3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(9):925-935. doi:10.7150/ijms.8951 This issue Cite
Review
Alanine Aminotransferase-Old Biomarker and New Concept: A Review
1. Department of Hepatobiliary Surgery, First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, Guangxi Province, China.
2. Department of Pediatrics, Women and children's hospital of Guangxi, Nanning, 530005, Guangxi province, China.
* These authors contributed to this work equally.
Received 2014-2-27; Accepted 2014-6-12; Published 2014-6-26
Abstract
Measurement of serum alanine aminotransferase (ALT) is a common, readily available, and inexpensive laboratory assay in clinical practice. ALT activity is not only measured to detect liver disease, but also to monitor overall health. ALT activity is influenced by various factors, including viral hepatitis, alcohol consumption, and medication. Recently, the impact of metabolic abnormalities on ALT variation has raised concern due to the worldwide obesity epidemic. The normal ranges for ALT have been updated and validated considering the metabolic covariates in the various ethnic districts. The interaction between metabolic and demographic factors on ALT variation has also been discussed in previous studies. In addition, an extremely low ALT value might reflect the process of aging, and frailty in older adults has been raised as another clinically significant feature of this enzyme, to be followed with additional epidemiologic investigation. Timely updated, comprehensive, and systematic introduction of ALT activity is necessary to aid clinicians make better use of this enzyme.
Keywords: serum alanine aminotransferase, ALT, activity
1. Introduction
Serum alanine aminotransferase (ALT) is a readily available, inexpensive, and routine biochemical assay used in clinical practice [1]. Initially, many concerns were voiced regarding the clinical significance of ALT activity on viral and toxic hepatitis, muscular dystrophy, and other muscular diseases [2], which might cause a substantial increase in the ALT level [3] in a relatively low percentage of the overall population. However, more and more metabolic disorders, such as obesity, hyperlipidemia, and diabetes mellitus (DM) have been observed independently associated with mild-to-moderate ALT elevation [4]. The series of metabolic disorders were referred to metabolic syndrome (MetS) [5], and featured insulin resistance and obesity. The clinical implication of ALT elevation in representing MetS has caused worldwide concern [4, 6, 7] in Western and Eastern countries with the rapidly increasing prevalence of obesity [8]. Currently, ALT measurement is not only widely used in detecting the incidence, development, and prognosis of liver disease with obvious clinical symptoms, but also provides reference on screening the overall health status during health check-ups [1, 9].
Some demographic factors, such as gender and age, might also interfere with the ALT level in the general population [10-13]. Even the laboratory method and the diurnal variation might interfere with the ALT level, which has been discussed in previous studies [14-16]. For a comprehensive association linked to many disease processes, co-morbidities, and concurrent diseases, the clinicians should carefully evaluate and explain the clinical implication underlying the abnormal ALT level when faced with an individual patient. The relative confounders should be adjusted. Thus, the overview provided in this paper was intended to summarize the clinical factors that influence the ALT level based on previous reports and provide better knowledge of this liver enzyme as a tool in clinical estimation.
2. Chemical Formation and Physiology of ALT
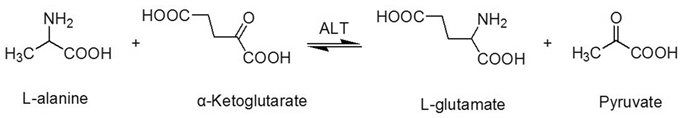
ALT is an enzyme that is mainly aggregated in the cytosol of the hepatocyte [17]. ALT consists of 496 amino acids, has a half-life of 47±10 hours [18, 19], and is coded by the ALT gene, which is on the long arm of chromosome 8 [19]. Physically, the ALT enzyme catalyzes the transfer of amino groups from L-alanine to α-ketoglutarate, and the converted products are L-glutamate and pyruvate (Figure 1) in the liver, which is a critical process of the tricarboxylic acid (TCA) cycle. In this process, the coenzyme, pyridoxal phosphate, is required [17, 20].
ALT is mainly aggregated in the cytosol of the hepatocyte. ALT activity in hepatic cells is approximately 3000 times higher than serum ALT activity [17]. When liver injury occurs, ALT is released from injured liver cells and causes a significant elevation in serum ALT activity. ALT also exists in muscles, adipose tissues, intestines, colon, prostate, and brain [21, 22]; however, the concentration of ALT in these organs is much lower than the liver [1, 23, 24].
3. Physiologic Factors Associate with the Serum ALT Level
The impact of some physiological factors has been shown to be associated with the serum ALT level. Extreme physical exertion can induce a short-term, reversible elevation in ALT. In a study focused on Thai boxers, the ALT level was 2-2.25-fold higher than the baseline value after a fight in 20 male adolescent Thai boxers [25]. In addition, an elevation in ALT was also observed in 9 runners who took part in a 1600 km ultra-marathon [26]; specifically, a 4-fold increase in ALT activity was observed on the 4th day of running compared to the baseline value. Many authors attribute the elevation in ALT after physical exertion to muscle injury [27-29]. In contrast, modest physical activity is helpful in normalizing ALT levels [30, 31]. In addition, the serum ALT values in those who exercise at customary levels were lower than those who do not exercise or exercise more strenuously than customary [3, 32]. ALT activity has a diurnal variation in a given population. Co'rdoba et al [14] observed a circadian variation in ALT activity, with the nadir value at 04:00h, and the peak value at 16:00h in 12 patients with chronic liver disease. All physiologic confounders should be taken into consideration and the outside environment should be standardized when comparison of ALT activity is performed.
4. Demographic Characteristics Influence the Serum ALT Level
Several demographic covariates may contribute to disparities in the ALT level. Specifically, age has been shown to be associated with the ALT level in prior cross-sectional and prospective studies [11-13]. Elinav et al [12] reported an inverted curve with peak ALT levels at 40-55 years in a group of Israeli participants. Further, Dong et al [11, 13] reported a significant decrease in a large sample of community participants through cross-sectional and prospective studies.
In addition, gender plays a crucial role in modulating discrepancies in ALT activity. In a study focused on blood donors without a diagnosis of hepatitis from London, the ALT level was much higher in males than females [33]. Many population-based health examinations have also shown significant discrepancies between genders under similar living conditions [4, 34-36]. Some scholars have attributed the gender-based differences in ALT levels to the hormonal differences between males and females [10].
Ethnicity difference in ALT levels has also been observed. In the Third National Health and Nutrition Examination Survey (NHANES III), Mexican-Americans had a higher prevalence of ALT elevation compared to other ethnicities [4, 37]. Indeed, the increased prevalence in ALT elevation might be correlated with the higher prevalence of metabolic syndrome (MetS) [37, 38], which is considered to be a major cause of ALT elevation in Mexican-Americans [6, 39, 40].
The transaminationreaction catalyzed by alanine aminotransferase.
5. Clinical Factors Associated with Serum ALT level
5.1 Hepatic-related causes
5.1.1 Viral hepatitis (mainly hepatitis B virus [HBV] and hepatitis C virus [HCV] infections)
Viral hepatitis infection is the leading or secondary cause of ALT elevation in populations worldwide [4, 35, 36]. ALT activity is an indicator of liver injury in patients with acute and chronic viral hepatitis [41].
With respect to HBV infection, ALT elevation is often observed in the process of the cytolytic immune response (acute phase) and the following ineffective HBV clearance (chronic phase) [42]. Liaw et al [42] have described a fluctuation in ALT activity during the process of HBV infection. ALT activity is a crucial reference indicator in treatment selection and the evaluation of prognosis in patients infected with HBV [42-44]. Nevertheless, controversy exists and Lai et al [45] reported significant fibrosis and inflammation in 37% of patients infected with HBV and persistently normal ALT levels [45]. Unlike HBV infections, the ALT level is less meaningful for diagnosis and prognosis of HCV treatment. More patients infected with HCV progress to chronic hepatitis with persistent hepatocyte injury [41]. Greater than 6 in 10 of common HCV carriers have normal ALT levels or mildly elevated ALT levels (< 2 times the upper limit of normal [ULN]) with rare hepatic histologic lesions confirmed by liver biopsy [46]. Recently, Ruhl et al [47] suggested that lowering the ULN of the ALT level (29 IU/L for men and 22 IU/L for women) was the best cut-off value to identify HCV infectors in the US population with a high prevalence of HCV infection. Otherwise, the HCV RNA titer is closely linked to the ALT elevation. Cathy et al [48] reported that approximately 68% of patients with positive HCV-RNA levels have ALT elevations in asymptomatic blood donors who tested positive for antibodies to the HCV (anti-HCV).
5.1.2 Alcohol intake
Excessive alcohol intake is another cause of ALT elevation in the general population. In an Italian population, 45.6% of altered liver tests have been attributed to excessive alcohol intake (≥28 g/day) [35], while in a US national population survey, excessive alcohol (>1 time/day) is a crucial cause of ALT elevation, second only to HCV infection [4], however, alcohol intake might be a time-and dose-dependent covariate that influences ALT activity. Short-term and light alcohol consumption was not shown to induce significant ALT elevation in adults [49, 50], however, ethnicity differences exist regarding the biological consequences of alcohol abuse [51, 52]. In a UK study based on alcohol abusers from different areas worldwide, adults from South Asia were shown to be more susceptible to alcohol-related liver damage and the ALT levels were higher than in European alcohol abusers [51]. Indeed, the effect of mild alcohol intake on ALT activity can be distinguished from binge drinking. Moderate alcohol intake does not contribute to significant ALT elevation, especially in a normal weight population, due to its potential effect on improvement of insulin sensitivity [53-55].
5.1.3 Some medications
Pratt et al [9] listed the medications that might cause ALT elevations. A randomized controlled trial (RCT) indicated that the estimated odds ratios (ORs) of ALT elevation in active treatment groups (including acetaminophen, hydromorphone+acetaminophen, morphine+acetaminophen, and oxycodone+acetaminophen) were 2.57-3.08 compared to the placebo group involving 343 healthy participants, even at the recommended dose [56]. Another commonly used medication, statins, also causes mild ALT elevation [57, 58]. The mechanism underlyingstatin-associated ALT elevation is still unclear. Some scholars have suggested that the ALT elevation in statin users is attributed to cholesterol reduction in hepatocytes and co-morbid conditions, rather than liver damage or dysfunction [59, 60]. Therefore, the long-term medications should be carefully considered when faced with an unexplained ALT elevation. Pratt et al [9] also indicated that cessation of drug treatment is the best way to confirm the relationship between a drug and ALT elevation.
5.1.4 Coffee consumption
Of note, coffee intake might be a protective factor against ALT elevation. In NHANES III, there was a 50% and 70% decrease in ALT elevation amongst participants who consumed >2 cups of coffee/day or ≥373 mg of caffeine, respectively, compared to participants who did not consume coffee [61]. Lee et al [62] attributed the protective effects of caffeine to antioxidant activity.
5.1.5 Non-alcoholic fatty liver disease (NAFLD)
NAFLD is a spectrum of clinical and pathologic changes, from fatty liver alone to steatohepatitis [63]. NAFLD is common in asymptomatic patients, and the prevalence ranges from 10 to 24% worldwide [64]. Considered as a manifestation of the MetS in liver [65, 66], NAFLD has been strongly associated with ALT activity in previous studies [4, 35, 36, 67]. NAFLD is the common cause of unexplained mild ALT elevation [3, 68]. NAFLD in asymptomatic patients is often serendipitously detected by liver biochemistry testing during routine health check-ups [69, 70]. Similar to the increasing prevalence of obesity [8], NAFLD is increasing and becoming a major health burden [71]. In spite of the non-linear correlation between the degree of ALT elevation and the histologic severity of NAFLD [72, 73], a mild ALT elevation is largely attributed to NAFLD. When faced with an unexplained ALT elevation (without viral hepatitis or a history of excessive alcohol intake), NAFLD should be considered in the differential diagnosis.
5.1.6 Autoimmune hepatitis
Autoimmune hepatitis is a less common liver disease than NAFLD [74], and the mechanism underlying autoimmune hepatitis is still unknown [75]. ALT elevation is an available auxiliary measurement in the diagnosis of autoimmune hepatitis [76].
Moreover, ALT activity is a crucial indicator in detecting the effect of immunosuppressive treatment, prognosis, and long-term survival in patients with autoimmune hepatitis. In a study based on 84 Japanese autoimmune hepatitis patients, a persistently low ALT level (≤40 U/l) was the threshold value that was associated with improved prognosis [77]. Another study also showed an association between persistent ALT elevation and poor survival in 69 autoimmune hepatitis patients [78]. ALT is considered to be a crucial non-invasive marker of inflammation in patients with autoimmune hepatitis [79].
5.2 Non-hepatic cause
5.2.1 Metabolic covariates
Except for apparent causes, such as viral hepatitis, alcohol intake, and some medications, the so-called unexplained causes of ALT elevation in some previous studies have mainly been attributed to MetS [4, 68]. Similar to the pandemic of obesity [8], MetS presents as a series of metabolic disorders, including glucose intolerance, central obesity, dyslipidaemia, and hypertension, has caused worldwide concern in the most recent decades.
Compared to the obvious cause of ALT elevation referred above, ALT elevation caused by MetS is mild and neglected. In a cross-sectional study, the ALT level in MetS patients, as defined by the National Cholesterol Education Programme Adult Treatment Panel III (NCEP-ATP-III) criteria, was approximately 30% higher than participants without MetS in a male population from south China [7]. This impact of MetS on ALT elevation, however, is progressive and cumulative with a linear trend [6, 34, 39]. Even within ULN values, which did not consider the impact of MetS and are higher than the updated values, the increasing prevalence of MetS is still correlated with the increasing ALT level in the general population. In a community-based Korean population, the ORs for MetS in the highest quintiles of ALT were 7.1-fold higher than the reference quintile in men and 2.1-fold higher in women [80]. All of the enrolled participants were selected within the ULN values (the ULN value is 30 U/l for males and 19 U/l for females) [80]. Another Korean national health survey also showed a significantly increased prevalence of MetS components, as defined by NCEP-ATP-III criteria, in the subgroup with high-normal ALT levels [81]. With respect to the lipoproteins, ALT was shown to be stably and significantly associated with intermediate-density lipoprotein (IDL) and apolipoprotein B (ApoB) after adjusting various covariates in different models [82]. These associations were commonly attributed to the stable and independent effects of insulin resistance and fatty liver disease in subjects with ALT elevations [6, 49].
In addition, the impact of MetS components on ALT activities varies to some extent. These distinguishing effects emerged after logistic regression using all of the MetS components as covariates. The impact of the individual MetS components on ALT elevation was disproportionate [7]. The body mass index (BMI) and waist circumstance (WC), representing the central obesity component of MetS, were more closely linked to the ALT elevation [4, 7, 36, 67, 83, 84], although, the underlying mechanism has not been elucidated. The possible explanation is that obesity, especially abdominal fat, is potentially involved in the visceral adipose deposition that causes hepatotoxic fatty acids [85]. In a US national population-based study [86], BMI lost significance when evaluating the association between obesity and ALT abnormities after adjusting for leptin, insulin, and triglyceride concentrations, rather than the WC, which was representative of the visceral adipose deposition, indicating that BMI might be an intrinsic association between obesity and ALT abnormalities. Another viewpoint was that the obesity-ALT elevation association was modulated by insulin resistance (IR). In a national health survey from a Korean adolescent population, the prevalence of IR status was positively correlated with the degree of obesity [87]. The OR of obesity-induced ALT elevation was significantly decreased after adjusting the homeostasis model (HOMA-IR) as an index of IR status [88]. Despite the ill-defined intrinsic mechanism, central obesity is the crucial MetS component that most influences the ALT level in general population.
5.2.2 Celiac disease and muscle injury
Chronic ALT elevation is also found in several non-hepatic disorders, such as celiac disease and muscle injury [27, 89, 90]. The intrinsic mechanism between celiac disease is not known. Approximately 40-57% of patients with celiac disease have abnormal liver tests [91-95]. Celiac disease patients with elevated ALT levels should be treated with gluten-free diet; doing so will restore ALT levels to normal in 75-95% of patients within 6 months [92, 94, 95]. It is well known that ALT elevation is often observed in patients with muscle necrosis [96] and the ALT elevation without evidence of liver disease should be considered due to muscle injury [27]. Otherwise, the increased creatine kinaseand lactate dehydrogenase activities that occurred following muscle injury should be assayed to identify the cause of ALT elevation [9, 27].
5.2.3 Hemochromatosis
As an ethnic specific disease mainly occurring in individuals of Nordic descent, hemochromatosis is a less common cause of ALT elevation. HFE gene mutations are the major cause of hereditary hemochromatosis, and iron overload is the main cause of hepatic injury. Measurements of serum ferritin, total iron-binding capacity, and the HEF mutation test can help diagnose hemochromatosis [97]. Liver biopsy might be necessary if the aforementioned tests are negative in patients highly suspected to have hemochromatosis to evaluate the status of liver injury from iron overload.
6. Interaction on ALT Level between Distinguished Covariates
Except for the independent univariate impact, ALT fluctuation has also been shown to be influenced by multivariate interaction in several studies [54, 55, 84]. Among 13,580 US participants, after excluding the patient with hepatitis B or C infection, or iron overload, obesity significantly increased the risk of alcohol-related abnormal aminotransferase activity. The prevalence of abnormal aminotransferase activity was increased from <5% in the normal weight group to near 30% in the obesity subgroup [54]. Similarly, these effects were also observed in the Finnish population [55]. Piton et al [84] also described the interaction of ALT activity by neural network and recommended the distinguishing ULN of the ALT level to be classified by BMI and gender. In addition, the age and gender interaction was also observed and expressed in mathematical formulas [12, 98]. In contrast, our prior cross-sectional study did not show a synergistic effect between MetS and HBV infection, which were also considered as critical covariates associated with ALT elevation [7].
7. Several Concerns that Clinicians should Raise
7.1 Age as a critical covariate that should be emphasized
As a covariate influencing ALT activity, the association between age and ALT activity has been described in previous studies [11-13]. The impact of age on ALT fluctuation is not only present based on quantitative discrimination, but also on the diagnostic value in predicting all-cause and disease-specific mortality. A previous authoritative review [1] indicated that elevated ALT might predict higher mortality in a general population; however, this opinion might be questionable, especially in old adults. Inverse relationships between ALT activity and mortality were observed in several studies focused on older population [57, 99, 100], and this opinion was confirmed by subsequent meta-analysis [101]. Dong et al [11, 13] raised the concept that ALT activity might be influenced by accelerated aging and frailty in older adults independent of its traditional role in screening liver function. Although age lost significance in many multi-covariate analyses [7, 35, 36], clinicians should carefully explain the extremely low ALT level, especially in older population owing to its potential implication on increasing mortality.
7.2 ULN of ALT as a hot scientific topic
Earlier ULNs for the ALT level were defined in blood donors with non-B, non-C hepatitis, and ranged from 40 to 50 U/l [102-105]. These definitions did not consider that metabolic covariates caused liver injury with slight-to-moderate ALT elevation [3, 106]. Because of the increasing prevalence of patients with metabolic disorders, the clinical significance of ALT values have been recognized. Metabolic covariates have been enrolled when evaluating the ULNs of ALT activity by many scholars in recent decades. Prati et al [107] first raised the concept that subjects with metabolic abnormalities should be excluded in evaluating the ULN of ALT level for the potential risk to the general health. In agreement with Prati et al [107], many scholars have re-evaluated the ULN of ALT values in specific populations, including adults and adolescents [10, 47, 108-123].
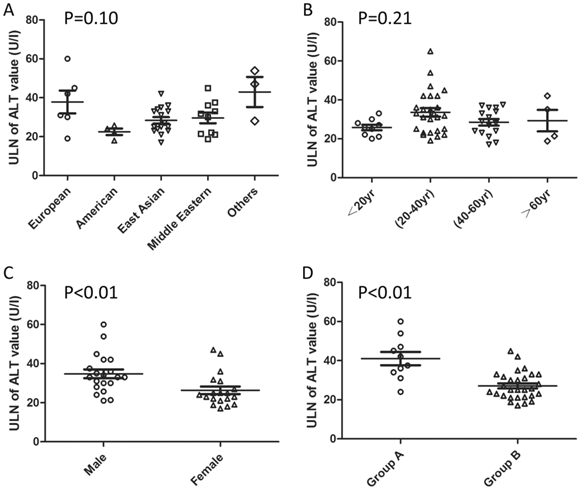
The detailed ULNs of ALT levels are distinct for the intrinsic differences in ethnicity, gender, and age distribution. In addition, the difference in definition of so-called “healthy subjects” and statistical methods might also contribute to the variations in the ULNs. Pacifico et al [15] had summarized the reported ULNs of serum ALT levels in published studies [10, 84, 107-110, 113-118, 121]. This review is incomplete due to omitting some additional references [47, 111, 112, 119, 120, 122, 123] and indirect comparisons. The etiology of ALT elevation can be attributed to viral hepatitis, excessive alcohol consumption, and metabolic disorders, including fatty liver disease [4, 35, 36, 83], in population-based studies. Subjects with the above-mentioned problems should be excluded when evaluating the ULNs of ALT values in a given population. Therefore, we re-summarized the reported ULNs of ALT values by extracting the key exclusion information in various districts as a supplement and presented the information by category (Table 1, Figure 2). As shown in Figure 2, the ULNs of ALT levels in studies excluding the subjects with metabolic abnormalities were greater than those without (41.0±10.8 vs. 27.1±7.0 U/l). About one third decrease on ULN of ALT definition when excluding the subjects with metabolic disorders. The adoption of an updated ULN of ALT activity in a subsequent investigation raised the sensitivity of the diagnosis of potential liver disease with acceptable specificity decrease [47, 107, 110, 114, 117].
Updated Upper Limit of Normal Serum Alanine Aminotransferase Value in Reported Studies.
| Authors, country, year [reference] | Number of enrolled participants(M/F) | Age of enrolled participants [year, mean±SD or mean (age range)] | Exclusion of viral hepatitis (No/Yes) | Exclusion of excessive alcohol consumption (No/Yes) | Exclusion of medication (No/Yes) | Exclusion of metabolic abnormity (No/Yes) | Exclusion of fatty liver disease by imaging tools (No/Yes) | Liver biopsy (No/Yes) | Statistical methods | ULN of ALT value (U/l) |
|---|---|---|---|---|---|---|---|---|---|---|
| Piton et al, France, 1998, [84] | 487/546 | 30±0.36 | Yes | No | No | No | No | No | 95th percentile one sided | BMIa≤23: 42 for men 31 for women BMI>23: 66 for men 44 for women |
| Prati et al, Italy, 2002, [107] | 3865/2970 | 29.8±9.5 | Yes | No | Yes | Yes | No | No | 95th percentile one sided | 30 for men 19 for women |
| Kariv R et al, Israel, 2006, [109] | 6124/11374 | 31.91±17.07 | Yes | No | Yes | Yes | No | No | 95th percentile one sided | 37.5 for all 44.9 for men 31.8 for women |
| Poorten et al, Australia,2007, [110] | 206/0 | 16.8±1.4 | Yes | Yes | Yes | Yes | No | No | 95th percentile one sided | 28 |
| Jamali et al, Iran,2008, [111] | 628/1300 | 40.7±14.7 | Yes | Yes | No | No | No | No | 95th percentile one sided | BMI<25 and non-diabetics: 36.1 for all 37.5 for men, 36 for women BMI>25: 51 for all 59 for men 45.25 for women |
| Kibaya et al, Kenya,2008, [112] | 1020/521 | 30(18-55) | No | No | No | No | No | No | 97.5th percentile one sided | 52 for all 53.9 for men 47 for women |
| Lee et al, Korea, 2010, [108] | 643/462 for pathological normality 346/313 met for Prati criteria | 29.1±9.0 for all 27.2±8.4 for men 31.6±9.3 for women | Yes | Yes | No | Yes | No | Yes | 97.5th percentile one sided | Normal histological liver donor: 35 for men 26 for women Subjects met the Prati criteria: 33 for men 25 for women |
| Schwimmer et al, USA, 2010, [114] | 548/434 | 14.5±1.8 for boys 15.1±1.8 for girls | Yes | Yes | Yes | Yes | No | No | 95th percentile one sided | 25.8 for boys 22.1 for girls |
| Kang et al, Korea,2011, [113] | 704/1041 | 41.8±12.5 | Yes | Yes | Yes | Yes | No | No | 95th percentile one sided | 28 for all 31 for men 23 for women |
| Poustchi et al, IRAN, 2011, [10] | 186/185 | 12.87±3.13 for all 12.58±3 for boys 13.17±3.41 for girls | Yes | Yes | No | Yes | No | No | 95th percentile one sided | 30 for boys 21 for girls |
| Volzke et al, Germany, 2011, [115] | 1180/1423 | 20-79 | Yes | Yes | No | No | Yes | No | 95th percentile one sided | 60 for men 45 for women 20-49 yr: 65 for men and 42 for women 50-79 yr: 42 for men and 35 for women |
| Park et al, Korea,2012,[116] | 880/836 | 14.4 (10-19) | Yes | Yes | No | Yes | No | No | 97.5th percentile one sided | 33 for boys 25 for girls |
| Ruhl et al, USA, 2012, [47] | 1607/2140 | 42.0±16.9 | Yes | Yes | Yes | Yes | No | No | 95th percentile one sided | 24 for men 18 for women |
| Wu et al, Taiwan, 2012, [117] | 1237/1657 | 52.4±13.1 | Yes | No | No | Yes | Yes | No | 95th percentile one sided | 21 for men 17 for women |
| Zheng et al, China, 2012, [118] | 4765/8872 | 35.3(19-44) | Yes | Yes | Yes | Yes | Yes | No | 95th percentile one sided | 35 for men 23 for women |
| Alhamoudi et al, Saudi Arabia,2013, [119] | 41/93 for pathological normality 52/11 for Prati criteria | 28.9±7.3 for all 28.5±7.3 for men 30.0±7.1 for women | Yes | Yes | Yes | Yes | No | Yes | 95th percentile one sided | Normal histological liver donor: 42 for men 31.1 for women Subjects met the Prati criteria: 33 for men 22 for women |
| Kabir et al, Iran,2013, [120] | 688/621 | 61.5 ± 7.9 for men 61.4 ± 7.9 for women | Yes | Yes | Yes | Yes | No | No | 95th percentile one sided | 21.4 for men 18.8 for women |
| Park et al, Korea,2013, [121] | 1355/1961 | 36.3(≥20) | Yes | Yes | Yes | Yes | No | No | 95th percentile one sided | 42 for men 25 for women |
| Sohn et al, Korea,2013, [122] | 297 663/113 577 | 24.6±6.4 for men 22.2±5.2 for women | Yes | Yes | Yes | No | No | No | 95th percentile one sided | 34 for men 24 for women 15-19 yr: 27 for men, 20 for women 20-24 yr: 31 for men, 21 for women 25-29 yr: 35 for men, 22 for women 30-34 yr: 36 for men, 23 for women 35-39 yr: 36 for men, 24 for women 40-44 yr: 34 for men, 28 for women 45-49 yr: 37 for men, 24 for women ≥50 yr:33 for men, 29 for women |
| Tanaka et al, Japan, 2013, [123] | 1462/2046 | 50.6±19.8 for men 48.8±19.4 for women | Yes | No | Yes | Yes | Yes | No | 95th percentile one sided | 36 for men 27 for women |
Abbreviation: ALT: alanine aminotransferase; BMI: body mass index; F: female; M: male; SD: standard deviation; ULN: upper limit of normal; U/l: unit per liter; yr: year.
a: the unit of BMI is kg/m2.
Pooled reported ULN of ALT values by categories. a the others is represented as Australian and African. b Group A includes the studies without exclusion of subjects with metabolic abnormity when evaluating the ULN of ALT value. c Group B includes the studies with exclusion of subjects with metabolic abnormity when evaluating the ULN of ALT value. P-value is based on the comparison of ALT ULN classified by different categories (by Mann-Whitney U test).
Although controversy exists in the definition of the ALT normal range for the debate on the focus of risk-benefit or cost-effectiveness [124], the metabolic disorders should be considered when defining the normal range of the ALT level. And the ULN of ALT level should be re-defined individually with more specific ethnicity to make best use of ALT in related disease detection. In addition, these updated thresholds in the general population should be validated in the follow-up studies to make the best balance between sensitivity and specificity.
7.3 ALT-cardiovascular disease (CVD) association as a plausible issue
Some scholars have summarized the previous studies and referred to the existing controversy on the association between ALT elevation and CVD incidence [125-127]. Some scholars have found a positive link between ALT elevation and CVD-related incidence in their own studies [128-130], while others did not [131-134]. Wang et al [135] attributed the unobserved significant ALT-CVD association to the presence of viral hepatitis infections and alcohol abusers amongst the enrolled participants, in agreement with the opinion of Stefano et al [125]. Otherwise, age, gender distribution, and ethnicity may also contribute to the heterogeneity of the ALT-CVD association in specific populations [99, 127, 136].
Inferior to gamma glutamyltransferase (GGT) [127], the ALT level is not the best indicator to screen for the incidence of CVD events in the general population. More evidence-based studies focused on ALT-CVD association are needed to disclose their inner relationship.
7.4 Key limitation of serum ALT assay in health check-ups
Although available in detecting underlying disease status, some limitation for the ALT assay should be noted. First, the histologic severity of NAFLD does not correlate with the ALT elevation. Patients with non-alcoholic steatohepatitis (NASH) were also observed in patients with normal ALT levels [63]. No significant difference on histologic severity was found between NAFLD patients with or without ALT elevation after matching gender and age of respective subgroups [137]. Second, a significant percentage of liver lesions were observed in patients with viral hepatitis and persistently normal ALT (PNALT). The percentage with corresponding liver fibrosis amongst patients infected with HCV/HBV with PNALT was 16% and 37%, respectively, while the percentage with corresponding cirrhosis was higher in patients infected with HBV (27%) [45,138]. Third, the ALT assay is a continuous variable and even the fluctuation in the normal range also indicates the potential risk for metabolic disorders or cardiovascular disease in a given population [139, 140]. Therefore, clinicians should interpret the sole ALT abnormality carefully, and multi-biomarker evaluation might enhance the diagnostic efficiency further [141].
7.5 What should clinicians do when faced with an ALT elevation
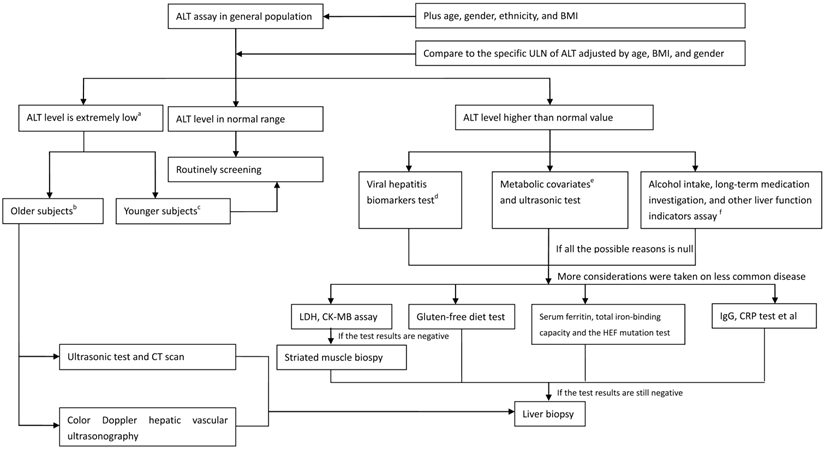
In spite of the limitations referred earlier, what appropriate measure should be taken for a clinician when faced with subjects who have ALT abnormalities? A flow diagram has been created to aid clinicians in treating adults with ALT elevations (Figure 3). When the ALT value is in normal range, annual routing test is recommended. When the ALT level is decreased to an extremely low level, especially in older adults, the accelerated process of aging and frailty, followed by reduced liver size and lowered liver blood flow, is suggested. Therefore, imaging measurements should include ultrasonography and CT scan to evaluate metabolic function of the liver. When the ALT level is elevated and exceeds the normal range, the common cause of ALT elevation, including viral hepatitis indicators, metabolic covariates, alcohol abuse, long-term medication history, and other liver functional indicators, should be investigated. Less common causes of ALT elevation, including hemochromatosis, autoimmune hepatitis, celiac disease, and muscle injury, should be identified when the inspection results referred before are negative. A liver biopsy might be the final instrument when the ALT abnormality cannot be explained. However, the clinicians should carefully evaluate and balance the patients' effect and risk they should afford when a liver biopsy is performed.
Acknowledgements
This work was supported in part by the National Nature Science Foundation of China (NSFC 81072321, 30760243, 30460143 and 30560133), 2009 Program for New Century Excellent Talents in University (NCET), Guangxi Nature Sciences Grant (GuiKeGong 1104003A-7), Guangxi Health Ministry Medicine Grant (Key-Scientific-Research-Grant Z201018), grants from Programs for Changjiang Scholars and Innovative Research Team in University (No.IRT1119) and Innovative Research Team in Guangxi Natural Science Foundation (No. 2011GXNSFF018005). No funding body had any influence or input into the study design, data collection, analysis, data interpretation, or writing of the report.
Strategy diagram of checking the subjects with ALT assay (on the ideal status). a the extremely low ALT value is represented as lower than the median value. b the Older subjects are indicative of the subjects aged > 70 years. c the Younger subjects are indicative of the subjects aged≤70 years. d Viral biomarkers includes the hepatitis B virus surface antigen, and the anti-HCV. e Metabolic covariates includes the BMI, triglyceride, high density lipoprotein cholesterol, glucose, and blood pressure et al expressed as a series of metabolic status.
Conflict of interest
The authors declare no conflict of interest.
References
1. Kim W, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47(4):1363-1370
2. Schwartz MK. [261c] Clinical aspects of aspartate and alanine aminotransferases. Methods in enzymology. 1971;17:866-875
3. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. Canadian medical association journal. 2005;172(3):367-379
4. Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. The American journal of gastroenterology. 2003;98(5):960-967
5. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. The Lancet. 2005;365(9468):1415-1428
6. Yun JE, Kim SY, Kang H-C, Lee SJ, Kimm H, Jee SH. Alanine aminotransferase is associated with metabolic syndrome independently of insulin resistance. Circulation journal: official journal of the Japanese Circulation Society. 2010;75(4):964-969
7. Liu Z, Hu Y, Yang X, Tan A, Gao Y, Qin X, Liang Y, Mo Z, Peng T. Combinative analysis of factors influence serum alanine aminotransferase activity in adult male population from southern China. Clinical biochemistry. 2012;45(18):1683-1688
8. James PT. Obesity: the worldwide epidemic. Clinics in dermatology. 2004;22(4):276-280
9. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. New England Journal of Medicine. 2000;342(17):1266-1271
10. Poustchi H, George J, Esmaili S, Esna-Ashari F, Ardalan G, Sepanlou SG, Alavian SM. Gender differences in healthy ranges for serum alanine aminotransferase levels in adolescence. PloS one. 2011;6(6e):21178
11. Dong MH, Bettencourt R, Barrett-Connor E, Loomba R. Alanine aminotransferase decreases with age: the Rancho Bernardo Study. PloS one. 2010;5(12e):14254
12. Elinav E, Ben-Dov IZ, Ackerman E, Kiderman A, Glikberg F, Shapira Y, Ackerman Z. Correlation between serum alanine aminotransferase activity and age: an inverted U curve pattern. The American journal of gastroenterology. 2005;100(10):2201-2204
13. Dong MH, Bettencourt R, Brenner DA, Barrett-Connor E, Loomba R. Serum levels of alanine aminotransferase decrease with age in longitudinal analysis. Clinical Gastroenterology and Hepatology. 2012;10(3):285-290
14. Córdoba J, O'Riordan K, Dupuis J, Borensztajn J, Blei AT. Diurnal variation of serum alanine transaminase activity in chronic liver disease. Hepatology. 1998;28(6):1724-1725
15. Pacifico L, Ferraro F, Bonci E, Anania C, Romaggioli S, Chiesa C. Upper limit of normal for alanine aminotransferase:< i> Quo vadis</i>? Clinica Chimica Acta. 2013;422:29-39
16. Ruhl CE, Everhart JE. Diurnal Variation in Serum Alanine Aminotransferase Activity in the United States Population. Journal of Clinical Gastroenterology. 2013;47(2):165
17. Sherman KE. Alanine aminotransferase in clinical practice: a review. Archives of internal medicine. 1991;151(2):260
18. Ishiguro M, Takio K, Suzuki M, Oyama R, Matsuzawa T, Titani K. Complete amino acid sequence of human liver cytosolic alanine aminotransferase (GPT) determined by a combination of conventional and mass spectral methods. Biochemistry. 1991;30(43):10451-10457
19. Sohocki MM, Sullivan LS, Harrison WR, Sodergren EJ, Elder FF, Weinstock G, Tanase S, Daiger SP. Human glutamate pyruvate transaminase (GPT): localization to 8q24. 3, cDNA and genomic sequences, and polymorphic sites. Genomics. 1997;40(2):247-252
20. Toney MD. Reaction specificity in pyridoxal phosphate enzymes. Archives of biochemistry and biophysics. 2005;433(1):279-287
21. Yanai I, Benjamin H, Shmoish M, Chalifa-Caspi V, Shklar M, Ophir R, Bar-Even A, Horn-Saban S, Safran M, Domany E. Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics. 2005;21(5):650-659
22. Yang RZ, Park S, Reagan WJ, Goldstein R, Zhong S, Lawton M, Rajamohan F, Qian K, Liu L, Gong DW. Alanine aminotransferase isoenzymes: molecular cloning and quantitative analysis of tissue expression in rats and serum elevation in liver toxicity. Hepatology. 2009;49(2):598-607
23. Flora KD, Keeffe EB. Evaluation of mildly abnormal liver tests in asymptomatic patients. J Insur Med. 1990;22(4):264-267
24. Weibrecht K, Dayno M, Darling C, Bird SB. Liver aminotransferases are elevated with rhabdomyolysis in the absence of significant liver injury. Journal of Medical Toxicology. 2010;6(3):294-300
25. Saengsirisuwan V, Phadungkij S, Pholpramool C. Renal and liver functions and muscle injuries during training and after competition in Thai boxers. British journal of sports medicine. 1998;32(4):304-308
26. Fallon K, Sivyer G, Sivyer K, Dare A. The biochemistry of runners in a 1600 km ultramarathon. British journal of sports medicine. 1999;33(4):264-269
27. Nathwani RA, Pais S, Reynolds TB, Kaplowitz N. Serum alanine aminotransferase in skeletal muscle diseases. Hepatology. 2005;41(2):380-382
28. Noakes T, Carter J. Biochemical parameters in athletes before and after having run 160 kilometres. South African medical journal= Suid-Afrikaanse tydskrif vir geneeskunde. 1976;50(40):1562
29. Edge K, Chinoy H, Cooper R. Serum alanine aminotransferase elevations correlate with serum creatine phosphokinase levels in myositis. Rheumatology. 2006;45(4):487-488
30. Hickman I, Jonsson J, Prins J, Ash S, Purdie D, Clouston A, Powell E. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53(3):413-419
31. BABA CS, ALEXANDER G, KALYANI B, PANDEY R, RASTOGI S, PANDEY A, CHOUDHURI G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. Journal of gastroenterology and hepatology. 2006;21(1):191-198
32. Dufour D. Effects of habitual exercise on routine laboratory tests. CLINICAL CHEMISTRY: 1998: AMER ASSOC CLINICAL CHEMISTRY 2101 L STREET NW, SUITE 202, WASHINGTON, DC 20037-1526 USA. 1998(A) 136-136
33. Mijovic V, Contreras M, Barbara J. Serum alanine aminotransferase (ALT) and gamma-glutamyltransferase (gamma-GT) activities in north London blood donors. Journal of clinical pathology. 1987;40(11):1340-1344
34. Cotler S, Dhamija M, Luc B, Siqueira F, Bartram A, Layden T, Wong S. The prevalence and clinical correlates of elevated ALT levels in an urban Chinatown community. Journal of Viral Hepatitis. 2010;17(2):148-152
35. Pendino GM, Mariano A, Surace P, Caserta CA, Fiorillo MT, Amante A, Bruno S, Mangano C, Polito I, Amato F. Prevalence and etiology of altered liver tests: A population-based survey in a Mediterranean town. Hepatology. 2005;41(5):1151-1159
36. Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, Yeh YH, Yueh SK. Prevalence and etiology of elevated serum alanine aminotransferase level in an adult population in Taiwan. Journal of gastroenterology and hepatology. 2007;22(9):1482-1489
37. Pan J-J, Qu H-Q, Rentfro A, McCormick JB, Fisher-Hoch SP, Fallon MB. Prevalence of metabolic syndrome and risks of abnormal serum alanine aminotransferase in Hispanics: a population-based study. PloS one. 2011;6(6e):21515
38. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA: the journal of the American Medical Association. 2002;287(3):356-359
39. Saito T, Nishise Y, Makino N, Haga H, Ishii R, Okumoto K, Ito J-i, Watanabe H, Saito K, Takeda H. Impact of metabolic syndrome on elevated serum alanine aminotransferase levels in the Japanese population. Metabolism. 2009;58(8):1067-1075
40. Browning JD, Szczepaniak LS, Dobbins R, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387-1395
41. Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nature Reviews Immunology. 2005;5(3):215-229
42. Liaw Y-F, Chu C-M. Hepatitis B virus infection. The Lancet. 2009;373(9663):582-592
43. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45(2):507-539
44. Dienstag JL. Hepatitis B virus infection. New England Journal of Medicine. 2008;359(14):1486-1500
45. Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. Journal of hepatology. 2007;47(6):760-767
46. Alter HJ, Conry-Cantilena C, Melpolder J, Tan D, Van Raden M, Herion D, Lau D, Hoofnagle JH. Hepatitis C in asymptomatic blood donors. Hepatology. 1997;26(S3):29S-33S
47. Ruhl CE, Everhart JE. Upper limits of normal for alanine aminotransferase activity in the United States population. Hepatology. 2012;55(2):447-454
48. Conry-Cantilena C, VanRaden M, Gibble J, Melpolder J, Shakil AO, Viladomiu L, Cheung L, DiBisceglie A, Hoofnagle J, Shih JW. Routes of infection, viremia, and liver disease in blood donors found to have hepatitis C virus infection. New England Journal of Medicine. 1996;334(26):1691-1696
49. Gunji T, Matsuhashi N, Sato H, Iijima K, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Risk factors for serum alanine aminotransferase elevation: A cross-sectional study of healthy adult males in Tokyo, Japan. Digestive and Liver Disease. 2010;42(12):882-887
50. Belfrage P, Berg B, Cronholm T, Elmqvist D, Hägerstrand I, Johansson B, Nilsson-Ehle P, Norden G, Sjövall J, Wiebe T. Prolonged administration of ethanol to young, healthy volunteers: effects on biochemical, morphological and neurophysiological parameters. Acta medica Scandinavica Supplementum. 1973;552:1
51. Wickramasinghe S, Corridan B, Izaguirre J, Hasan R, Marjot D. Ethnic differences in the biological consequences of alcohol abuse: a comparison between south Asian and European males. Alcohol and alcoholism. 1995;30(5):675-680
52. Stewart SH. Racial and Ethnic Differences in Alcohol-Associated Aspartate Aminotransferase and {gamma}-Glutamyltransferase Elevation. Archives of internal medicine. 2002;162(19):2236
53. Alatalo PI, Koivisto HM, Hietala JP, Puukka KS, Bloigu R, Niemelä OJ. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. The American journal of clinical nutrition. 2008;88(4):1097-1103
54. Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clinical Gastroenterology and Hepatology. 2005;3(12):1260-1268
55. Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women. JAMA: the journal of the American Medical Association. 2002;287(19):2559-2562
56. Watkins PB, Kaplowitz N, Slattery JT, Colonese CR, Colucci SV, Stewart PW, Harris SC. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily. JAMA: the journal of the American Medical Association. 2006;296(1):87-93
57. Ford I, Mooijaart SP, Lloyd S, Murray HM, Westendorp RG, de Craen AJ, Packard CJ, Buckley B, Barlow C, Preiss D. The inverse relationship between alanine aminotransferase in the normal range and adverse cardiovascular and non-cardiovascular outcomes. International journal of epidemiology. 2011;40(6):1530-1538
58. Tolman KG. The liver and lovastatin. The American journal of cardiology. 2002;89(12):1374-1380
59. Sniderman AD. Is there value in liver function test and creatine phosphokinase monitoring with statin use? The American journal of cardiology. 2004;94(9):30-34
60. Cohen DE, Anania FA, Chalasani N. An assessment of statin safety by hepatologists. The American journal of cardiology. 2006;97(8):S77-S81
61. Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128(1):24-32
62. Lee K-G, Mitchell A, Shibamoto T. Antioxidative activities of aroma extracts isolated from natural plants. Biofactors. 2000;13(1):173-178
63. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413-1419
64. Angulo P. Nonalcoholic fatty liver disease. New England Journal of Medicine. 2002;346(16):1221-1231
65. Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844-1850
66. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917-923
67. Liu C-M, Tung T-H, Liu J-H, Chen V, Lin C-H, Hsu C-T, Chou P. A community-based epidemiological study of elevated serum alanine aminotransferase levels in Kinmen, Taiwan. World J Gastroenterol. 2005;11(11):1616-1622
68. Liangpunsakul S, Chalasani N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: results from the third National Health and Nutrition Survey (NHANES III). The American journal of the medical sciences. 2005;329(3):111-116
69. Sass DA, Chang P, Chopra KB. Nonalcoholic fatty liver disease: a clinical review. Digestive diseases and sciences. 2005;50(1):171-180
70. Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clinical Gastroenterology and Hepatology. 2004;2(12):1048-1058
71. Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202-1219
72. Powell EE, Cooksley WGE, Hanson R, Searle J, Halliday JW, Powell W. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11(1):74-80
73. Sonsuz A, Basaranoglu M, Ozbay G. Relationship between aminotransferase levels and histopathological findings in patients with nonalcoholic steatohepatitis. The American journal of gastroenterology. 2000;95(5):1370-1371
74. Muri Boberg K. Prevalence and epidemiology of autoimmune hepatitis. Clinics in liver disease. 2002;6(3):635-647
75. Krawitt EL. Autoimmune hepatitis. New England Journal of Medicine. 2006;354(1):54-66
76. Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193-2213
77. Miyake Y, Iwasaki Y, Terada R, Takagi S, Okamaoto R, Ikeda H, Sakai N, Makino Y, Kobashi H, Takaguchi K. Persistent normalization of serum alanine aminotransferase levels improves the prognosis of type 1 autoimmune hepatitis. Journal of hepatology. 2005;43(6):951-957
78. Miyake Y, Iwasaki Y, Terada R, Okamaoto R, Ikeda H, Makino Y, Kobashi H, Takaguchi K, Sakaguchi K, Shiratori Y. Persistent elevation of serum alanine aminotransferase levels leads to poor survival and hepatocellular carcinoma development in type 1 autoimmune hepatitis. Alimentary pharmacology & therapeutics. 2006;24(8):1197-1205
79. Fabbri A, Lenzi M. Non-invasive markers of inflammation in autoimmune hepatitis. Liver International. 2013;33(9):1295-1297
80. Jeong S, Nam H, Rhee J, Shin J, Kim J, Cho K. Metabolic syndrome and ALT: a community study in adult Koreans. International journal of obesity. 2004;28(8):1033-1038
81. Suh S-Y, Choi S-E, Ahn H-Y, Yang H-M, Kim Y-I, Sung N-J. The association between normal alanine aminotransferase levels and the metabolic syndrome: 2005 Korean National Health and Nutrition Examination Survey. Metabolism. 2009;58(12):1731-1736
82. Lorenzo C, Hanley A, Rewers M, Haffner S. The association of alanine aminotransferase within the normal and mildly elevated range with lipoproteins and apolipoproteins: the Insulin Resistance Atherosclerosis Study. Diabetologia. 2013;56(4):746-757
83. Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. The American journal of gastroenterology. 2006;101(1):76-82
84. Piton A, Poynard T, Imbert-Bismut F, Khalil L, Delattre J, Pelissier E, Sansonetti N, Opolon P. Factors associated with serum alanine transaminase activity in healthy subjects: consequences for the definition of normal values, for selection of blood donors, and for patients with chronic hepatitis C. Hepatology. 1998;27(5):1213-1219
85. Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. In: Seminars in liver disease: 2001. 2001:15-22
86. Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. GASTROENTEROLOGY-BALTIMORE THEN PHILADELPHIA-. 2003;124(1):71-79
87. Park SH, Heo NY, Park JH, Kim TO, Yang SY, Moon YS, Kim CH, Suk KT, Kim DJ, Lee HY. Obesity, insulin resistance, and the risk of an elevated alanine aminotransferase activity in the Korean adolescent population. Journal of Pediatric Endocrinology and Metabolism. 2012;25(9-10):945-949
88. Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419
89. Bardella MT, Vecchi M, Conte D, Del Ninno E, Fraquelli M, Pacchetti S, Minola E, Landoni M, Cesana BM, De Franchis R. Chronic unexplained hypertransaminasemia may be caused by occult celiac disease. Hepatology. 1999;29(3):654-657
90. Abdo A, Meddings J, Swain M. Liver abnormalities in celiac disease. Clinical Gastroenterology and Hepatology. 2004;2(2):107-112
91. Hagander B, Berg N, Brandt L, Norden A, Sjölund K, Stenstam M. Hepatic injury in adult coeliac disease. The Lancet. 1977;310(8032):270-272
92. Bardella MT, Fraquelli M, Quatrini M, Molteni N, Bianchi P, Conte D. Prevalence of hypertransaminasemia in adult celiac patients and effect of gluten-free diet. Hepatology. 1995;22(3):833-836
93. Bonamico M, Pitzalis G, Culasso F, Vania A, Monti S, Benedetti C, Mariani P, Signoretti A. Hepatic damage in celiac disease in children]. Minerva pediatrica. 1986;38(21):959
94. Novacek G, Miehsler W, Wrba F, Ferenci P, Penner E, Vogelsang H. Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. European journal of gastroenterology & hepatology. 1999;11(3):283-288
95. Jacobsen M, Fausa O, Elgjo K, Schrumpf E. Hepatic lesions in adult coeliac disease. Scandinavian journal of gastroenterology. 1990;25(7):656-662
96. Janssen G, Kuipers H, Willems G, Does R, Janssen M, Geurten P. Plasma activity of muscle enzymes: quantification of skeletal muscle damage and relationship with metabolic variables. International journal of sports medicine. 1989;10(S 3):S160-S168
97. Powell LW, George DK, McDonnell SM, Kowdley KV. Diagnosis of hemochromatosis. Annals of Internal Medicine. 1998;129(11_Part_2):925-931
98. Grossi E, Colombo R, Cavuto S, Franzini C. Age and gender relationships of serum alanine aminotransferase values in healthy subjects. The American journal of gastroenterology. 2006;101(7):1675-1676
99. Schooling CM, Kelvin EA, Jones HE. Alanine transaminase has opposite associations with death from diabetes and ischemic heart disease in NHANES III. Annals of epidemiology. 2012;22(11):789-798
100. Elinav E, Ackerman Z, Maaravi Y, Ben-Dov IZ, Ein-Mor E, Stessman J. Low Alanine Aminotransferase Activity in Older People Is Associated with Greater Long-Term Mortality. Journal of the American Geriatrics Society. 2006;54(11):1719-1724
101. Liu Z, Ning H, Que S, Wang L, Qin X, Peng T. Complex Association between Alanine Aminotransferase Activity and Mortality in General Population: A Systematic Review and Meta-Analysis of Prospective Studies. PloS one. 2014;9(3e):91410
102. Aach RD, Szmuness W, Mosley JW, Hollinger FB, Kahn RA, Stevens CE, Edwards VM, Werch J. Serum alanine aminotransferase of donors in relation to the risk of non-A, non-B hepatitis in recipients: the transfusion-transmitted viruses study. New England Journal of Medicine. 1981;304(17):989-994
103. Alter HJ, Purcell RH, Holland PV, Alling DW, Koziol DE. Donor transaminase and recipient hepatitis: impact on blood transfusion services. Jama. 1981;246(6):630-634
104. KAHN RA, JOHNSON G, AACH RD, HINES A, ELLIS FR, MILLER WV. The distribution of serum alanine aminotransferase levels in a blood donor population. American journal of epidemiology. 1982;115(6):929-940
105. Siest G, Schiele F, Galteau M-M, Panek E, Steinmetz J, Fagnani F, Gueguen R. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clinical chemistry. 1975;21(8):1077-1087
106. Mathiesen U, Franzen L, Frydén A, Foberg U, Bodemar G. The clinical significance of slightly to moderately increased liver transaminase values in asymptomatic patients. Scandinavian journal of gastroenterology. 1999;34(1):85-91
107. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Annals of Internal Medicine. 2002;137(1):1-10
108. Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51(5):1577-1583
109. Kariv R, Leshno M, Beth-Or A, Strul H, Blendis L, Kokia E, Noff D, Zelber-Sagie S, Sheinberg B, Oren R. Re-evaluation of serum alanine aminotransferase upper normal limit and its modulating factors in a large-scale population study. Liver International. 2006;26(4):445-450
110. Van der Poorten D, Kenny DT, Butler T, George J. Liver disease in adolescents: A cohort study of high-risk individuals. Hepatology. 2007;46(6):1750-1758
111. Jamali R, Pourshams A, Amini S, Deyhim M-R, Rezvan H, Malekzadeh R. The upper normal limit of serum alanine aminotransferase in Golestan Province, northeast Iran. Arch Iran Med. 2008;11(6):602-607
112. Kibaya RS, Bautista CT, Sawe FK, Shaffer DN, Sateren WB, Scott PT, Michael NL, Robb ML, Birx DL, de Souza MS. Reference ranges for the clinical laboratory derived from a rural population in Kericho, Kenya. PloS one. 2008;3(10e):3327
113. Kang HS, Um SH, Seo YS, An H, Lee KG, Hyun JJ, Kim ES, Park SC, Keum B, Kim JH. Healthy range for serum ALT and the clinical significance of “unhealthy” normal ALT levels in the Korean population. Journal of gastroenterology and hepatology. 2011;26(2):292-299
114. Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, Sirlin CB. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138(4):1357-1364 e1352
115. Völzke H, Alte D, Ittermann T, Schmidt CO, Rettig R, Mayerle J, Lowenfels AB, Lerch MM, Nauck M. Subjects with sonographical hepatic steatosis should be excluded from studies to establish upper reference levels of serum transaminases. Liver International. 2011;31(7):985-993
116. Park SH, Park HY, Kang JW, Park J, Shin KJ. Aminotransferase upper reference limits and the prevalence of elevated aminotransferases in the Korean adolescent population. Journal of pediatric gastroenterology and nutrition. 2012;55(6):668-672
117. Wu WC, Wu CY, Wang YJ, Hung HH, Yang HI, Kao WY, Su CW, Wu JC, Chan WL, Lin HC. Updated thresholds for serum alanine aminotransferase level in a large-scale population study composed of 34 346 subjects. Alimentary pharmacology & therapeutics. 2012;36(6):560-568
118. Zheng M-H, Shi K-Q, Fan Y-C, Liu W-Y, Lin X-F, Li L-F, Chen Y-P. Upper limits of normal for serum alanine aminotransferase levels in Chinese Han population. PloS one. 2012;7(9):e43736
119. Al-hamoudi W, Ali S, Hegab B, Elsiesy H, Hashim A, Al-Sofayan M, Khalaf H, Al-Bahili H, Al-Masri N, Al-Sebayel M. Revising the Upper Limit of Normal for Levels of Serum Alanine Aminotransferase in a Middle Eastern Population with Normal Liver Histology. Digestive diseases and sciences. 2013;58(8):2369-2375
120. Kabir A, Pourshams A, Khoshnia M, Malekzadeh F. Normal Limit for Serum Alanine Aminotransferase Level and Distribution of Metabolic Factors in Old Population of Kalaleh, Iran. Hepatitis monthly. 2013 13(10)
121. Park SH, Heo NY, Kim CH, Suk KT, Kim DJ, Lee HY. Upper Reference Limits for Aminotransferase Activities and the Prevalence of Elevated Aminotransferase Activities in a Korean Population. Journal of Clinical Gastroenterology. 2013;47(1):76-82
122. Sohn W, Jun DW, Kwak MJ, Park Q, Lee KN, Lee HL, Lee OY, Yoon BC, Choi HS. Upper limit of normal serum alanine and aspartate aminotransferase levels in Korea. Journal of gastroenterology and hepatology. 2013;28(3):522-529
123. Tanaka K, Hyogo H, Ono M, Takahashi H, Kitajima Y, Ono N, Eguchi T, Fujimoto K, Chayama K, Saibara T. The upper limit of normal serum alanine aminotransferase levels in Japanese subjects. Hepatology Research. 2013
124. Kaplan MM. Alanine aminotransferase levels: what's normal? Annals of internal medicine. 2002;137(1):49-51
125. Bellentani S, Bedogni G, Tiribelli C. Liver and heart: A new link? Journal of hepatology. 2008;49(2):300-302
126. Yilmaz Y. Liver function tests: Association with cardiovascular outcomes. World journal of hepatology. 2010;2(4):143
127. Lioudaki E, S Ganotakis E, P Mikhailidis D. Liver enzymes: potential cardiovascular risk markers? Current pharmaceutical design. 2011;17(33):3632-3643
128. Goessling W, Massaro JM, Vasan RS, D'Agostino Sr RB, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. 2008;135(6):1935
129. Monami M, Balzi D, Lamanna C, Melani C, Cocca C, Lotti E, Fedeli A, Masotti G, Marchionni N, Mannucci E. Prognostic value of serum liver enzymes levels in type 2 diabetic patients. Diabetes/metabolism research and reviews. 2007;23(8):625-630
130. Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, Diamant M. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191(2):391-396
131. Monami M, Bardini G, Lamanna C, Pala L, Cresci B, Francesconi P, Buiatti E, Rotella CM, Mannucci E. Liver enzymes and risk of diabetes and cardiovascular disease: results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism. 2008;57(3):387-392
132. Olynyk JK, Knuiman MW, Divitini ML, Davis TM, Beilby J, Hung J. Serum alanine aminotransferase, metabolic syndrome, and cardiovascular disease in an Australian population. The American journal of gastroenterology. 2009;104(7):1715-1722
133. Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor D. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake analysis of the British women's heart and health study and meta-analysis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(12):2729-2735
134. Emdin M, Passino C, Michelassi C, Donato L, Pompella A, Paolicchi A. Additive prognostic value of gamma-glutamyltransferase in coronary artery disease. International journal of cardiology. 2009;136(1):80-85
135. Wang C-C, Kao J-H. Alanine Aminotransferase, Metabolic Syndrome, and Cardiovascular Disease: A Missing Link&quest. The American journal of gastroenterology. 2010;105(1):224-224
136. Adibi P, Sadeghi M, Mahsa M, Rozati G, Mohseni M. Prediction of coronary atherosclerotic disease with liver transaminase level. Liver International. 2007;27(7):895-900
137. Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, Sterling RK, Shiffman ML, Stravitz RT, Sanyal AJ. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286-1292
138. Kyrlagkitsis I, Portmann B, Smith H, O'Grady J, Cramp ME. Liver histology and progression of fibrosis in individuals with chronic hepatitis C and persistently normal ALT. The American journal of gastroenterology. 2003;98(7):1588-1593
139. Siddiqui MS, Sterling RK, Luketic VA, Puri P, Stravitz RT, Bouneva I, Boyett S, Fuchs M, Sargeant C, Warnick GR. Association Between High-Normal Levels of Alanine Aminotransferase and Risk Factors for Atherogenesis. Gastroenterology. 2013;145(6):1271-1279 e1273
140. Park HK, Hwang JS, Moon JS, Lee JA, Kim DH, Lim JS. Healthy Range of Serum Alanine Aminotransferase and Its Predictive Power for Cardiovascular Risk in Children and Adolescents. Journal of pediatric gastroenterology and nutrition. 2013;56(6):686-691
141. Ozer JS, Chetty R, Kenna G, Palandra J, Zhang Y, Lanevschi A, Koppiker N, Souberbielle BE, Ramaiah SK. Enhancing the utility of alanine aminotransferase as a reference standard biomarker for drug-induced liver injury. Regulatory Toxicology and Pharmacology. 2010;56(3):237-246
Author contact
![]() Corresponding author: Tao Peng, Department of Hepatobiliary Surgery, First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, Guangxi Province, China. E-mail: pengtaocncom Tel: (+86)-771-5350190, Fax: (+86)-771-5350031.
Corresponding author: Tao Peng, Department of Hepatobiliary Surgery, First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, Guangxi Province, China. E-mail: pengtaocncom Tel: (+86)-771-5350190, Fax: (+86)-771-5350031.

 Global reach, higher impact
Global reach, higher impact