3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(10):1001-1008. doi:10.7150/ijms.8705 This issue Cite
Research Paper
The Effects of ABCG2 on the Viability, Proliferation and Paracrine Actions of Kidney Side Population Cells under Oxygen-Glucose Deprivation
1. Department of Nephrology, Xijing Hospital, Fourth Military Medical University, Xi'an, 710032, China;
2. State Key Laboratory of Cancer Biology, Department of Medical Genetics and Developmental Biology, Fourth Military Medical University, Xi'an, 710032, China;
3. Institute of Materia Medica, School of Pharmacy, Fourth Military Medical University, Xi'an, 710032, China;
4. Department of Nephrology, Tangdu Hospital, Fourth Military Medical University, Xi'an, 710032, China.
* These authors (Hong-Bao Liu, Qiu-Hong Meng, De-Wei Du and Ji-Feng Sun) contributed equally to this work.
Received 2014-1-28; Accepted 2014-7-1; Published 2014-7-20
Abstract
Bcrp1/ABCG2 is exclusively expressed in side population (SP) cells, however, it has not been fully elucidated whether it has an impact on the viability, proliferation and paracrine actions in kidney SP cells under oxygen-glucose deprivation (OGD) followed by reoxygenation. In this study, we found that 2-h OGD did not injure SP cells (sub-lethal OGD) but induced SP cells proliferation 48 and 72 h after reoxygenation; whereas 4-h OGD markedly injured the cells (lethal OGD) and led to apoptosis 24-72 h after reoxygenation. Fumitremorgin C, an inhibitor of ABCG2, attenuated both the proliferation and viability of SP cells. Sub-lethal and lethal OGD induced the increase in the secretion of vascular endothelial growth factor, insulin-like growth factor 1, hepatocyte growth factor, and stromal cell-derived factor-1α in kidney SP cells, which was inhibited by Fumitremorgin C. Collectively, these findings provide evidence for a crucial role for the ABCG2 expression in the viability, proliferation and paracrine actions of kidney SP cells after OGD.
Keywords: Stem cells, Side population, Kidney, Proliferation, Apoptosis, Paracrine, ischemia, hypoxia, oxygen-glucose deprivation.
1. Introduction
Side population (SP) cells, which are originally identified in bone marrow using flow cytometers equipped with UV sources, are defined as a group of cells displaying low Hoechst33342 fluorescence and hematopoietic stem cell properties [1]. Subsequently, the SP cells resident in the tissues of many adult organs have been successfully isolated, which have been identified to be a stem/progenitor-like cell population, possessing strong potential of proliferation, self-renewal and multipotentiality [2]. In recent years, resident SP cells have also been found in kidneys of both animal and human, which showed multipotentiality and can promoting the repair process of the damaged kidney when transplanted in vivo [3-10], suggesting that the cells could be good targets for clinical renal regenerative medicine.
The ability of SP cells in excluding Hoechst 33342 dye is dependent on the expression of Bcrp1/ABCG2 [11]. Studies on cells from a variety of sources showed that SP cells have a superior anti-apoptotic ability compared with the non-SP cells, and which was associated with the expression of ABCG2, suggesting that the expression of ABCG2 on SP cells probably have functional effects [12, 13]. Our previous study [10] indicated that the kidney SP cells can obviously improve the renal function, cell proliferation and viability in mice with renal ischemia/reperfusion (I/R) injury, and the MEK/ERK-ABCG2 pathway is involved in protecting kidney SP cells from I/R injury. However, whether the expression of ABCG2 has some effects on the viability, proliferation and paracrine actions in kidney SP cells remains unknown.
In the present study, therefore, we performed oxygen-glucose deprivation (OGD) followed by reoxygenation to induce ischemic-like injury in the cultured kidney SP and non-SP cells, and attempted to investigate the role of ABCG2 on the viability, proliferation and paracrine actions of SP cells after OGD.
2. Materials and methods
2.1. Isolation of kidney SP cells
6 to 8-week-old male C57BL/6 mice were provided by the Laboratory Animal Center of the Fourth Military Medical University (Xi'an, China). They were housed individually in cages in an animal house at 22 °C under a light/dark cycle of 12/12 hours, with standard food and water provided ad libitum, which were approved as the protocol by the institutional animal care and use committee. And all the procedures in our animal study were conducted under approved guidelines of the animal ethics committee of the university.
The protocol was based on a report by Challen et al. [4]. Briefly, anesthetized mice were perfused with normal saline via the abdominal aorta, and the minced kidneys were digested enzymatically in Hank's balanced salt solution (HBSS) (Invitrogen, Sweden) containing 1.2 U/mL Dispase II (Roche, Italy), 0.01% DNAse type I (Sigma, USA), and 7.5 mg/mL collagenase B (Roche) at 37 °C for 20 min. Then the single cell suspension was filtered through 40 μm strainers (BD Falcon 2350; BD Pharmingen, USA) to remove debris, and washed in HBSS containing 10 mmol/L HEPES (Sigma) and 2% fetal bovine serum (FBS) (Gibco, USA). Then cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) (HyClone, USA) containing 2% FBS, 10 mM HEPES, and 5 μg/mL Hoechst 33342 (Sigma), with or without 50 μM verapamil (Sigma). Cells were then incubated at 37 °C for 90 min, with a definite concentration of 1×106 cells/mL. For fluorescence-activated cell sorting (FACS) analysis, propidium iodide (PI, 2 μg/ml) (BD Pharmingen) was added previously so that to exclude dead cells and ensure all quantified cells were viable.
2.2. Cell culture
The kidney SP and non-SP cells were seeded in DMEM media containing 10% fetal bovine serum (FBS), 5 pM thyronine, 5 μg/mL insulin, 5 μg/mL transferrin, 50 nM hydrocortisone, 50 nM selenium, and 50 nM prostaglandin (all Sigma), incubated at 37 °C in a 21% O2 / 74% N2 / 5% CO2 incubator for 48 h. OGD followed by reoxygenation (OGD/R) is used as an in vitro model of I/R injury[14-16]. The OGD group was incubated in glucose-free DMEM and placed in a hypoxic chamber (Billups-Rothenberg, Del Mar, CA, USA) filled with an anoxic gas mixture (95% N2 / 5% CO2) for 1, 2, 4, 6 h while the control normoxia group was incubated in DMEM supplemented with 5.5 mM glucose and placed in a cell culture incubator for the same periods of time. At the end of OGD, the plates were taken out from the hypoxic chamber, the glucose and FBS were added and the cells were incubated under normoxic conditions for different time periods to generate reoxygenation. To determine the involvement of ABCG2, the ABCG2 inhibitor Fumitremorgin C (FTC, 0.2-10 μM, Sigma) were continuously applied from 30 min before OGD to the end of reoxygenation.
2.3 RT-PCR Analysis
Total RNA was extracted from the kidney SP and non-SP cells using RNAzol (Molecular Research Center, Cincinnati, OH, USA) following the manufacturer's instructions and complementary DNA (cDNA) was synthesized using oligo dT primers with reverse transcriptase (Promega Corp., Fitchurg, WI, USA). The primers used were as follows: ABCG2 (235 bp), forward, 5'-CCA TAG CCA CAG GCC AAA GT-3' and reverse, 5'-GGG CCA CAT GAT TCT TCC AC-3'; β-actin (375 bp), forward, 5'-TCG TGC GTG ACA TCA AAG AGA-3' and reverse, 5'-GAA CCG CTC GTT GCC AAT AGT-3'. PCR conditions were as follows: 94 °C for 5 min; 35 cycles at 94 °C for 45 s, 58 °C for 45 s, and 72 °C for 60 s; final elongation at 72 °C for 10 min. The PCR products were separated by electrophoresis in 2% agarose gels and stained with ethidium bromide (both Invitrogen).
2.4 Western Blot Analysis
Nuclear protein of the kidney SP and non-SP cells was extracted and protein concentration was determined using the BCA Protein Assay (Thermo Scientific). Protein samples were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred onto polyvinylidene difluoride membrane (all Invitrogen). The membrane was incubated with antibodies against ABCG2 (BXP-53, Santa Cruz, CA, USA), proliferating cell nuclear antigen (PCNA) (Santa Cruz, CA, USA) and β-actin (Cell Signaling Technology Inc.). Proteins of interest were detected with horseradish peroxidase-conjugated secondary antibodies (Abcam) and were developed using the enhanced chemiluminescence Plus kit (Amersham, Freiburg, Germany).
2.5 Cell viability assay
The cell viability was tested by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (Sigma). In order to better compare the cell metabolic activities in different controls, the MTT results in other groups were presented as a percentage compared to SP cells in a same control group.
2.6 Cell apoptosis assay
Apoptosis assays were performed using an Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (catalog No. 556419; BD Pharmingen) according to the manufacturer's instructions. Briefly, kidney SP and non-SP cells in the dish (105 cells/well) were collected and resuspended in binding buffer. Annexin V-FITC and PI were added, and the reaction was incubated in the dark for 15 min. Cells were analyzed using a FACScan flow cytometer.
2.7. Cell cycle studies
Kidney SP and non-SP cells in S-G2M phase were identified using immunostaining of the cell cycling marker, Ki 67, and the DNA binding dye, PI. Briefly, we pelleted the isolated kidney SP and non-SP cells through centrifugation, and the the cells were permeabilized by using the Citofix/Cytoperm kit (Pharmingen). After that the permeabilized cells were then stained with FITC conjugated anti-Ki 67 goat polyclonal antibodies (2 μg/mL) (Santa Cruz Biotechnology, Inc) or PI (10 μg/mL) for 30 minutes on ice. We used the respective isotype control (Santa Cruz Biotechnology, Inc) as a negative control. And then cells were washed twice with HBSS and analyzed using FACS as described below.
2.8. FACS analysis
FACS was carried out using a dual-laser FACS Vantage (BD Biosciences, USA). Hoechst 33342 was excited by an 355 nm UV laser, and the fluorescence emission signals were collected with a 675nm long-pass filter (Hoechst red) and a 450/20 band-pass filter (Hoechst blue), and we used a 610 nm dichroic mirror short-pass to separate the wavelengths of emission light. The fluorescences of PE, FITC, and PI were detected using a 488 nm argon laser, and the live cell gate was identified as the cells being excluded from the PI positive cells.
2.9. Enzyme linked immunosorbent assay (ELISA)
The levels of vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF), and stromal cell-derived factor-1 (SDF-1α) in the supernatants of SP and non-SP were measured by ELISA assay using a commercially available ELISA kit (R&D Systems, USA) referring to the manufacturer's recommendation. The cell suspensions containing about 1 × 106 cells were treated with OGD/R (2-h OGD+ 48-h reoxygenation or 4-h OGD+ 24-h reoxygenation), respectively. And all the proteins were quantified by using BCA protein assay reagent (Pierce, USA). Optical density values were measured at 450nm with wavelength correction at 570 nm. All standards of samples were measured twice.
2.10. Statistical analysis
All values are expressed as means ± SD. Differences between data means of all assays were compared by use of analysis of variance (ANOVA) or Student's t-test using the SPSS statistical software package (SPSS, Inc., Chicago, IL, USA). A threshold of statistical significance was set at P < 0.05.
3. Results
3.1 OGD/R-induced kidney SP cell proliferation and apoptosis
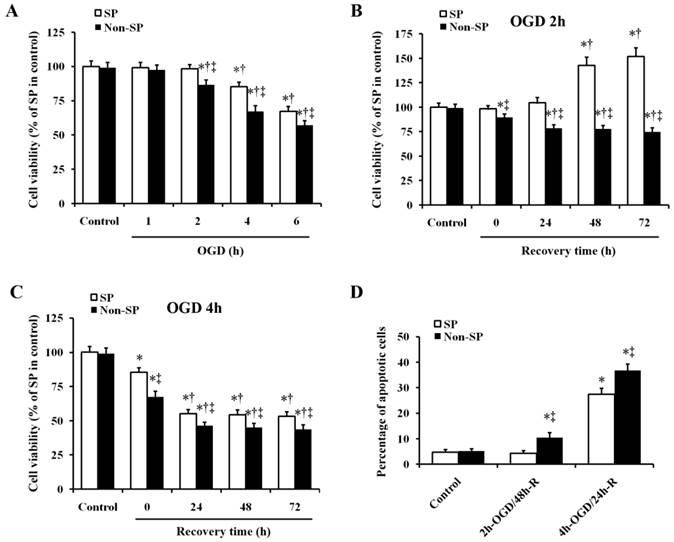
We determined the effects of exposure to various durations of OGD on the viability of kidney SP and non-SP cells. The results showed that OGD time-dependently decreased the viability of kidney SP and non-SP cells. The viability of both the two types of cells was not affected at 1 h after OGD. And 2-h OGD decreased the viability of non-SP cells, but the same decrease did not happen in SP cells at the same time. Both at 4 and 6 h after OGD, the viability of SP cells decreased as well as that of the non-SP cells. And at all the three time points of 2, 4 and 6 h after OGD, the viability of SP cells significantly higher than that of corresponding non-SP cells, respectively (Fig. 1A). Then we treated the kidney SP and non-SP cells with 2-h and 4-h OGD and observed viability at 24, 48, and 72 h after recovery. Treatment of SP cells with 2-h OGD increased the viability at 48 and 72 h after reoxygenation (indicating SP cell proliferation, Fig. 1B); however, 4-h OGD decreased the SP cell viability at 24-72 h after reoxygenation (indicating SP cell injury, Fig. 1C). As for non-SP cells, no matter 2-h or 4-h OGD treatment decreased the cell viability at 24-72 h after reoxygenation (indicating non-SP cell injury, Fig. 1B and 1C). In addition, apoptosis of SP cells were induced by 4-h OGD and 24-h reoxygenation (4-h OGD/24-h R), but not by 2-h OGD and 48-h reoxygenation (2-h OGD/48-h R) (Fig.1D). While both the 4-h OGD/24-h R and 2-h OGD/48-h R treatments induced the apoptosis in non-SP cells compared with corresponding normal control, respectively (Fig.1D). On the basis of these results, we induced SP cell proliferation by 2-h OGD/48-h R (defined as sublethal OGD/R), and SP cell apoptosis by 4-h OGD/24-h R (defined as lethal OGD/R) in the following experiments.
3.2 Involvement of ABCG2 in OGD/R-induced kidney SP cell proliferation and apoptosis
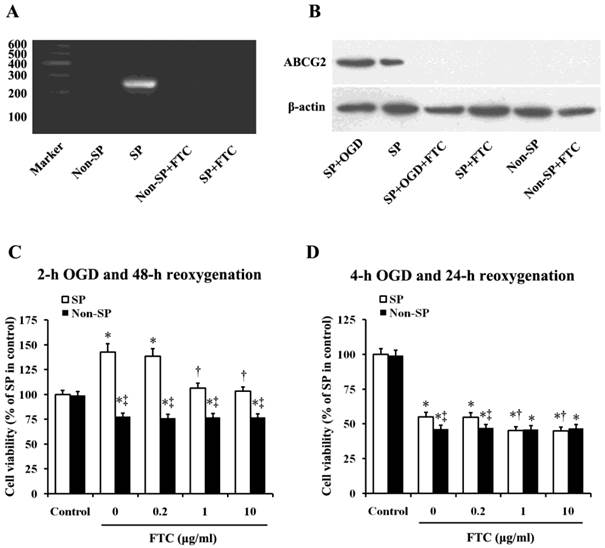
To determine whether ABCG2 is involved in OGD/R-induced kidney SP cell proliferation and apoptosis, we observed the effects of Fumitremorgin C (FTC), a selective BCRP1/ABCG2 inhibitor [17-20], on cell proliferation/apoptosis. As the articles previously published by us [9, 10], in this study, we further confirmed the exclusive expression of ABCG2 in kidney SP cells, but not non-SP cells by using RT-PCR analysis. The results showed that the administration of FTC (10 μM) block the mRNA expression of ABCG2 in kidney SP cells. (Fig. 2A).Then the western blot analysis further proved the exclusive expression of ABCG2 in SP cells, which showed that the expression of ABCG2 was significantly increased, when performed the treatment of sub-lethal OGD (2h) on the cells, but FTC (10 μM) markedly blocked the expression of ABCG2 in SP cells, whether the cells treated with sub-lethal OGD (2h) or not (Fig.2B). FTC (1 and 10 μM) attenuated sub-lethal OGD/R-induced SP cell proliferation (Fig.2C), and further aggravated lethal OGD/R-induced SP cell injury (Fig.2D).
Effect of OGD/R on cell viability and apoptosis in kidney SP and non-SP cells. (A) Kidney SP and non-SP cells were treated by OGD for 1-6 h; the SP cell viability was gradually decreased at 2, 4, and 6 h. (B) After 2-h OGD, the SP cell viability was significantly increased at 48 and 72 h after reoxygenation. (C) After 4-h OGD, the viability was reduced at 24-72 h after reoxygenation. (D) In vitro survival analysis of kidney SP and non-SP cells in basal conditions and after OGD/R. Bar graph described from the FACS-based Annexin V/propidium iodide apoptosis assay. The cells without OGD/R were used as controls. OGD, oxygen-glucose deprivation; 2-h OGD/48-h R, 2-h OGD and 48-h reoxygenation; 4-h OGD/24-h R, 4-h OGD and 24-h reoxygenation. Data are expressed as mean ±SD; n=6; * P < 0.05 versus control (without OGD); † P < 0.05 versus OGD alone; ‡ P < 0.5 versus SP.
Effects of ABCG2 expression on OGD/R-induced changes in cell viability. (A) RT-PCR analysis of the kidney SP and non-SP cells for ATP-binding cassette, subfamily G, member 2 (ABCG2; 235bp). The cells were treated with or without Fumitremorgin C (FTC, 10 μM), an inhibitor of ABCG2. (B) Western blot analysis was performed to detect ABCG2 expression in the kidney SP cells treated with or without OGD (2h). FTC (10 μM) was used to inhibit ABCG2 expression. (C) After 2-h OGD and 48-h reoxygenation, cell viability was significantly increased, which was attenuated by FTC (1-10 μM). (D) After 4-h OGD and 24-h reoxygenation, the viability was significantly reduced, which was futher depravated by FTC (1-10 μM). The cells without OGD/R were used as controls. FTC, Fumitremorgin C; OGD, oxygen-glucose deprivation. Data are expressed as mean ±SD; n=6; * P < 0.05 versus control (without OGD); † P < 0.05 versus OGD/R alone; ‡ P < 0.05 versus SP.
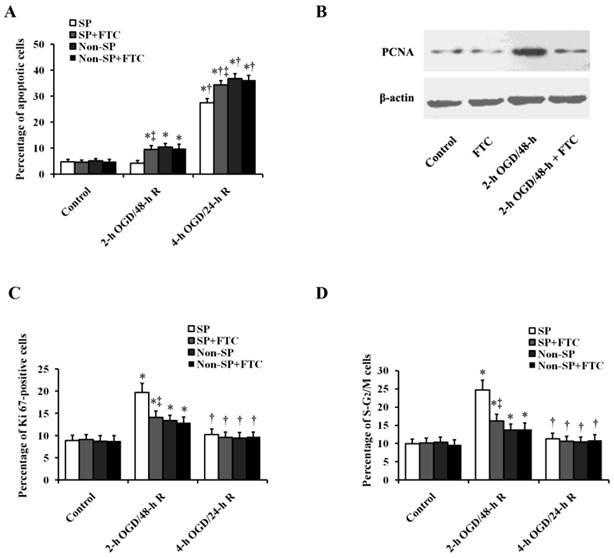
The apoptosis assay showed that the viable percentage of SP cells was comparable to that of non-SP cells treated with or without FTC (10 μM) under normoxic condition, suggesting that FTC is not toxic to kidney SP and non-SP cells (Fig. 3A). Sub-lethal OGD/R did not increase the ratio of apoptotic (Annexin V+) cells in SP cells, while FTC significantly increased the ratio of apoptotic (Annexin V+) cells in SP cells with sub-lethal OGD/R treatment. Similarly, FTC also further aggravated the SP cell apoptosis induced by lethal OGD/R treatment (Fig. 3A). When we inhibited the expression of ABCG2, apoptosis of SP cells was increased, which suggesting a role for ABCG2 in protecting SP cells against OGD/R injury. But FTC did not influence the OGD-induced non-SP cell apoptosis (Fig. 3A). The proliferation was verified by western blot analysis, in which sub-lethal OGD/R significantly increased the expression of proliferating cell nuclear antigen (PCNA) in SP cells, but had no obvious effect in non-SP cells, and the administration of FTC significantly decreased the expression of PCNA in SP cells, but did not affect the expression of PCNA in non-SP cells (Fig.3B). In addition, we examined the expression of the cell cycling markers, Ki67 and PI, and analyzed the cell percentage of the cells in S-G2/M phase. Flow cytometric analysis showed SP cells treated with sub-lethal OGD/R induced distinctly increase of the fraction of SP cells in S-G2/M phase, while the increase was relatively slight in non-SP cells, which indicated that compared non-SP cells sub-lethal OGD induced more SP cells to undergo mitosis. However, lethal OGD/R did not change the fraction of both kidney SP and non-SP cells entering in S-G2/M phase (Fig.3C and 3D). The increase of kidney SP cells of S-G2/M induced by sub-lethal OGD/R was decreased by the pretreatment of FTC, suggesting that FTC blocked SP cells to enter in division phase.
3.3. ABCG2 contributes to the OGD/R-induced paracrine action in kidney SP cells
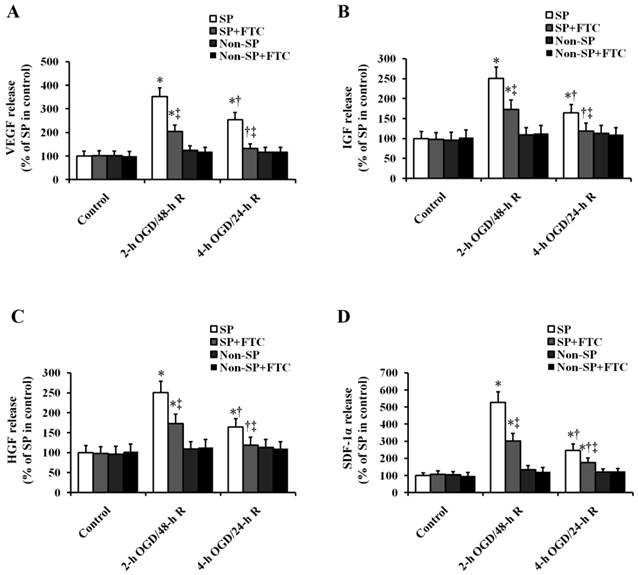
We first determined the role of OGD/R in the secretion of chemokine and mitogen factors in kidney SP cells (Fig. 4). In vitro sub-lethal and lethal OGD/R induced the increase in the secretion of VEGF, IGF-1, HGF and SDF-1α in kidney SP cells, but had no effect on the non-SP cells (Fig. 4). Compared with lethal OGD/R, sub-lethal OGD/R induced the more secretion of factors mentioned above. Then, we were looking forward to determining the role of ABCG2 in OGD/R-induced paracrine action of kidney SP cells. Just as shown in Fig. 4, the secretion of chemokine and mitogen factors induced by OGD/R was blocked by FTC.
4. Discussion
Our study for the first time confirmed that the role of ABCG2 on kidney SP cell viability, proliferation and paracrine function under OGD/R.
Bone marrow-derived stem cells and renal resident stem cells are postulated to participate in recovery after kidney damage [2, 21-23]. In recent years, we and other authors [3-10] have confirmed the existence of SP cells in normal kidneys. Affymetrix microarrays showed that the immunophenotype of kidney SP cells was distinct from that of bone marrow SP cells, suggesting that the kidney SP are a renal resident cell population [4, 5]. Although the SP cell populations are heterogeneous, which contains renal tubular epithelial cells, stromal cells and monocyte-macrophage cells, etc., they all have multi-linear differentiation potential and renal protective capacity [4-7, 9, 10]. Challen GA, et al [4] successfully induced the differentiation of SP cells into osteocytes and adipocytes in their cell culture assays in vitro, and they injected SP cells into an adriamycin-nephropathy model resulting in the reduction of the albuminuria:creatinine ratios. Hishikawa K, et al [6] demonstrated that the renal function was significantly improved by the infusion of SP cells in cisplatin-induced reversible acute renal failure mice model. Their quantitative real-time PCR shows that the secretion of renal protective factors was increased significantly in the kidney of acute renal failure mice, suggesting the kidney SP cells possessed the potential on the kidney regeneration. Our previous study [10] also indicated that exogenous infusion of kidney SP cells significantly improved the renal function, promoting the proliferation and decreasing the apoptosis of the surviving tubular cells in acute renal I/R injury mice model. Therefore, kidney SP cells have potential to be the target cells of renal reconstruction therapies in clinic. Given the newly discovered evidence that the predominant mechanism of kidney repair after ischemic tubular injury involved the regeneration by surviving tubular epithelial cells [23], the further study of the biological characteristics in these renal residential stem/progenitor cell population under ischemic and hypoxic conditions may open a novel way to the treatment of acute kidney injury.
Effects of ABCG2 expression on OGD/R-induced cell apoptosis, proliferation and cell cycle in kidney SP and non-SP cells. (A) In vitro survival analysis of kidney SP and non-SP cells treated with or without FTC (10 μM) in basal conditions and after OGD/R. Bar graph described from the FACS-based Annexin V/propidium iodide apoptosis assay. (B) Western blot analysis was performed to detect proliferating cell nuclear antigen (PCNA) levels in the kidney SP cells treated with or without OGD. FTC (10 μM) was used to inhibit ABCG2 expression. (C-D) Fractions of S-G2M kidney SP and non-SP cells were obtained by immunostaining for Ki67 (C) and PI (D). FTC (10 μM) was used to inhibit ABCG2 expression. The cells without OGD/R were used as controls. FTC, Fumitremorgin C; OGD, oxygen-glucose deprivation; 2-h OGD/48-h R, 2-h OGD and 48-h reoxygenation; 4-h OGD/24-h R, 4-h OGD and 24-h reoxygenation. Data are expressed as mean ±SD; n=6; * P < 0.05 versus control (without OGD); † P < 0.05 versus 2-h OGD/48-h R; ‡ P < 0.05 versus SP or non-SP cells treated without FTC.
Effect of ABCG2 expression on OGD/R-induced paracrine actions in kidney SP and non-SP cells. Cultured cells were subjected to 2-h OGD/48-h R or 4-h OGD/24-h R, and then the secretion levels of VEGF, IGF-1, HGF, and SDF-1α were quantified by ELISA. FTC (10 μM) was used to inhibit ABCG2 expression. The cells without OGD/R were used as controls. FTC, Fumitremorgin C; OGD, oxygen-glucose deprivation; 2-h OGD/48-h R, 2-h OGD and 48-h reoxygenation; 4-h OGD/24-h R, 4-h OGD and 24-h reoxygenation.. * P < 0.05 versus control (without OGD); † P < 0.05 versus 2-h OGD/48-h R; ‡ P < 0.05 versus SP or non-SP cells treated without FTC.
OGD/R has been commonly used as an in vitro model of I/R injury in numerous studies [14-16]. Our results showed that in vitro sub-lethal OGD/R did not injure SP cells but induced SP cells proliferation; whereas lethal OGD/R induced the marked decrease in the viability of SP and non-SP cells. Sub-lethal and lethal OGD/R induced the increase in the secretion of VEGF, IGF-1, HGF, and SDF-1α in kidney SP cells but not in non-SP cells. These results suggested that there was a significant difference between kidney SP and non-SP cells after OGD/R in cell viability, proliferation, and paracrine actions.
The SP cells constitutively express ABCG2, which are a member of the ATP-binding cassette transporter family and a very important determinant of the SP phenotype [1]. Our study also identified that kidney SP cells, but not non-SP cells, preferentially and exclusively expressed ABCG2. Importantly, in this study, we shows that the ABCG2 inhibitor, FTC, significantly increased sub-lethal and lethal OGD-induced apoptosis, and reduced the OGD-induced proliferation and paracrine ability of SP cells, suggesting that ABCG2 is crucial in prompting some biological functions of the kidney SP cells. Further clarify the signal pathways and key targets in regulating the viability, proliferation and paracrine actions in kidney SP cells, will be our future research directions.
Acknowledgements
This work was supported by grants from National Nature Science Foundation of China (Nos. 81370016 and 81000309), and Midwestern Excellent Young Scientist Foundation of Chinese Medical Doctor Association (2012).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ. et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nature medicine. 2001;7:1028-34
2. Bussolati B, Tetta C, Camussi G. Contribution of stem cells to kidney repair. American journal of nephrology. 2008;28:813-22
3. Addla SK, Brown MD, Hart CA, Ramani VA, Clarke NW. Characterization of the Hoechst 33342 side population from normal and malignant human renal epithelial cells. American journal of physiology. 2008;295:F680-7
4. Challen GA, Bertoncello I, Deane JA, Ricardo SD, Little MH. Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity. J Am Soc Nephrol. 2006;17:1896-912
5. Hishikawa K, Marumo T, Miura S, Nakanishi A, Matsuzaki Y, Shibata K. et al. Musculin/MyoR is expressed in kidney side population cells and can regulate their function. The Journal of cell biology. 2005;169:921-8
6. Hishikawa K, Marumo T, Miura S, Nakanishi A, Matsuzaki Y, Shibata K. et al. Leukemia inhibitory factor induces multi-lineage differentiation of adult stem-like cells in kidney via kidney-specific cadherin 16. Biochemical and biophysical research communications. 2005;328:288-91
7. Imai N, Hishikawa K, Marumo T, Hirahashi J, Inowa T, Matsuzaki Y. et al. Inhibition of histone deacetylase activates side population cells in kidney and partially reverses chronic renal injury. Stem cells (Dayton, Ohio). 2007;25:2469-75
8. Inowa T, Hishikawa K, Takeuchi T, Kitamura T, Fujita T. Isolation and potential existence of side population cells in adult human kidney. Int J Urol. 2008;15:272-4
9. Liu H, Liu W, Liu S, Meng Q, Zhang N, Wang H. et al. Reconstitution of kidney side population cells after ischemia-reperfusion injury by self-proliferation and bone marrow-derived cell homing. Evid Based Complement Alternat Med. 2013;2013:370961
10. Liu WH, Liu HB, Gao DK, Ge GQ, Zhang P, Sun SR. et al. ABCG2 protects kidney side population cells from hypoxia/reoxygenation injury through activation of the MEK/ERK pathway. Cell transplantation. 2013;22:1859-68
11. Huls M, Russel FG, Masereeuw R. The role of ATP binding cassette transporters in tissue defense and organ regeneration. The Journal of pharmacology and experimental therapeutics. 2009;328:3-9
12. Martin CM, Ferdous A, Gallardo T, Humphries C, Sadek H, Caprioli A. et al. Hypoxia-inducible factor-2alpha transactivates Abcg2 and promotes cytoprotection in cardiac side population cells. Circulation research. 2008;102:1075-81
13. Pfister O, Oikonomopoulos A, Sereti KI, Sohn RL, Cullen D, Fine GC. et al. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circulation research. 2008;103:825-35
14. Gu JH, Ge JB, Li M, Xu HD, Wu F, Qin ZH. Poloxamer 188 protects neurons against ischemia/reperfusion injury through preserving integrity of cell membranes and blood brain barrier. PloS one. 2013;8:e61641. doi:10.1371/journal.pone.0061641
15. Rizvi M, Jawad N, Li Y, Vizcaychipi MP, Maze M, Ma D. Effect of noble gases on oxygen and glucose deprived injury in human tubular kidney cells. Experimental biology and medicine. 2010;235:886-91 doi:10.1258/ebm.2010.009366
16. Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. American journal of physiology. 2012;303:F377-85 doi:10.1152/ajprenal.00354.2011
17. Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer research. 2000;60:47-50
18. Nakanishi T, Doyle LA, Hassel B, Wei Y, Bauer KS, Wu S. et al. Functional characterization of human breast cancer resistance protein (BCRP, ABCG2) expressed in the oocytes of Xenopus laevis. Molecular pharmacology. 2003;64:1452-62 doi:10.1124/mol.64.6.1452
19. Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. Journal of cell science. 2005;118:1715-24 doi:10.1242/jcs.02279
20. Robey RW, Honjo Y, van de Laar A, Miyake K, Regis JT, Litman T. et al. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochimica et biophysica acta. 2001;1512:171-82
21. Liu H, Liu S, Li Y, Wang X, Xue W, Ge G. et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PloS one. 2012;7:e34608
22. Liu H, Xue W, Ge G, Luo X, Li Y, Xiang H. et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochemical and biophysical research communications. 2010;401:509-15
23. Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS. et al. Intrinsic epithelial cells repair the kidney after injury. Cell stem cell. 2008;2:284-91
Author contact
![]() Corresponding author: Hua Han, PhD, Department of Medical Genetics and Developmental Biology, Fourth Military Medical University, Xi'an 710032, China. E-mail: huahanedu.cn or Jian-Bo Wang, PhD, Institute of Materia Medica, School of Pharmacy, Fourth Military Medical University, Xi'an 710032, China. E-mail: yyswjbedu.cn.
Corresponding author: Hua Han, PhD, Department of Medical Genetics and Developmental Biology, Fourth Military Medical University, Xi'an 710032, China. E-mail: huahanedu.cn or Jian-Bo Wang, PhD, Institute of Materia Medica, School of Pharmacy, Fourth Military Medical University, Xi'an 710032, China. E-mail: yyswjbedu.cn.

 Global reach, higher impact
Global reach, higher impact