Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(8):758-764. doi:10.7150/ijms.8919 This issue Cite
Research Paper
Association between Plasma Adiponectin Levels and Decline in Forced Expiratory Volume in 1 s in a General Japanese Population: The Takahata Study
1. Department of Cardiology, Pulmonology, and Nephrology;
2. Global Center of Excellence Program Study Group, Yamagata University School of Medicine, 2-2-2 Iida-Nishi Yamagata 990-9585, Japan.
Received 2014-2-24; Accepted 2014-5-2; Published 2014-5-21
Abstract
Background: Adiponectin is an anti-inflammatory and cardio-protective cytokine. However, several studies have demonstrated that plasma adiponectin levels were inversely associated with pulmonary function in patients with chronic obstructive pulmonary disease, suggesting a proinflammatory or pulmonary-destructive role. It is still unclear whether adiponectin is a potent biomarker predicting declines in pulmonary function. The aim of this study was to investigate the association between adiponectin and pulmonary function among Japanese individuals who participated in an annual health check-up.
Methods: Spirometry and blood sampling, including measurements of plasma adiponectin, were performed for 3,253 subjects aged 40 years or older who participated in a community-based annual health check-up in Takahata, Japan from 2004 to 2006. In 2011, spirometry was re-performed, and the data from 872 subjects (405 men and 467 women) were available for a longitudinal analysis.
Results: Plasma adiponectin levels were found to be significantly associated with age, body mass index (BMI), and alanine aminotransferase (ALT), triglycerides (TG), and high-density lipoprotein-cholesterol (HDL-c) levels among both men and women in the study population. Plasma adiponectin levels were found to be associated with lifetime cigarette consumption (Brinkman index, BI) in men only. Plasma adiponectin levels were inversely correlated with forced expiratory volume in 1 s (FEV1) per forced vital capacity in both men and women. In addition, the annual change in FEV1 was inversely associated with plasma adiponectin levels in both genders. A multiple linear regression analysis revealed that this association was independent of other confounding factors such as age, BMI, BI, ALT, TG, and HDL-c.
Conclusions: The results of the present study suggest that adiponectin levels are predictive of declines in FEV1 in the general population.
Keywords: Adiponectin, Decline in FEV1, General population, Pulmonary function, Spirometry.
Introduction
Long-term cigarette smoking induces many pulmonary diseases. Chronic obstructive pulmonary disease (COPD) is one of the most common respiratory disorders in the elderly population [1]. Given the tremendous impact of COPD on death and chronic disability, the prevention of COPD is particularly important. COPD is thought to involve chronic inflammation not only in the respiratory system, but also in the rest of the body resulting from the spread of inflammatory mediators from the lungs [2].
Metabolic syndrome is a predominant comorbidity of COPD [3-7]. Adipocytokines, including adiponectin, play a significant role in the pathogenesis of metabolic syndrome [8], and were noted as factors contributing to COPD. Tomoda et al. demonstrated an increase in plasma adiponectin levels in underweight COPD patients [9]. Recently, it was demonstrated that higher adiponectin levels were associated with a higher risk of all-cause mortality in COPD patients [10]. In addition, the Lung Health Study analysis demonstrated that adiponectin is a complex biomarker in COPD that is associated with a decreased risk of cardiovascular events, but an increased risk of respiratory mortality [11]. These results suggest that adiponectin plays a proinflammatory or pulmonary-destructive role in the pathogenesis of COPD.
However, studies using adiponectin gene-targeted mice have yielded controversial results. Two studies reported that adiponectin-null mice developed a COPD-like phenotype [12, 13]. In contrast, others have demonstrated that adiponectin knockout mice were protected from the development of emphysema induced by long-term exposure to cigarette smoke [14]. These contrasting results from animal studies complicate the interpretation of the role of adiponectin. Thus, whether adiponectin plays a pulmonary-protective or pulmonary-destructive role in the pathogenesis of COPD remains unclear.
To date, the relationship between plasma adiponectin levels and pulmonary function has not been demonstrated in a general population. This study aimed to investigate this relationship in order to determine the role of adiponectin in the lungs.
Methods
Study population
This study formed part of the Molecular Epidemiological Study of the Regional Characteristics of the 21st Century Centers of Excellence (COE) Program and the Global Centers of Excellence Program in Japan [3, 15-22]. The study was approved by the Ethics Committee of Yamagata University School of Medicine, and all participants provided their written informed consent.
The study used data from an annual community health check-up in which all residents of Takahata, a town in northern Japan, aged 40 years or older were invited to participate. From 2004 to 2006, 1,579 men and 1,941 women (total, 3,520 subjects) enrolled in the study and underwent initial spirometric examinations. We excluded 267 subjects from the analysis because their spirometry data did not meet the specified criteria. The data from 3,253 subjects (1,500 men and 1,753 women) were utilized in the final statistical analysis. The subjects' medical histories, smoking habits, current use of medications, and clinical symptoms were documented using a self-reported questionnaire. The lifetime consumption of cigarettes was expressed as the Brinkman index (BI: number of cigarettes per day × years of smoking) [3, 19]. In 2011, spirometry was re-performed on 873 subjects. After the exclusion of 1 subject resulting from an error in spirometry measurements, the data from 872 subjects (405 men and 467 women) were used for a longitudinal analysis.
Measurements
Fasting blood samples were taken from the antecubital vein of the subjects, and immediately transferred to chilled tubes. Plasma adiponectin levels were measured by using an enzyme immunoassay (Otsuka Pharmaceutical Co., Tokyo, Japan) [23].
Spirometric parameters (FVC and FEV1) were measured using standard techniques, with subjects performing FVC maneuvers on a CHESTAC-25 part II EX instrument (Chest Corp., Tokyo, Japan) according to the guidelines of the Japanese Respiratory Society (JRS) [24]. A bronchodilator was not administered prior to spirometry. The highest value of at least 3 FVC maneuvers by each subject was used for the analysis. The results were assessed by 2 pulmonary physicians, who visually inspected the flow-volume curves and excluded subjects with inadequate data as defined by the JRS criteria [24]. Subjects' pulmonary functions were classified as follows: normal, %FVC ≥ 80 and FEV1/FVC ≥ 0.7; restrictive, %FVC < 80 and FEV1/FVC ≥ 0.7; obstructive, %FVC ≥ 80 and FEV1/FVC < 0.7; and mixed disorder, %FVC < 80 and FEV1/FVC < 0.7. The annual change in FEV1 (%/year) was calculated as ([value of 2011 spirometry - value of initial spirometry]/value of initial spirometry) × 100/ time between observations (years).
Statistical analyses
For continuous variables, data are presented as the mean and standard deviation (SD). For multiple comparisons, a 1-way analysis of variance was used along with Tukey's test. Correlations between 2 variables were evaluated using Pearson's product moment correlation coefficient. A multiple linear regression analysis was performed to determine whether plasma adiponectin levels contributed to annual changes in FEV1 after adjustment for all other variables included in the model. Statistical significance was inferred for 2-sided P-values < 0.05. All statistical analyses were performed using JMP version 8 software (SAS Institute Inc., Cary, NC, USA).
Results
The median (interquartile range) of plasma adiponectin levels was 7.1 µg/mL (5.2, 10.0) in men and 10.5 μg/mL (7.5, 15.0) in women. Table 1 shows the characteristics of the subjects according to their adiponectin level quartile. Age and high-density lipoprotein-cholesterol (HDL-c) increased in accordance with increased adiponectin level quartile in both men and women. In contrast, body mass index (BMI), alanine aminotransferase (ALT), triglycerides (TG), and FEV1/FVC decreased with increasing adiponectin level quartile in both men and women. BI decreased with increases in adiponectin level only in men. FVC% predicted and FEV1% predicted in both men and women and BI in women remained unchanged with increases in adiponectin levels.
Table 2 shows the mean plasma adiponectin levels according to the spirometric classifications (i.e., normal, restrictive, obstructive, and mixed ventilatory disorders). Plasma adiponectin levels in patients with obstructive disorders were significantly higher than those in normal subjects (Tables 2A and B). The plasma adiponectin levels in women with obstructive disorders were higher than in those with restrictive disorders (Table 2B).
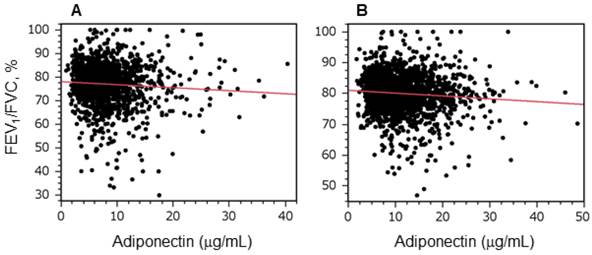
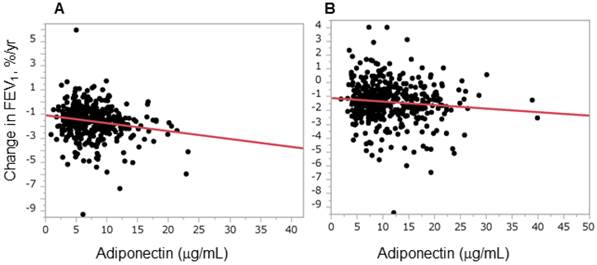
Figure 1 shows the relationship between plasma adiponectin levels and FEV1/FVC in men (Figure 1A) and women (Figure 1B). There was a significant inverse relationship between adiponectin levels and FEV1/FVC in both men and women. Because the degree of airflow obstruction is usually evaluated by FEV1, we evaluated whether adiponectin is predictive for airflow obstruction progression in a general population by assessing the relationship between plasma adiponectin levels and longitudinal changes in FEV1. An inverse relationship was observed in both men and women (Figure 2). A multiple linear regression analysis revealed that adiponectin was a significant determinant of annual changes in FEV1 in both men and women, and independent of other confounding factors such as age, BMI, BI, ALT, TG, and HDL-c (Table 3). Next, the relationships between adiponectin and annual changes in FEV1 were assessed separately according to smoking status. As shown in Table 4, this relation was significant in men who had never smoked and in current smokers, but not in men who were ex-smokers. In women, this relation did not reach statistical significance for any smoking status, although there was a trend that higher adiponectin levels were associated with a greater decline in FEV1 in women who had never smoked (P = 0.0594, women who never smoked, Table 4).
Characteristics of the subjects according to adiponectin level quartile.
| Men | |||||
| Q1 (n = 390) | Q2 (n = 390) | Q3 (n = 394) | Q4 (n = 398) | P-value | |
| Age, years | 59.2 (9.9) | 61.5 (9.9)* | 62.9 (10.2)* | 67.9 (9.8)*#¶ | < 0.0001 |
| BMI, kg/m2 | 24.5 (2.7) | 23.9 (2.9)* | 23.4 (2.8)* | 22.1 (2.9)* #¶ | < 0.0001 |
| BI, cigarettes × years | 485.7 (487.4) | 467.8 (495.3) | 433.5 (529.9) | 369.9 (447.9)* | 0.0158 |
| ALT, U/L | 30.3 (15.7) | 27.6 (19.3) | 24.1 (12.0)* # | 21.3 (11.1)* #¶ | < 0.0001 |
| TG, mg/dL | 151.8 (93.4) | 126.4 (84.1)* | 108.3 (69.2)* # | 86.3 (51.0)* #¶ | < 0.0001 |
| HDL-c, mg/dL | 50.4 (12.0) | 53.8 (14.1)* | 57.4 (14.0)* # | 62.9 (14.8)* #¶ | < 0.0001 |
| %FVC | 96.2 (12.9) | 97.4 (14.1) | 98.3 (15.0) | 97.1 (17.1) | 0.2494 |
| %FEV1 | 94.8 (15.7) | 96.4 (15.8) | 95.6 (17.7) | 95.0 (20.2) | 0.5937 |
| FEV1/FVC, % | 77.7 (7.6) | 77.9 (7.9) | 76.4 (9.5) | 76.1 (10.2) # | 0.0081 |
| Women | |||||
| Q1 (n = 463) | Q2 (n = 484) | Q3 (n = 476) | Q4 (n = 482) | P-value | |
| Age, years | 60.1 (10.3) | 61.1 (10.3) | 63.2 (10.0)* # | 64.4 (10.3)* # | < 0.0001 |
| BMI, kg/m2 | 24.7 (3.3) | 23.8 (3.3)* | 23.4 (3.3)* | 22.1 (3.2)* #¶ | < 0.0001 |
| BI, cigarettes × years | 22.7 (99.9) | 20.4 (112.9) | 9.4 (54.5) | 16.7 (90.3) | 0.14 |
| ALT, U/L | 24.8 (14.8) | 21.2 (11.7)* | 20.5 (13.1)* | 19.2 (10.3)* | < 0.0001 |
| TG, mg/dL | 115.7 (52.9) | 105.7 (50.3)* | 93.4 (39.6)* # | 79.6 (34.4)* #¶ | < 0.0001 |
| HDL-c, mg/dL | 55.8 (12.9) | 58.9 (12.6)* | 62.0 (13.7)* # | 69.2 (14.5)* #¶ | < 0.0001 |
| %FVC | 98.3 (13.9) | 100.4 (13.2) | 100.5 (14.5) | 100.4 (15.5) | 0.0686 |
| %FEV1 | 99.1 (14.9) | 100.1 (14.9) | 99.9 (15.3) | 99.9 (16.6) | 0.7634 |
| FEV1/FVC, % | 81.0 (5.8) | 79.9 (6.1) | 79.4 (6.6)* | 79.4 (7.4)* | 0.0011 |
Men: Q1 [adiponectin (μg/mL) < 5.2]; Q2 [5.2 ≤ adiponectin (μg/mL) < 7.1]; Q3 [7.1 ≤ adiponectin (μg/mL) < 10.0]; Q4 [adiponectin (μg/mL) ≥ 10.0] .
Women: Q1 [adiponectin (μg/mL) < 7.5]; Q2 [7.5 ≤ adiponectin (μg/mL) < 10.5]; Q3 [10.5 ≤ adiponectin (μg/mL) < 15.0]; Q4 [adiponectin (μg/mL) ≥ 15.0].
*: P < 0.05 vs. Q1, #: P < 0.05 vs. Q2, ¶: P < 0.05 vs. Q3.
Values are expressed as the mean and standard deviation (SD). Differences among the quartiles were evaluated by a 1-way analysis of variance and Tukey's method.
BMI, body mass index; BI, Brinkman index; ALT, alanine aminotransferase; TG, triglycerides; HDL-c, high density lipoprotein-cholesterol; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s.
Mean plasma adiponectin levels according to the type of spirometric disorder.
| Men | |||||
| Normal (n = 1161) | Restrictive (n = 96) | Obstructive (n = 184) | Mixed (n = 55) | P-value | |
| Adiponectin (μg/mL) | 7.9 (4.5) | 8.9 (5.3) | 9.5 (5.0)* | 8.6 (3.9) | < 0.0001 |
| Women | |||||
| Normal (n = 1525) | Restrictive (n = 96) | Obstructive (n = 80) | Mixed (n = 18) | P-value | |
| Adiponectin (μg/mL) | 11.6 (5.8) | 11.1 (6.1) | 13.5 (6.0)*# | 14.8 (7.5) | 0.0025 |
Adiponectin concentrations were not available for 384 men and 410 women.
Values are expressed as the mean (SD). Differences between the groups were evaluated by a 1-way analysis of variance and Tukey's method.
*: P < 0.05 vs. the normal group, #: P < 0.05 vs. the restrictive group.
Multiple linear regression analysis: correlations between variables and annual changes in FEV1.
| Men | Women | |||
|---|---|---|---|---|
| Explanatory variables | β | P | β | P |
| Age, years | -0.108 | 0.057 | -0.063 | 0.194 |
| BMI, kg/m2 | 0.000 | 0.994 | -0.088 | 0.100 |
| BI, cigarettes × years | -0.023 | 0.685 | -0.050 | 0.289 |
| ALT, U/L | -0.045 | 0.432 | 0.010 | 0.845 |
| TG, mg/dL | 0.032 | 0.605 | 0.006 | 0.910 |
| HDL-c, mg/dL | -0.020 | 0.757 | 0.020 | 0.717 |
| Adiponectin, μg/mL | -0.166 | 0.006 | -0.120 | 0.020 |
Dependent variable: annual change in FEV1 (%/year).
β, standard partial regression coefficient; BMI, body mass index; BI, Brinkman index; ALT, alanine aminotransferase; TG, triglycerides; HDL-c, high density lipoprotein-cholesterol.
Univariate linear regression analysis: correlations between annual change in FEV1 and plasma adiponectin levels according to gender and smoking status.
| Gender | Smoking status from 2004-2006 | n | β | P |
|---|---|---|---|---|
| Men | never | 148 | -0.28 | 0.0006 |
| Men | current | 106 | -0.25 | 0.0087 |
| Men | past | 151 | 0.002 | 0.9713 |
| Women | never | 426 | -0.09 | 0.0594 |
| Women | current | 21 | -0.291 | 0.1998 |
| Women | past | 9 | -0.28 | 0.4653 |
Explanatory variable: plasma adiponectin level (μg/mL).
Dependent variable: annual change in FEV1 (%/year).
β, standard partial regression coefficient.
Plasma adiponectin levels were inversely correlated with forced expiratory volume in 1 s per forced vital capacity. Graphs show the relationships between forced expiratory volume in 1 s [FEV1]/forced vital capacity [FVC] and plasma adiponectin levels in men (A) and women (B). Correlations were evaluated using Pearson's product moment correlation coefficient. A statistically significant relationship was observed in both men and women (A, r = -0.07, P = 0.01; B, r = -0.08, P = 0.001).
Plasma adiponectin levels were inversely correlated with annual changes in forced expiratory volume in 1 s. The graphs show the relationships between annual decline in forced expiratory volume in 1 s (FEV1) and plasma adiponectin levels in men (A) and women (B). Correlations were evaluated using Pearson's product moment correlation coefficient. A statistically significant relationship was observed in both men and women (A, r = -0.17, P = 0.001; B, r = -0.10, P = 0.033).
Discussion
In the present study, we demonstrated an inverse correlation between plasma adiponectin levels and FEV1/FVC in a general Japanese population (Figure 1). In addition, plasma adiponectin levels were inversely associated with annual changes in FEV1 (Figure 2), even after adjusting for confounding factors such as age, BMI, BI, ALT, TG, and HDL-c (Table 3). This association was significant not only in men who were current smokers, but also in those who had never smoked (Table 4).
Adiponectin is an adipocytokine that is derived from adipocytes. Adiponectin inhibits the expression of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-alpha [25]. Low levels of adiponectin have been reported to be related to high levels of C-reactive protein, a biomarker of inflammation, in patients with obesity, type 2 diabetes, or coronary artery disease [25]. In addition, adiponectin alters the phenotype of macrophages from pro-inflammatory macrophages (M1) to anti-inflammatory macrophages (M2) [26]. Therefore, there is accumulating evidence that suggests the role of adiponectin in anti-inflammatory, anti-atherosclerotic, and cardio-protective pathways [25].
To date, 3 institutes have reported on pulmonary phenotypes in adiponectin-deficient mice. Summer's and Nakanishi's groups demonstrated the development of emphysematous changes in adiponectin-deficient mice [12,13]. In null mice, alveolar macrophage activation and the secretion of matrix metalloproteinase-12, a macrophage-derived protease that promotes emphysema, were confirmed [13]. In addition, they observed emphysematous development in accordance with aging; further, the extra-pulmonary phenotypes were commonly observed in human COPD patients, such as weight loss, fat atrophy, and osteoporosis [12]. These results are in accordance with evidence that adiponectin has anti-inflammatory properties, and that the loss of adiponectin results in a reduced protective capacity against the development of emphysema. However, as many studies have shown, circulating adiponectin levels in patients with COPD were not reduced; in fact, they were increased [9, 27-29]. Even if adiponectin is anti-inflammatory and pulmonary-protective, the elevation of adiponectin levels is not enough to suppress the impairment of pulmonary function in COPD patients. In contrast, Miller et al. demonstrated that adiponectin deficiency protected mice from tobacco-induced inflammation and increased emphysema [14]. Thus, their new evidence showing the pro-inflammatory effects of adiponectin challenges the established theory regarding the anti-inflammatory role of adiponectin. They demonstrated that adiponectin deficiency suppressed the production of pro-inflammatory cytokines such as TNF-alpha and keratinocyte-derived chemokines in the lungs, which resulted in the protection of the lungs from cigarette smoke in adiponectin-null mice. Their study on adiponectin-deficient mice agrees with clinical evidence that shows an elevation of adiponectin in COPD patients.
Globular adiponectin is a proteolytic cleavage fragment consisting of the globular C-terminal domain of full-length adiponectin [30]. Proteases secreted from leukocytes are thought to cleave adiponectin to generate a globular fragment of adiponectin. In contrast to full-length adiponectin, it has been reported that globular adiponectin has the potential to induce inflammation [14, 31, 32]. In addition, the receptor for globular adiponectin is adiponectin receptor 1, and has been reported to be expressed in airway epithelial cells in COPD patients [33, 34]. Because the kit for adiponectin detection in the present study used 2 antibodies that recognize the collagenous domain of adiponectin, the measured adiponectin concentration demonstrated in the present study did not contain those in globular form. According to the instruction manual of another commercial enzyme-linked immunosorbent assay kit for human globular adiponectin (Alpha Diagnostic International, San Antonio TX, USA) [35], the mean serum level of globular adiponectin was 0.16 μg/mL (0.06-0.65 μg/mL). Although the concentrations of globular adiponectin of subjects in the present study are currently unknown, those in subjects with reduced FEV1 values may be elevated by the proteolytic milieu in obstructive subjects [1], resulting in the promotion of airway inflammation linked to airflow obstruction. Further investigations are required regarding this point.
Previously, we demonstrated that hyperhomocysteinemia and lower serum iron levels were predictive of a rapid decline in FEV1 in men who were continuing smokers using the Takahata Study database between 2004-2006 (Visit 1) and 2009 (Visit 2) (n = 147) [16, 19]. In 2011 (Visit 3), we re-performed the pulmonary function measurements for 873 participants in this study. Not only men who were current smokers, but also women and other subjects with smoking status were included in the analyses. During the follow-up period, many active smokers quit smoking. During Visit 1, the active smokers included 521 men (total male subjects, 1500) and 99 women (total female subjects, 1753) [17, 18]. Finally, during Visit 3, there were 50 men who were continuing smokers (total male subjects, 405) and 9 women (total female subjects, 467). Neither homocysteine nor serum iron levels were associated with changes in FEV1 between Visit 1 and Visit 3, suggesting that these variables predict declines only in continuing smokers (data not shown). In contrast, adiponectin predicted declines in FEV1 for both genders and for any smoking status.
The present study has other limitations because it was not hospital-based. Further, the lack of the information regarding chest radiography findings, the final pulmonary disease diagnosis, and current medication use, which could affect adiponectin values, may be potential limitations. In addition, there is the possibility of a sampling bias among the subjects who participated in the present study, specifically resulting from the socioeconomic status of these patients.
In conclusion, we demonstrated an inverse relationship between plasma adiponectin levels and FEV1/FVC in a healthy Japanese population. In addition, adiponectin levels were inversely associated with annual changes in FEV1, even after the adjusting for possible confounding factors. In contrast to clinical evidence regarding the cardio-protective role of adiponectin, many studies demonstrated elevation of adiponectin in clinical COPD patients, suggesting pro-inflammatory and destructive roles in the lungs. The results of the present study were in accordance with previous clinical studies regarding adiponectin levels in COPD patients. Since adiponectin levels were significantly associated with annual declines in FEV1, they predict declines in pulmonary function in the general population, both in smokers and in those who have never smoked.
Acknowledgements
We thank Taiko Aita, Emiko Nakamura, and Eiji Tsuchida for their excellent technical assistance. We also thank the following contributors: Michiko Nishiwaki (Yamagata City Hospital, Saiseikan), Toshihiro Wada (Yamagata City Hospital, Saiseikan), Jun-Ichi Machiya (Nihonkai General Hospital), Noriyuki Hirama (NHO Yamagata National Hospital), Noriaki Takabatake (Tohoku Central Hospital), and Makoto Sata (National Cerebral and Cardiovascular Center, Japan).
Funding
This study was supported by a grant-in-aid from the Global COE Program of the Japan Society for the Promotion of Science, and grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (19590880, 20590892, and 23390220).
Ethics committee approval
This study was approved by the Institutional Ethics Committee of Yamagata University and all participants gave written informed.
Conflicts of interest
The authors have declared that no competing interest exists.
References
1. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease; updated 2011. GOLD Global Initiative for Chronic Obstructive Lung Disease. http://www.goldcopd.org/
2. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33:1165-85
3. Sato M, Shibata Y, Abe S, Inoue S, Igarashi A. et al. Retrospective analysis of the relationship between decline in FEV1 and abdominal circumference in male smokers: The Takahata Study. Int J Med Sci. 2013;10:1-7
4. Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P. et al. The metabolic syndrome in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2005;25:226-32 discussion 33-4
5. Watz H, Waschki B, Kirsten A, Muller KC, Kretschmar G. et al. The metabolic syndrome in patients with chronic bronchitis and COPD: frequency and associated consequences for systemic inflammation and physical inactivity. Chest. 2009;136:1039-46
6. Clini E, Crisafulli E, Radaeli A, Malerba M. COPD and the metabolic syndrome: an intriguing association. Intern Emerg Med. 2011 Epub ahead of print
7. Lam KB, Jordan RE, Jiang CQ, Thomas GN, Miller MR. et al. Airflow obstruction and metabolic syndrome: the Guangzhou Biobank Cohort Study. Eur Respir J. 2010;35:317-23
8. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29-33
9. Tomoda K, Yoshikawa M, Itoh T, Tamaki S, Fukuoka A. et al. Elevated circulating plasma adiponectin in underweight patients with COPD. Chest. 2007;132:135-40
10. Waschki B, Kirsten A, Holz O, Muller KC, Meyer T. et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140:331-42
11. Yoon HI, Li Y, Man SF, Tashkin D, Wise RA. et al. The complex relationship of serum adiponectin to COPD outcomes COPD and adiponectin. Chest. 2012;142:893-99
12. Nakanishi K, Takeda Y, Tetsumoto S, Iwasaki T, Tsujino K. et al. Involvement of endothelial apoptosis underlying chronic obstructive pulmonary disease-like phenotype in adiponectin-null mice: implications for therapy. Am J Respir Crit Care Med. 2011;183:1164-75
13. Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T. et al. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1035-42
14. Miller M, Pham A, Cho JY, Rosenthal P, Broide DH. Adiponectin-deficient mice are protected against tobacco-induced inflammation and increased emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299(L):834-42
15. Shibata Y, Watanabe T, Osaka D, Abe S, Inoue S. et al. Impairment of pulmonary function is an independent risk factor for atrial fibrillation: the Takahata study. Int J Med Sci. 2011;8:514-22
16. Shibata Y, Inoue S, Igarashi A, Yamauchi K, Abe S. et al. Elevated serum iron is a potent biomarker for spirometric resistance to cigarette smoke among Japanese males: the Takahata study. PLoS One. 2013;8:e74020
17. Shibata Y, Inoue S, Igarashi A, Yamauchi K, Abe S. et al. A lower level of forced expiratory volume in 1 second is a risk factor for all-cause and cardiovascular mortality in a Japanese population: the takahata study. PLoS One. 2013;8:e83725
18. Osaka D, Shibata Y, Abe S, Inoue S, Tokairin Y. et al. Relationship between habit of cigarette smoking and airflow limitation in healthy Japanese individuals: the Takahata study. Intern Med. 2010;49:1489-99
19. Nunomiya K, Shibata Y, Abe S, Inoue S, Igarashi A. et al. Hyperhomocysteinaemia predicts the decline in pulmonary function in healthy male smokers. Eur Respir J. 2013;42:18-27
20. Nemoto T, Shibata Y, Osaka D, Abe S, Inoue S. et al. Impact of cigarette smoking on maximal expiratory flows in a general population: the Takahata study. Intern Med. 2011;50:2547-55
21. Kishi H, Shibata Y, Osaka D, Abe S, Inoue S. et al. FEV6 and FEV1/FEV6 in Japanese participants of the community-based annual health check: the Takahata study. Intern Med. 2011;50:87-93
22. Aida Y, Shibata Y, Osaka D, Abe S, Inoue S. et al. The relationship between serum uric acid and spirometric values in participants in a health check: the Takahata study. Int J Med Sci. 2011;8:470-8
23. Nishise Y, Saito T, Makino N, Okumoto K, Ito JI. et al. Relationship between alcohol consumption and serum adiponectin levels: the Takahata study--a cross-sectional study of a healthy Japanese population. J Clin Endocrinol Metab. 2010;95:3828-35
24. The Committee of Pulmonary Physiology JRS. Guidelines for Pulmonary Function Tests: Spirometry, flow-volume curve, diffusion capacity of the lung. Tokyo. 2004
25. Aprahamian TR, Sam F. Adiponectin in cardiovascular inflammation and obesity. Int J Inflam. 2011;2011:376909
26. Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA. et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;285:6153-60
27. Chan KH, Yeung SC, Yao TJ, Ip MS, Cheung AH. et al. Elevated plasma adiponectin levels in patients with chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2010;14:1193-200
28. Breyer MK, Rutten EP, Locantore NW, Watkins ML, Miller BE. et al. Dysregulated adipokine metabolism in chronic obstructive pulmonary disease. Eur J Clin Invest. 2012;42:983-91
29. Kirdar S, Serter M, Ceylan E, Sener AG, Kavak T. et al. Adiponectin as a biomarker of systemic inflammatory response in smoker patients with stable and exacerbation phases of chronic obstructive pulmonary disease. Scand J Clin Lab Invest. 2009;69:219-24
30. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772-83
31. Chedid P, Hurtado-Nedelec M, Marion-Gaber B, Bournier O, Hayem G. et al. Adiponectin and its globular fragment differentially modulate the oxidative burst of primary human phagocytes. Am J Pathol. 2012;180:682-92
32. Tomizawa A, Hattori Y, Kasai K, Nakano Y. Adiponectin induces NF-kappaB activation that leads to suppression of cytokine-induced NF-kappaB activation in vascular endothelial cells: globular adiponectin vs. high molecular weight adiponectin. Diab Vasc Dis Res. 2008;5:123-7
33. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T. et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-9
34. Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol. 2009;182:684-91
35. Alpha_Diagnostic_International Instruction Manual No.200-120-AGH: Human Serum Adipolean or globular adiponectin/Acrp30 (gAcrp30). http://www.4adi.com/objects/catalog/product/extras/200-120-AGH.pdf
Author contact
![]() Corresponding author: Dr. Yoko Shibata, 2-2-2 Iida-Nishi, Yamagata City, Yamagata 990-9585, Japan. Telephone: +81-23-628-5302 Fax: +81-23-628-5305 Email: shibataid.yamagata-u.ac.jp.
Corresponding author: Dr. Yoko Shibata, 2-2-2 Iida-Nishi, Yamagata City, Yamagata 990-9585, Japan. Telephone: +81-23-628-5302 Fax: +81-23-628-5305 Email: shibataid.yamagata-u.ac.jp.

 Global reach, higher impact
Global reach, higher impact