3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(8):841-849. doi:10.7150/ijms.8128 This issue Cite
Research Paper
Activation of the PI3K/Akt/mTOR/p70S6K Pathway is Involved in S100A4-induced Viability and Migration in Colorectal Cancer Cells
1. Key Laboratory of Laboratory Medical Diagnostics, Ministry of Education, Department of Laboratory Medicine, Chongqing Medical University, Chongqing 400016, China;
2. Department of Laboratory, the First People's Hospital of Jiulongpo District, Chongqing 400050, China.
* Haiyan Wang and Liang Duan contributed equally to this work.
Received 2013-11-14; Accepted 2014-5-14; Published 2014-6-8
Abstract
The S100 protein family member S100A4 regulates various cellular functions. Previous studies have shown that elevated expression of S100A4 is associated with progression and metastasis of colorectal cancer (CRC). However, little is known about whether and how S100A4 contributes to CRC development. In our present study, the elevated expression of S100A4 in CRC tissues compared to matched adjacent normal tissues was confirmed by immunohistochemistry, semi-quantitative RT-PCR and Western blot. Adenovirus-mediated S100A4 overexpression obviously enhanced viability and migration of CRC cells, which was detected by MTT assay and transwell assay, respectively. Additionally, S100A4 overexpression increased the phosphorylation levels of Akt, mTOR and p70S6K. These effects of S100A4 were abolished by treatment with either the specific PI3K/Akt inhibitor LY294002, or the specific mTOR/p70S6K inhibitor rapamycin. Furthermore, overexpression of S100A4 resulted in upregulation of VEGF and downregulation of E-cadherin, which were strongly reversed by either LY294002 or rapamycin. Altogether, our results demonstrate that activation of the PI3K/Akt/mTOR/p70S6K signaling pathway is involved in S100A4-induced viability, migration, upregulation of VEGF and downregulation of E-cadherin in CRC cells.
Keywords: colorectal cancer, S100A4, PI3K/Akt/mTOR/p70S6K, viability, migration.
Introduction
Colorectal cancer (CRC) is the most predominant malignant digestive tumor worldwide, in which distant metastasis accounts for the leading cause of mortality in CRC patients [1]. In the United States, the 5-year survival rate is about 90% in patients with local tumor, 69% in patients with regional lymph node metastasis, and 12% in patients with distant metastasis [2]. Therefore, there is an urgent need to investigate the pathological mechanism in CRC for developing therapy and prevention strategies against this malignancy.
S100A4, also known as metastasin (Mts1), p9Ka, CAPL, calvasculin, fibroblast-specific protein (FSP1), pEL-98, 18A2, and 42A [3], is a member of the low-molecular calcium binding S100 protein family, which consists of at least 21 members that possess a various biological functions including cell survival, motility, adhesion and migration [4, 5, 6]. Aberrant expression of S100A4 was found in different types of tumors including CRC [7-11], gastric cancer [12], breast cancer [13], pancreatic carcinoma [14] and lung squamous cell carcinoma [15]. Overexpression of S100A4 has been implicated in tumor growth, angiogenesis, epithelial-mesenchymal transition (EMT), extracellular matrix remodeling and metastasis [16, 17]. Thus, S100A4 is assumed to be a marker for poor prognosis and high risk of distant metastasis [3, 7, 8]. However, the precise role and potential molecular mechanism of S100A4 in CRC tumorigenesis still remain to be fully elucidated.
The phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) signaling pathway is associated with multiple cellular functions such as cell proliferation, differentiation and intracellular trafficking, all of which are involved in cancer development. Mammalian target of rapamycin (mTOR), an important downstream target of PI3K/Akt, positively regulates the serine/threonine kinase p70 S6 kinase (p70S6K) and eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1), which constitutes a main pathway for cell proliferation, survival, differentiation and angiogenesis [18, 19]. Previous study has demonstrated that Akt, mTOR and p70S6K are significantly activated in CRC tumor tissues [20-23].
In this study, we investigated the effects of S100A4 on viability and migration in CRC cells and the potential role of the PI3K/Akt/mTOR/p70S6K signaling pathway in these functions of S100A4.
Materials and Methods
Human colorectal cancer tissue samples
Tumor tissues and matched adjacent normal mucosa tissues from five randomly clinical-diagnosed CRC patients were provided by the First Affiliated Hospital of Chongqing Medical University, Chongqing, China. No patient underwent any therapy before surgery. This study was approved by the Ethics Committee of Chongqing Medical University (protocol number 2012-19). All tumor tissue samples were acquired at the time of surgery, immediately frozen in liquid nitrogen and kept at -80˚C.
Cell culture
Human CRC cell lines SW480, LoVo and human embryonic kidney cell line HEK293 were purchased from American Type Culture Collection (Manassas, VA, USA). All cells were cultured in high-glucose Dulbecco's Modified Eagle Medium (Invitrogen, CA, USA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, Saint Louis, MO, USA), 100 U/ml penicillin and 100 μg/ml streptomycin (Sigma-Aldrich, Saint Louis, MO, USA) at 37˚C in a humidified atmosphere of 5% CO2.
Reagents
Adenovirus expressing S100A4 and green fluorescent protein (Ad-S100A4), and adenovirus expressing green fluorescent protein (Ad-GFP) were kindly provided by Dr. Tongchuan He (Medical Center, Chicago University, Chicago, USA). The PI3K/Akt inhibitor LY294002 and the mTOR/p70S6K inhibitor rapamycin were purchased from Sigma-Aldrich (Saint Louis, MO, USA). All antibodies used were: goat anti-S100A4 antibody (Cat#19948, Santa Cruz, CA, USA), mouse anti-β-actin antibody (Cat#47778, Santa Cruz, CA, USA), mouse anti-E-cadherin antibody (Cat#8426, Santa Cruz, CA, USA), rabbit anti-Akt antibody (Cat#4691, Cell Signaling, MA, USA), rabbit anti-p-Akt (Ser473) (Cat#4060, Cell Signaling, MA, USA), rabbit anti-mTOR antibody (Cat#2983, Cell Signaling, MA, USA), rabbit anti-p-mTOR (Ser2448)(Cat#2971, Cell Signaling, MA, USA), rabbit anti-p70S6K antibody (Cat#2708, Cell Signaling, MA, USA), rabbit anti-p-p70S6K (Thr421/Ser424)(Cat#9204, Cell Signaling, MA, USA), rabbit anti-goat IgG (Cat#2306, Zhongshan Golden Bridge, Beijing, China), goat anti-mouse IgG (Cat#2305, Zhongshan Golden Bridge, Beijing, China), goat anti-rabbit IgG (Cat#2301, Zhongshan Golden Bridge, Beijing, China).
Immunohistochemistry
The expression of S100A4 in human CRC tissues and matching adjacent normal mucosa tissues was examined by immunohistochemistry. After fixed with formalin and embedded with paraffin, the sections were deparaffinized in xylene and dehydrated in a gradually decreasing concentrations of ethanol series, then incubated in 10 mM sodium citrate buffer in a microwave oven (500 W) for 20 min. After inactivation of the endogenous peroxidase activity with 3% hydrogen peroxide at room temperature for 10 min, the sections were blocked with 10% normal goat serum for 1 h, followed by incubated with primary S100A4 antibody (1:100) overnight at 4˚C. Subsequently, the sections were washed with phosphate buffered saline (PBS) three times, incubated with biotinylated secondary antibody at 37˚C for 30 min, and visualized with 0.05% 3,3'-diaminobenzidine hydrochloride (DAB). Finally, the sections were counterstained with 10% hematoxylin, and photographs were taken using a microscope with a digital camera.
Semi-quantitative RT-PCR
Total RNA from human CRC tissues was isolated using Trizol Reagent (Invitrogen, CA, USA). RT-PCR was performed by using the TaqMan reverse transcription kit according to the manufacturer's instructions (TaKaRa Biotechnology, Dalian, China) and the T100 Thermal Cycler (Bio-Rad, CA, USA). PCR products were identified by electrophoresis with 1.5% agarose gels and recorded using the Gel Doc 1000 imaging system (Bio-Rad, CA, USA). GAPDH RNA was served as an input control. The following primers were used: S100A4, forward primer: 5'-TCAGAACTAAAGGAGCTGCTGACC-3', reverse primer: 5'-TTTCTTCCTGGGCTGCTTATCTGG-3'; GAPDH, forward primer: 5'-CAGCGACACCCACTCCTC-3', reverse primer: 5'-TGAGGTCCACCACCCTGT-3'.
Western blot
Human CRC tissues and CRC cell lines SW480 and LoVo were collected, and lysed in ice-cold RIPA lysis buffer (Beyotime, Shanghai, China) containing protease inhibitor and phosphatase inhibitor (Roche, Mannheim, Germany). Protein extracts were separated by 6%-15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membranes (Millipore Corporation, MA, USA). The membranes were blocked in TBST containing 5% skimmed milk for 2 h, followed by incubation with primary antibodies (1:1000) overnight at 4˚C. Subsequently, the membranes were washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) for 1 h. After washed with TBST, the interest proteins were detected using enhanced chemiluminescence (Millipore Corporation, MA, USA), followed by exposure on the Gel Doc 1000 Electrophoresis Documentation (Bio-Rad, CA, USA).
Amplification and infection of recombinant adenoviruses
The recombinant adenoviruses Ad-S100A4 and Ad-GFP were amplified in HEK293 cells. Ad-S100A4 was used to increase S100A4 expression in CRC cells while Ad-GFP was used as a negative control.
Cell viability assay
The CRC cells were seeded at a density of 1.5×103 cells per well in 96-well plates and incubated at 37˚C. At 24 h, 48 h, 72 h after infection with adenoviruses, and with or without chemical inhibitors in DMEM containing 1% FBS, 20μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT, Promega, WI, USA) was added to each well. After incubation for 4 h, the culture medium supernatant was removed and the formazan was dissolved with dimethyl sulfoxide for 10 min at room temperature. The spectrophotometric absorbance was measured at 492 nm using a microtiter plate reader (Tecan Sunrise, DE, USA).
Cell migration assay
The cell migration assay was performed with 24-well Millipore transwell chambers (Millipore Corporation, MA, USA). CRC cells (4×104 cells per well) at 24 h after Ad-S100A4 infection, with or without inhibitors, were trypsinized, washed, and suspended in 400 μl of serum-free DMEM and seeded in the upper chamber. The lower chamber was filled with 600 μl of DMEM containing 20% FBS as a chemoattractant. After incubation at 37˚C for 24 h, the migrated cells on the lower surface of membrane were washed with PBS, fixed in 4% paraformaldehyde for 20 min, stained with crystal violet solution (Sigma-Aldrich, Saint Louis, MO, USA). The migrated cells were photographed and counted.
Statistical analysis
All experiments were repeated at least thrice, and the values were expressed as mean ± standard deviation (mean ± SD). All data were analyzed by using SPSS version 15.0 software (SPSS Inc, IL, USA). Statistical significance was evaluated using the independent sample t-test, and a value of P<0.05 was considered to be statistically significant.
Results
Increased S100A4 expression in human CRC tissues
To validate that S100A4 expression is elevated in CRC, we examined the mRNA and protein levels of S100A4 in human CRC and matched adjacent normal mucosa tissues. As expected, elevated mRNA and protein levels of S100A4 were detected in CRC tissues from five randomly clinical-diagnosed CRC patients by semi-quantitative RT-PCR and Western blot, respectively (Fig. 1A, 1B). Elevated S100A4 protein expression was further confirmed by immunohistochemistry, in which S100A4 was mainly detected in the cytoplasm and cell membrane in tumor cells (Fig. 1C). In contrast, S100A4 was undetectable in matched adjacent normal tissues (Fig. 1A, 1B, 1C).
S100A4 overexpression promotes viability and migration in CRC cells
To explore the effects of S100A4 on viability and migration in CRC cells, we first infected two CRC cell lines SW480 and LoVo with Ad-S100A4 for S100A4 overexpression (Fig. 2A). While compared with the Ad-GFP group, S100A4 overexpression strongly increased the viability of SW480 and LoVo cells at 48 h and 72 h (Fig. 2B). Twenty-four hour after viral infection, the numbers of migrated SW480 or LoVo cells in S100A4 overexpression groups were much more than that in the GFP groups (Fig. 2C).
S100A4 expression is increased in human CRC tissues. (A) S100A4 mRNA in human CRC tissues and matched adjacent normal mucosa tissues from five randomly clinical-diagnosed colorectal cancer patients was detected by semi-quantitative RT-PCR. N, matched adjacent normal mucosa tissues; T, tumor tissues. (B) S100A4 Protein was detected by Western blot. N, matched adjacent normal mucosa tissues; T, tumor tissues. (C) S100A4 expression was detected by immunohistochemistry. Representative images are shown. The black arrow showed normal glandular epithelial cells (upper) and the red arrow showed tumor glandular epithelial cells (bottom), ×400.
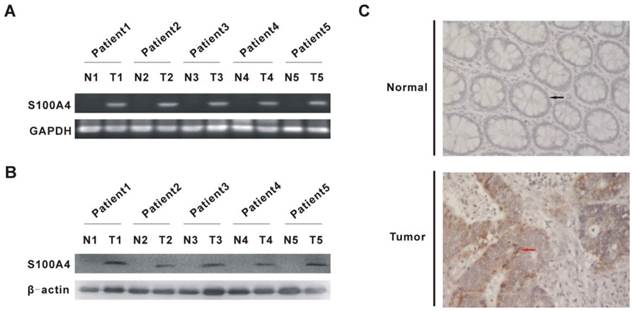
S100A4 promotes viability and migration in CRC cells. (A) Increased protein levels of S100A4 in SW480 and LoVo cells after infection with Ad-S100A4 were detected by Western blot. **P<0.01 vs GFP group. (B) Effects of S100A4 overexpression on viability of SW480 and LoVo cells by MTT assay. *P<0.05 vs GFP group. (C) Effects of S100A4 overexpression on migration of SW480 and LoVo cells by transwell migration assay, ×200. *P<0.05, **P<0.01 vs GFP group.
S100A4 activates the PI3K/Akt/mTOR/p70S6K signaling pathway in CRC cells
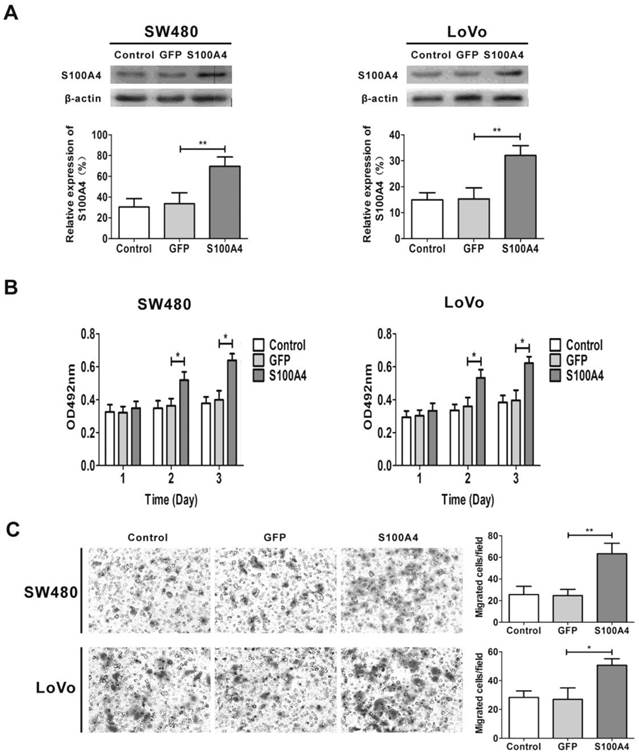
To explore the signaling pathways involved in S100A4-induced viability and migration in CRC cells, we focused on the PI3K/Akt signaling pathway that plays a critical role in cancer cell viability, migration and cancer progression. Interestingly, S100A4 overexpression enhanced phosphorylation levels of Akt, mTOR and p70S6K in SW480 and LoVo cells. However, S100A4 had no significant effect on total protein levels of Akt, mTOR and p70S6K (Fig. 3A). Furthermore, the S100A4-induced phosphorylation levels of Akt, mTOR and p70S6K were suppressed by LY294002 (10μM), while rapamycin (100nM) only reduced the phosphorylation levels of mTOR and p70S6K (Fig. 3B).
S100A4 induces cell viability and migration via the PI3K/Akt/mTOR/p70S6K signaling pathway
Because S100A4 induced viability and migration of CRC cells and activated the PI3K/Akt/mTOR/p70S6K signaling pathway, we proceeded to examine whether the activation of the PI3K/Akt/mTOR/p70S6K signaling pathway is involved in S100A4-induced cell viability and migration in CRC cells. Ad-S100A4-infected SW480 and LoVo cells were treated with the LY294002 or rapamycin, and then cell viability and migration were measured. We found that both LY294002 and rapamycin significantly suppressed S100A4-induced cell viability and migration (Fig. 4A, 4B).
S100A4 regulates expression of VEGF and E-cadherin via the PI3K/Akt/mTOR/p70S6K signaling pathway
Previous studies showed that the expression of VEGF and E-cadherin was regulated by the PI3K/Akt/mTOR signaling pathway, and VEGF and E-cadherin are involved in viability and migration of various tumor cells [44, 45, 47]. We next examined the effect of S100A4 on the expression of VEGF and E-cadherin. The results showed that S100A4 overexpression obviously increased VEGF expression and decreased E-cadherin expression in SW480 and LoVo cells (Fig. 5A), which were abolished by treatment with either LY294002 or rapamycin (Fig. 5B).
S100A4 activates the PI3K/Akt/mTOR/p70S6K pathway in CRC cells. (A) Increased phosphorylation levels of Akt, mTOR, p70S6K in Ad-S100A4-infected SW480 and LoVo cells were detected by Western blot. (B) Effects of LY294002 and rapamycin on S100A4-induced activation of Akt, mTOR, p70S6K in SW480 and LoVo cells.
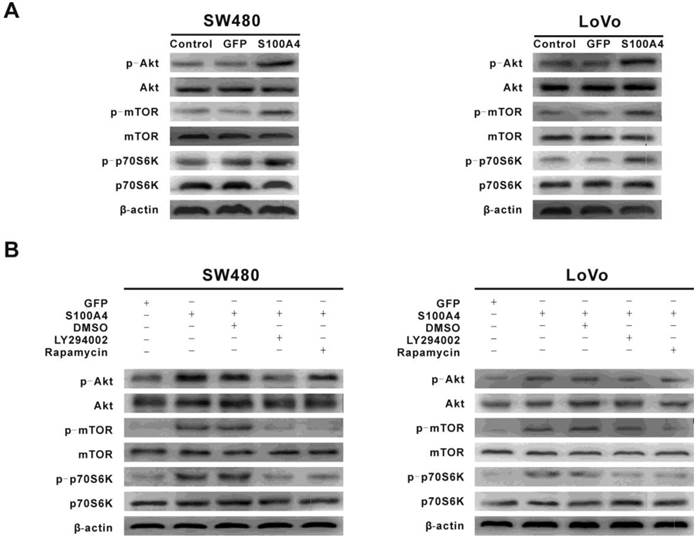
LY294002 and rapamycin suppress S100A4-induced viability and migration of CRC cells. (A) Effects of LY294002 and rapamycin on S100A4-induced viability of SW480 and LoVo cells were detected by MTT assay. *P<0.05 vs S100A4 group. (B) Effects of LY294002 and rapamycin on S100A4-induced migration of SW480 and LoVo cells were detected by transwell assay, ×200. *P<0.05, **P<0.01 vs S100A4 group.
Discussion
In this study, we confirmed that S100A4 expression is upregulated in CRC tissues and that S100A4 overexpression promotes viability and migration of CRC cells. In addition, S100A4 induced upregulation of VEGF expression and downregulation of E-cadherin expression. We also provided evidence showing that activation of the PI3K/Akt/mTOR/p70S6K signaling pathway is involved in S100A4-induced cell viability, migration, and VEGF upregulation and E-cadherin downregulation in CRC cells. These results suggest that activation of the PI3K/Akt/mTOR/p70S6K signaling pathway is involved in the oncogenic property of S100A4.
Over the past decades, molecular genetic studies have revealed gene alterations including a number of well-known oncogenes and tumor suppressors underlying the pathogenesis mechanism of CRC [24, 25]. Substantial clinical evidences have implied S100A4 as an important gene associated with CRC progression, metastasis, and survival [7, 8, 10]. Consistent with previous reports, mRNA and protein expression of S100A4 in human CRC tissues were obviously increased. Accumulating evidences suggest that S100A4 modulates a wide range of cellular processes ranging from angiogenesis to cell motility, adhesion, migration, and invasion [9]. For instance, S100A4 regulates the interaction between myosin and actin to impact rearrangement of the cytoskeleton [26]. Several studies have reported that S100A4 contributes to cell invasion and angiogenesis through upregulation of matrix metalloproteinases (MMPs) [27-29]. Additionally, downregulation of S100A4 expression in colorectal cancer cells suppressed cell growth, invasion and metastasis formation [30, 31]. In agreement with these studies, our data showed that CRC cells SW480 and LoVo with S100A4 overexpression had significantly increased viability and migration.
Regulation of VEGF and E-cadherin expression by S100A4 involves the PI3K/Akt/mTOR/p70S6K pathway in CRC cells. (A) Effects of S100A4 on protein levels of VEGF and E-cadherin in SW480 and LoVo cells were detected by Western blot. *P<0.05, **P<0.01 vs GFP group. (B) Effects of LY294002 and rapamycin on S100A4-induced protein levels of VEGF and E-cadherin in SW480 and LoVo cells were detected by Western blot.
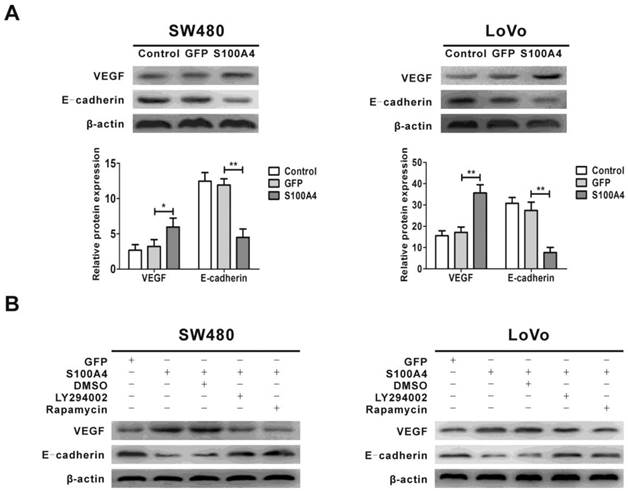
It is well known that vascular endothelial growth factor (VEGF) is one of the most potent endothelial cell mitogens [32]. It not only increases formation of vascular capillary lumens, but also promotes cancer cell proliferation, invasion, and subsequent metastasis [33]. Previous studies have shown the overexpression of VEGF in human CRC, and VEGF could be a prominent angiogenic factor for induction of angiogenesis during CRC development [34, 35]. In transgenic mice, S100A4 has been shown to be a potent stimulator of angiogenesis [36]. In gastric carcinoma, positive correlation between expression of S100A4 and VEGF was found, which was associated with lymph node metastasis and prognosis [37]. S100A4 silencing suppressed proliferation, angiogenesis and invasion in thyroid cancer cells, which was associated with downregulation of VEGF [38]. High expression of S100A4 and VEGF were concurrently detected in colorectal cancer [39]. Our data showed that VEGF was upregulated by S100A4 overexpression in CRC cells through activation of the PI3K/Akt/mTOR/p70S6K pathway. On the other hand, VEGF is recognized as a potent PI3K/Akt activating factor [40]. While how S100A4 activates the PI3K/Akt/mTOR/p70S6K pathway is currently unclear, it is interesting to explore the possibility in future study that a VEGF autocrine establishes a positive feedback loop for S100A4-induced activation of this pathway and CRC cell viability and migration.
A possible mechanism by which S100A4 induces migration of cancer cells is to inhibit cell adhesion [41]. E-cadherin plays an important role in cell adhesion [42]. It has been reported that S100A4-mediated esophageal squamous cell invasion and metastasis involving E-cadherin downregulation [43]. Increased S100A4 expression combined with decreased E-cadherin expression was associated with tumor progression, distant metastasis and survival in CRC [44]. In the present study, we found that S100A4 reduced E-cadherin expression in CRC cells SW480 and LoVo cells. Downregulation of E-cadherin expression induced by S100A4 may inhibit intercellular adhesion, thus contributing to tumor cells' migration phenotype [45].
The PI3K/Akt signaling pathway plays a pivotal role in cell proliferation, apoptosis, survival, migration, invasion and metastasis [46]. A number of studies suggest that aberrant activation of the PI3K/Akt signaling pathway is associated with human CRC [47, 48]. The mTOR kinase lies downstream of the PI3K/Akt signaling pathway and facilitates phosphorylation of p70S6K and 4E-BP1 [49]. Previous findings demonstrated frequent changes of the PI3K/Akt/mTOR pathway in human CRC [50, 51]. The PI3K/Akt/mTOR pathway regulates VEGF expression in different tumors for angiogenesis [52, 53]. In human esophageal squamous cells S100A4 regulates E-cadherin expression through Akt [54]. In this report, we found that the phosphorylation levels of Akt, mTOR and p70S6K were activated by S100A4 overexpression and suppressed by the PI3K/Akt inhibitor LY294002 in SW480 and LoVo cells. The mTOR/p70S6K inhibitor rapamycin only inhibited the phosphorylation levels of mTOR and p70S6K. Moreover, inhibitor of PI3K or mTOR activity blocked S100A4-induced viability, migration, VEGF upregulation and E-cadherin downregulation of CRC cells. These results establish that S100A4 induces viability and migration in CRC cells through the PI3K/Akt/mTOR/p70S6K signaling pathway.
Altogether, our results suggest that activation of the PI3K/Akt/mTOR/p70S6K pathway contributes to S100A4-induced viability and migration in CRC cells. Therefore, this S100A4-mediated colorectal carcinogenesis signaling pathway could be a therapeutic and preventive target against CRC.
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 30772548), and the 973 Program of the Ministry of Science and Technology of China (No. 2011CB707906). We thank Dr. Tongchuan He for the Ad-GFP and Ad-S100A4 recombinant adenoviruses.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Stein U, Schlag PM. Clinical, biological, and molecular aspects of metastasis in colorectal cancer. Recent Results Cancer Res. 2007;176:61-80
2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29
3. Garrett SC, Varney KM, Weber DJ. et al. S100A4, a mediator of metastasis. J Biol Chem. 2006;281:677-80
4. Takenaga K, Nakamura Y, Endo H. et al. Involvement of S100-related calcium-binding protein pEL98 (or mts1) in cell motility and tumor cell invasion. Jpn J Cancer Res. 1994;85:831-9
5. 5. Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637-68
6. 6. Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540-51
7. 7. Kang YG, Jung CK, Lee A, et al. Prognostic significance of S100A4 mRNA and protein expression in colorectal cancer. Surg Oncol. 2012;105:119-24
8. 8. Huang LY, Xu Y, Cai GX, et al. S100A4 over-expression underlies lymph node metastasis and poor prognosis in colorectal cancer. World J Gastroenterol. 2011;17:69-78
9. 9. Boye K, Nesland JM, Sandstad B, et al. Nuclear S100A4 is a novel prognostic marker in colorectal cancer. Eur J Cancer. 2010;46:2919-25
10. 10. Kwak JM, Lee HJ, Kim SH, et al. Expression of protein S100A4 is a predictor of recurrence in colorectal cancer. World J Gastroenterol. 2010;16:3897-904
11. 11. Hemandas AK, Salto-Tellez M, Maricar SH, et al. Metastasis-associated protein S100A4--a potential prognostic marker for colorectal cancer. J Surg Oncol. 2006;93:498-503
12. 12. Wang YY, Ye ZY, Zhao ZS, et al. High-level expression of S100A4 correlates with lymph node metastasis and poor prognosis in patients with gastric cancer. Ann Surg Oncol. 2010;17:89-97
13. 13. de Silva Rudland S, Martin L, Roshanlall C, et al. Association of S100A4 and osteopontin with specific prognostic factors and survival of patients with minimally invasive breast cancer. Clin Cancer Res. 2006;12:1192-200
14. 14. Ikenaga N, Ohuchida K, Mizumoto K, et al. S100A4 mRNA is a diagnostic and prognostic marker in pancreatic carcinoma. J Gastrointest Surg. 2009;13:1852-58
15. 15. Tsuna M, Kageyama S, Fukuoka J, et al. Significance of S100A4 as a prognostic marker of lung squamous cell carcinoma. Anticancer Res. 2009;29:2547-54
16. 16. Schneider M, Hansen JL, Sheikh SP. S100A4: a common mediator of epithelial-mesenchymal transition, fibrosis and regeneration in diseases. J Mol Med. 2008;86:507-22
17. 17. Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528-35
18. 18. Li W, Tan D, Zhang Z, et al. Activation of Akt-mTOR-p70S6K pathway in angiogenesis in hepatocellular carcinoma. Oncol Rep. 2008;20:713-9
19. 19. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9-22
20. 20. Johnson SM, Gulhati P, Rampy BA, et al. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J Am Coll Surg. 2010;210:767-76
21. 21. Baba Y, Nosho K, Shima K, et al. Phosphorylated AKT expression is associated with PIK3CA mutation, low stage and favorable outcome in 717 colorectal cancers. Cancer. 2011;117:1399-408
22. 22. Wang D, Chen J, Guo FJ, et al. Clinical significance of mTOR and p-mTOR protein expression in human colorectal carcinomas. Asian Pacific J Cancer Prev. 2011;12:2581-4
23. 23. Nozawa H, Watanabe T, Nagawa H. Phosphorylation of ribosomal p70 S6 kinase and rapamycin sensitivity in human colorectal cancer. Cancer Letters. 2007;251:105-13
24. 24. Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449-60
25. 25. Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479-507
26. 26. Li ZH, Bresnick AR. The S100A4 metastasis factor regulates cellular motility via a direct interaction with myosin-IIA. Cancer Res. 2006;66:5173-80
27. 27. Mathisen B, Lindstad RI, Hansen J, et al. S100A4 regulates membrane induced activation of matrix metalloproteinase-2 in osteosarcoma cells. Clin Exp Metastasis. 2003;20:701-11
28. 28. Saleem M, Kweon MH, Johnson JJ, et al. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci USA. 2006;103:14825-30
29. 29. Yammani RR, Carlson CS, Bresnick AR, et al. Increase in production of matrix metalloproteinase 13 by human articular chondrocytes due to stimulation with S100A4: role of the receptor for advanced glycation end products. Arthritis Rheum. 2006;54:2901-11
30. 30. Dahlmann M, Sack U, Herrmann P, et al. Systemic shRNA mediated knock down of S100A4 in colorectal cancer xenografted mice reduces metastasis formation. Oncotarget. 2012;3:783-97
31. 31. Huang L, Xu Y, Cai G, et al. Downregulation of S100A4 expression by RNA interference suppresses cell growth and invasion in human colorectal cancer cells. Oncol Rep. 2012;27:917-22
32. 32. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-57
33. 33. Dallas NA, Fan F, Gray MJ, et al. Functional significance of vascular endothelial growth factor receptors on gastrointestinal cancer cells. Cancer Metastasis Rev. 2007;26:433-41
34. 34. Garcia V, Garcia JM, Silva J, et al. Levels of VEGF-A mRNA in plasma from patients with colorectal carcinoma as possible surrogate marker of angiogenesis. J Cancer Res Clin Oncol. 2008;134:1165-71
35. 35. Diaz-Rubio E, Schmoll HJ. Introduction. Critical role of anti-angiogenesis and VEGF inhibition in colorectal cancer. Oncology. 2005;69:1-3
36. 36. Semov A, Moreno MJ, Onichtchenko A, et al. Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem. 2005;280:20833-41
37. 37. Feng LZ, Zheng XY, Zhou LX, et al. Correlation between expression of S100A4 and VEGF-C, and lymph node metastasis and prognosis in gastric carcinoma. J Int Med Res. 2011;39:1333-43
38. 38. Jia W, Gao XJ, Zhang ZD, et al. S100A4 silencing suppresses proliferation, angiogenesis and invasion of thyroid cancer cells through downregulation of MMP-9 and VEGF. Eur Rev Med Pharmacol Sci. 2013;17:1495-508
39. 39. Roh SA, Choi EY, Cho DH, et al. Growth and invasion of sporadic colorectal adenocarcinomas in terms of genetic change. J Korean Med Sci. 2010;25:353-60
40. 40. Ruan GX, Kazlauskas A. VEGF-A engages at least three tyrosine kinases to activate PI3K/Akt. Cell Cycle. 2012;11:2047-8
41. 41. Sherbet GV, Lakshmi MS. S100A4 (MTS1) calcium binding protein in cancer growth, invasion and metastasis. Anticancer Res. 1998;18:2415-21
42. 42. Andersen H, Mejlvang J, Mahmood S, et al. Immediate and delayed effects of E-cadherin inhibition on gene regulation and cell motility in human epidermoid carcinoma cells. Mol Cell Biol. 2005;25:9138-50
43. 43. Zhang HY, Zheng XZ, Wang XH, et al. S100A4 mediated cell invasion and metastasis of esophageal squamous cell carcinoma via the regulation of MMP-2 and E-cadherin activity. Mol Biol Rep. 2012;39:199-208
44. 44. Lee SJ, Choi SY, Kim WJ, et al. Combined aberrant expression of E-cadherin and S100A4, but not beta-catenin is associated with disease-free survival and overall survival in colorectal cancer patients. Diagn Pathol. 2013;8:99-121
45. 45. Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987-91
46. 46. Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501
47. 47. Parsons DW, Wang TL, Samuels Y, et al. Colorectal cancer: mutations in a signaling pathway. Nature. 2005;436:792
48. 48. Rychahou PG, Jackson LN, Silva SR, et al. Targeted molecular therapy of the PI3K pathway: therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833-42
49. 49. Ollikainen M, Gylling A, Puputti M, et al. Patterns of PIK3CA alterations in familial colorectal and endometrial carcinoma. Int J Cancer. 2007;121:915-20
50. 50. Ekstrand AI, Jonsson M, Lindblom A, et al. Frequent alterations of the PI3K/AKT/mTOR pathways in hereditary nonpolyposis colorectal cancer. Fam Cancer. 2010;9:125-9
51. 51. Frost P, Shi Y, Hoang B, et al. AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells. Oncogene. 2007;26:2255-62
52. 52. Xie SR, Wang Y, Liu CW, et al. Liquiritigenin inhibits serum-induced HIF-1alpha and VEGF expression via the AKT/mTOR-p70S6K signaling pathway in Hela cells. Phytother Res. 2012;26:1133-41
53. 53. Zhang K, Zhang M, Zhao H, et al. S100A4 regulates motility and invasiveness of human esophageal squamous cell carcinoma through modulating the AKT/Slug signal pathway. Dis Esophagus. 2012;25:731-9
54. 54. Lau MT, Leung PC. The PI3K/Akt/mTOR signaling pathway mediates insulin-like growth factor 1-induced E-cadherin down-regulation and cell proliferation in ovarian cancer cells. Cancer Lett. 2012;326:191-8
Author contact
![]() Corresponding author: Dr. Lan Zhou, Department of Laboratory Medicine, Chongqing Medical University, Yixueyuan Road, Chongqing 400016, China. Tel: +86 023-68485992 Fax: +86 023-68485239 E-mail: zhoulanedu.cn
Corresponding author: Dr. Lan Zhou, Department of Laboratory Medicine, Chongqing Medical University, Yixueyuan Road, Chongqing 400016, China. Tel: +86 023-68485992 Fax: +86 023-68485239 E-mail: zhoulanedu.cn

 Global reach, higher impact
Global reach, higher impact