reported that PPARα and PPARγ activators induce the osteoblastic maturation of MC3T3-E1 mouse osteoprecursor cells. However, they observed that reduced ALP activity and calcium content occurred at higher PPARγ activator concentrations. In human bone marrow-derived mesenchymal stem cells, Yu et al [15] reported that PPARγ inhibitors reduced the extent of adipogenesis, but did not significantly affect osteogenesis. They observed that PPARγ inhibition did not significantly influence expression of the major osteogenic transcription factor Runx2.
In the present study, treatment with the PPARα agonist WY14643 largely did not affect the histochemical activity of ALP, mineralization, and calcium content in the periosteal-derived cells that were cultured in osteogenic induction medium. Although PPARγ agonist pioglitazone treatment did not stimulate the ALP activity in these cells, pioglitazone significantly increased Runx2 mRNA expression at day 3, and ALP mRNA expression at day 3 and 1 and 2 weeks of culture. Conversely, pioglitazone significantly decrease Runx2 mRNA expression in periosteal-derived osteoblastic cells between weeks 1 and 3. In addition, pioglitazone clearly enhanced mineralization and calcium content in the periosteal-derived osteoblastic cells. Especially, pioglitazone at the highest concentration (≥10 µM) employed in this study appreciably enhanced alizarin red-positive mineralization of periosteal-derived osteoblastic cells. Considering that ALP and Runx2 are early markers of osteoblast differentiation, whereas osteocalcin secretion and matrix mineralization are associated with the late phase of osteoblast differentiation, our results suggest that pioglitazone enhances osteoblastic differentiation of the cultured human periosteal-derived cells by increasing Runx2 and ALP expression at earlier times and increasing mineralization at later time points.
The PPARα antagonist GW6471 and the PPARγ antagonist T0070907 decreased the histochemical detection of ALP activity and ALP mRNA expression in the periosteal-derived osteoblastic cells. GW6471 and T0070907 tended to decrease alizarin red-positive mineralization and calcium content of periosteal-derived osteoblastic cells in a dose-dependent manner during the culture period. These PPAR antagonists also decreased OC expression in the periosteal-derived cells at 2 weeks of culture. These results suggest that both of GW6471 and T0070907 inhibit osteoblastic differentiation of the periosteal-derived cells by decreasing ALP activity and mineralization of the periosteal-derived cells.
To our knowledge, evidence regarding the effects of PPAR ligands on cultured human periosteal-derived cells has been limited. The primary findings of this study are that PPARγ agonist stimulates osteoblastic differentiation of cultured human periosteal-derived cells and PPARα and PPARγ antagonists inhibit osteoblastic differentiation in these cells. Further study is needed to clarify the mechanisms of PPAR-regulated osteogenesis.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A2038795). This study was also supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1596).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Park BW, Hah YS, Kim DR, Kim JR, Byun JH. Osteogenic phenotypes and mineralization of cultured human periosteal-derived cells. Arch Oral Biol. 2007;52(10):983-989
2. Park BW, Hah YS, Kim DR, Kim JR, Byun JH. Vascular endothelial growth factor expression in cultured periosteal-derived cells. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2008;105(5):554-560
3. Ryu YM, Hah YS, Park BW, Kim DR, Roh GS, Kim JR, Kim UK, Rho GJ, Maeng GH, Byun JH. Osteognic differentiation of human periosteal-derived cells in a three-dimensional collagen scaffold. Mol Biol Rep. 2011;38(5):2887-2894
4. Beresford JN, Bennett JH, Devlin C, Owen MEC. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102(2):341-351
5. Schilling T, Nöth U, Klein-Hitpass L, Jakob F, Schütze N. Plasticity in adipogenesis and osteogenesis of human mesenchymal stem cells. Mol Cell Endocrinol. 2007;271(1-2):1-17
6. Takada I, Suzawa M, Matsumoto K, Kato S. Suppression of PPAR transactivation switches cell fate of bone marrow stem cells from adipocytes into osteoblasts. Ann N Y Acad Sci. 2007;1116:182-195
7. Takahashi T. Overexpression of Runx2 and MKP-1 stimulates transdifferentiation of 3T3-L1 preadipocytes into bone-forming osteoblasts in vitro. Calcif Tissue Int. 2011;88(4):336-347
8. Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66(2):236-253
9. Jackson SM, Demer LL. Peroxisome proliferator-activated receptor activators modulate the osteoblastic maturation of MC3T3-E1 preosteoblasts. FEBS Lett. 2000;471(1):119-124
10. Takano M, Otsuka F, Matsumoto Y, Inagaki K, Takeda M, Nakamura E, Tsukamoto N, Miyoshi T, Sada KE, Makino H. Peroxisome proliferator-activated receptor activity is involved in the osteoblastic differentiation regulated by bone morphogenetic proteins and tumor necrosis factor-α. Mol Cell Endocrinol. 2012;348(1):224-232
11. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846-855
12. Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143(6):2376-2384
13. Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401-406
14. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146(3):1226-1235
15. Yu WH, Li FG, Chen XY, Li JT, Wu YH, Huang LH, Wang Z, Li P, Wang T, Lahn BT, Xiang AP. PPARγ suppression inhibits adipogenesis but does not promote osteogenesis of human mesenchymal stem cells. Int J Biochem Cell Biol. 2012;44(2):377-384
16. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78(3):783-809
17. Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19(2):129-137
18. Liu LF, Shen WJ, Zhang ZH, Wang LJ, Kraemer FB. Adipocytes decrease Runx2 expression in osteoblastic cells: roles of PPARγ and adiponectin. J Cell Physiol. 2010;225(3):837-845
19. Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274(45):32048-32054
20. Okamoto H, Iwamoto T, Kotake S, Momohara S, Yamanaka H, Kamatani N. Inhibition of NF-kappaB signaling by fenofibrate, a peroxisome proliferator-activated receptor-alpha ligand, presents a therapeutic strategy for rheumatoid arthritis. Clin Exp Rheumatol. 2005;23(3):323-330
21. Chan BY, Gartland A, Wilson PJ, Buckley KA, Dillon JP, Fraser WD, Gallagher JA. PPAR agonists modulate human osteoclast formation and activity in vitro. Bone. 2007;40(1):149-159
22. Syversen U, Stunes AK, Gustafsson BI, Obrant KJ, Nordsletten L, Berge R, Thommesen L, Reseland JE. Different skeletal effects of the peroxisome proliferator activated receptor (PPAR)alpha agonist fenofibrate and the PPARgamma agonist pioglitazone. BMC Endocr Disord. 2009;9:10
23. Stunes AK, Westbroek I, Gustafsson BI, Fossmark R, Waarsing JH, Eriksen EF, Petzold C, Reseland JE, Syversen U. The peroxisome proliferator-activated receptor (PPAR) alpha agonist fenofibrate maintains bone mass, while the PPAR gamma agonist pioglitazone exaggerates bone loss, in ovariectomized rats. BMC Endocr Disord. 2011;11:11
24. Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50(5):1087-1094
25. Kawaguchi H, Akune T, Yamaguchi M, Ohba S, Ogata N, Chung UI, Kubota N, Terauchi Y, Kadowaki T, Nakamura K. Distinct effects of PPARgamma insufficiency on bone marrow cells, osteoblasts, and osteoclastic cells. J Bone Miner Metab. 2005;23(4):275-279
26. He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100(26):15712-15717
27. Sheng H, Rui XF, Sheng CJ, Li WJ, Cheng XY, Jhummon NP, Yu YC, Qu S, Zhang G, Qin L. A novel semisynthetic molecule icaritin stimulates osteogenic differentiation and inhibits adipogenesis of mesenchymal stem cells. Int J Med Sci. 2013;10(6):782-789
28. Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, Reid IR. The peroxisome-proliferatoractivated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92(4):1305-1310
29. Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G; Diabetes Outcome Progression Trial (ADOPT) Study Group. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT). Diabetes Care. 2008;31(5):845-851
30. Gruntmanis U, Fordan S, Ghayee HK, Abdullah SM, See R, Ayers CR, McGuire DK. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone increases bone resorption in women with type 2 diabetes: a randomized, controlled trial. Calcif Tissue Int. 2010;86(5):343-349
Author contact
![]() Corresponding authors: June-Ho Byun (Department of Oral and Maxillofacial Surgery, Institute of Health Sciences, Biomedical center (BK21), 660-702, Gyeongsang National University School of Medicine, Jinju, Republic of Korea, Tel: 82-55-750-8258, Fax: 82-55-761-7024, E-mail: surbyunac.kr) or Dong Kyun Woo (College of Pharmacy and Research Institute of Pharmaceutical Sciences, Gyeongsang National University, Jinju, Republic of Korea, Tel: 82-55-772-2428, E-mail: dongkyun.wooac.kr).
Corresponding authors: June-Ho Byun (Department of Oral and Maxillofacial Surgery, Institute of Health Sciences, Biomedical center (BK21), 660-702, Gyeongsang National University School of Medicine, Jinju, Republic of Korea, Tel: 82-55-750-8258, Fax: 82-55-761-7024, E-mail: surbyunac.kr) or Dong Kyun Woo (College of Pharmacy and Research Institute of Pharmaceutical Sciences, Gyeongsang National University, Jinju, Republic of Korea, Tel: 82-55-772-2428, E-mail: dongkyun.wooac.kr).

 Global reach, higher impact
Global reach, higher impact