3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2014; 11(2):172-179. doi:10.7150/ijms.6875 This issue Cite
Research Paper
Intracellular Distributing and Interferon-γ Secretion of Human Interleukin-18 in BxPC-3 Cells
1. Department of General Surgery, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou 310016, China
2. Life Science College, Zhejiang Chinese Medical University, Hangzhou 310053, China
* Both authors have contributed equally to this work.
Received 2013-6-10; Accepted 2013-11-11; Published 2014-1-7
Abstract
Objective: To investigate the characteristics of interleukin-18 (IL-18) in vitro, explore IL-18, interferon-γ (IFN-γ) and interleukin-2 (IL-2) secretive activity in BxPC-3 line cells with interleukin-18 mutants.
Methods: Human IL-18 full-length gene (hIL-18-F) and the hIL-18 presumed mature protein gene (hIL-18-M) were inserted into the expression vector pEGFP-N1, to construct recombinant plasmids as Mu0, Mu1, Mu2, Mu3, and Mu4, and the recombinant plasmids were then transferred into BxPC-3 line cells. There are significant differences between Mu1, Mu2 and the pEGFP-C1 control group (P<0.05) by 3-(4,5-dimethiazol- 2-yl)- 2,5-diphenyltetrazolium bromide (MTT) for a proliferation assay, and the fluorescence of the Mu1 and Mu 2 appeared targeted to the membranous region in the BxPC-3 cells after transfected 24h by confocal laser scanning microscope (OLSM).To characterize the intracellular distribution of hIL-18, recombinant IL-18 were each fused to the enhanced green fluorescent protein gene, and expressed in BxPC-3 cells.
Results: Results showed that the Mu1 tended to the membranous region in BxPC-3 cells, this indicates that the N-terminal former amino acid peptide helped ChIL-18 target to BxPC-3 cellS membranes. ELISA results demonstrated that IFN-γ and IL-18 secreted levels of BxPC-3 cells transfecting with recombinant plasmid showed an significant difference (P<0.01); refers to IL-2 expression, the two BxPC-3 cells groups transfecting with recombinant plasmid have no significant function (P>0.05).
Conclusions: The results showed that hIL-18 and hIL-18 presumed mature protein can induce the secretion of IFN-γ in BxPC-3 cells, and increase the expression of IL-18, but they have no effects on IL-2.
Keywords: hIL-18 mutants, BxPC-3 cells, intracellular distribution, IFN-γ
1. Introduction
Interleukin-18 (IL-18) gene was first isolated and cloned from propioibacteriumacnes-treated and lipopolysaccharide-treated mouse livers in 1995 by 0kamura [1]. IL-18 was discovered as an interferon (IFN-γ)-inducing factor and had a critical role in inflammatory and immune response [2]. It stimulates natural killer (NK) and T cells and enhances Th1 immune response. IL-18 is a proinflammatory cytokine with biological properties similar to IL-12 [3]. As a member of the IL-1 family, other functions ascribed to IL-18 include induction of IL-1β, tumor necrosis factor-α (TNF-α), production of granulocyte/macrophage colony stimulation factor (GM-CSF) and IL-2 by T cells [4], as well as enhancement of NK cell cytotoxicity and neutrophil activity, enhancement of Fas ligand expression by Th1 cells, and anti-tumor effects were reported [5]. The expression and secretion of IL-18 is shown in various kinds of cells from immune cells such as macrophages, T cells and neutrophils to cancer cells [6], it induces IFN-γ production from T cells without the requirement for T-cell receptor (TCR) engagement [7]. So, the crucial function of IL-18 as an immunomodulatory molecule is used for antitumor.
Recently, some cancer cells such as prostate cancer cells and esophageal cancers have been reported to secrete IL-18 [8,9], so the role of IL-18 needs to be investigated in cancers. Some reports showed that IL-18 increases in the serum of the majority of cancer patients and has been associated with disease progression [10], suppression of IL-18 bioactivity as a novel therapeutic concept for the treatment of chronic inflammatory [11]. However, a large number of tests showed that IL-18 eliminates spontaneous cancer or pathogen infected cells [12,13], and IL-18 was applied to an adjuvant of vaccines.
IL-18 is a multifunctional cytokine that induces IFN-γ secretion and play an important role in antitumor immunity [14]. Pro-IL-18, does not contain a known signal peptide-sequence, and is converted into functional active mature protein by proteolytic cleavage of IL-1β converting enzyme (ICE) resulting in an 18.3 kDa final molecule [12], variations in the DNA sequences of IL-18 may lead to altered IL-18 production and/or activity, we constructed IL-18 mutants by cutting the hIL-18 cDNA, to investigate the secretion characteristics of IFN-γ, IL-2, IL-18, and protein distribution, antigenicity with hIL-18 mutants in pancreatic cancer BxPC-3 cells line.
2. Materials and methods
2.1 Plasmids and cell culture
Eukaryotic expression vector pEGFP-C1 was purchased from Clontech (Palo Alto, USA). pGEM-T-hIL-18 was conserved in our laboratory. E. coli DH-5α were purchased from TAKARA (Dalian,China). BxPC-3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco,Gaithersburg, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 IU/ml penicillin and 100 μg/ml streptomycin at 37 ºC with 5% CO2.
2.2 Construction of recombinant eukaryotic expression plasmids
According to the sequence of hIL-18 cDNA gene provided by GenBank, four various hIL-18 sequences were amplified with primer pairs (Table 1) from pGEM-T-hIL-18 (presaved in our lab), respectively. The cutting ATG ORF of hIL-18 were amplified with primer pairs P1/P2 forming sequence Mu0 (sMu0). Assuming that the hIL-18 protein is cleaved at the 36th amino acid residue after the conserved aspartate residue, we amplify a cDNA fragment encoding an N-terminal truncated form (sMu1) of hIL-18 with primer pairs P3/P4. To investigate the influences with an incomplete sequence of hIL-18, N-terminal 56 amino acid peptide truncated form (sMu2) and C-terminal 20 amino acid peptide truncated form (sMu3) were amplified by PCR, middle mino acid peptide (P124-P143) truncated form (sMu4) of hIL-18 was synthesized by TaKaRa Biotechnology (Dalian) Company. Each fragments of hIL-18 were inserted into pEGFP-C1 after digestion with restriction enzymes, resulting in recombinant plasmids Mu0 (containing the complete ORF of hIL-18), Mu1, Mu2, Mu3 and Mu4 (Figure 1, table 1 showed primers).
All recombinant plasmid were identified with restriction enzymes, and sequencing by TaKaRa Biotechnology (Dalian) Company. The fused proteins of green fluorescent protein (GFP) and IL-18 can be observed by confocal laser scanning microscope.
Sequence of primers used in the experiments
| Primer | Nucleotide sequence | Location | Restriction enzyme |
|---|---|---|---|
| P1 | TCA CTCGAG CTGCTGCTGAACCAGTAGAAG | 4-24 | XhoⅠ |
| P2 | CGA GAATTC CTAGTCTTCGTTTTGAACAGTG | 583-604 | EcoRⅠ |
| P3 | CT GGTACC TACTTTGGCAAGCTTGAATCTAA | 112-134 | KpnⅠ |
| P4 | CA GGATCC CTAGTCTTCGTTTTGAACAGTGA | 583-605 | BamHⅠ |
| P5 | G GAATTC ATTCATTGACCAAGGAAATCGGC | 172-194 | EcoRⅠ |
| P6 | TC GGTACC CTAGTCTTCGTTTTGAACAGTG | 583-604 | KpnⅠ |
| P7 | GG GGTACC CTGCTGCTGAACCAGTAGAAG | 4-24 | KpnⅠ |
| P8 | GGGATCC TAAATGAGTTTAAAAAGGTCTC | 523-544 | BamHⅠ |
Restriction enzyme sites are in italic.
Recombinant plasmids construction of Mu0, Mu1, Mu2, Mu3, Mu4.
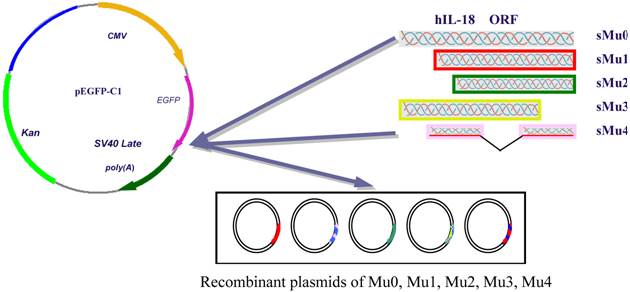
2.3 Transfection of five recombinant plasmids and pEGFP-C1 into BxPC-3 cells
The pancreatic cancer cell line BxPC-3 was obtained from the Shanghai Institute for Biological Sciences, Chinese Academy of Science (SIBS/CAS), and cultured in RPMI1640 (HyClone, Logan, USA) supplemented with 10% FBS, 100 IU/ml penicillin and 100 μg/ml streptomycin at 37ºC with 5% CO2. After 48 h, BxPC-3 cells were centrifugated at 1000 g for 5 min, and 6-well plated at 1×105cells/well in growth medium without antibiotics. Five recombinant plasmids and pEGFP-C1(0.8ug/well) were transferred into adherent BxPC-3 cells using Lipofectamine™ 2000 (Invitrogen, Carlsbad,USA) as its instructions [15]. The transfection rate assayed was about between 29% and 31% by fluorescent microscopy.
2.4 Proliferation response of BxPC-3 cells transfected with recombinant plasmids by MTT assay
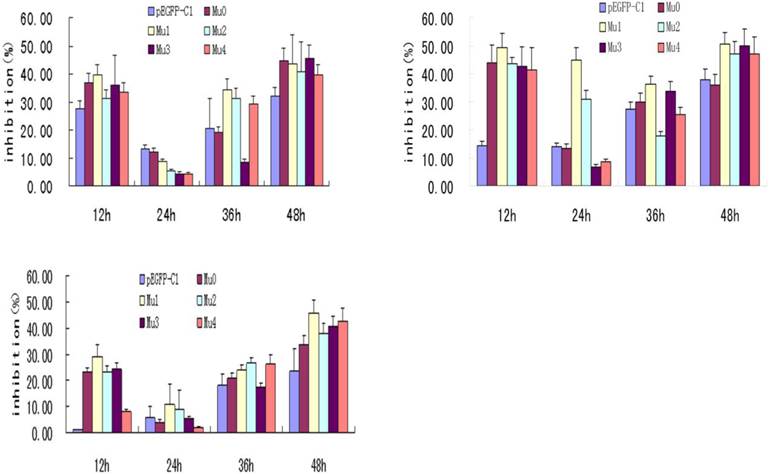
BxPC-3 cells were cultured for four days in RPMI1640 medium containing 10% FBS. Cell suspension was inoculated to each well of 96-well plates (each well has a total volume of 100 μL, density of 1×104 cells/well. After 24 h, five recombinant plasmids and pEGFP-C1 were transfected into BxPC-3 cells, of which the treatment concentration were 0.4ug/well, 0.2ug/well and 0.1ug/well. Each well repeated three times. At different post-transfection time points (12 h, 24 h, 36 h, 48 h ), cells were observed under a contrast phase microscope before adding MTT solution, prepared fresh as 5 mg/ml in ddH2O and filtered through a 0.22 μm filter, and kept for 10 min at 60ºC. 10 µl of MTT solution was added to each well incubated in the dark at 37ºC for 4 h. Then MTT/medium was removed and 150 µl DMSO was added to each well. The OD values were measured at 570 nm using Model 680 Microplate reader as figure 2 (Bio-RAD).
2.4 Analysis of hIL-18 expression by RT-PCR and enzyme-linked immunosorbent assay (ELISA)
Five recombinant plasmids and pEGFP-C1 were transfected into BxPC-3 cells. At different post-transfection time points, the cells transfected were digested with 0.25% trypsin and 0.02% EDTA and collected into 1.5 ml Eppendorf tubes. The transcriptions of target genes were determined by reverse transcription (RT)-PCR with primers β-actin and hIL-18 full length respectively using the total RNA extracted from cells 12 h, 24 h, 36 h, 48 h post-transfection as the template (Figure 3). At the same time,the cultivation supernatant was transferred from each of all the wells to each of 1.5 ml Eppendorf tubes and measured by ELISA. Briefly, Enzyme-linked immunosorbent assay (ELISA) were carried out to determine protein expression using hIL-8 ELISA Kit, IFN-γ ELISA Kit and IL-2 ELISA Kit (Cusabio, Wuhan, China) according to the manufacturer's protocol. Each well repeated three times.
2.5 Intracellular Distribution of five Fused GFP-IL-18 in BxPC-3 cells
The cell culture was similar as above, 100 μL of cell suspension was inoculated to each well of 96-well plates and transfected with five recombinant plasmids (Mu0, Mu1, Mu2, Mu3, Mu4) and pEGFP-C1 (as control), At different post-transfection time points (12 h, 24 h, 36 h, 48 h), cells were examined under laser confocal microscopy 510 (Zeiss, Oberkochen, Germany) for Green fluorescent distribution.
2.6 Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS ®) version 18.0 (SPSS, Inc. Chicago, IL, USA). The differences in the measurement data were compared using the Student's t test, and comparisons between groups were tested using the χ2 test or the Fisher exact probability test. P < 0.05 was considered statistically significant.
Effects of transfection of five recombinant plasmids on BxPC-3 cells' proliferation. The treatment concentration of A to C were 0.4ug/well, 0.2ug/well and 0.1ug/well. The statistics showed that the five recombinant plasmids all had the most effective inhibition at 24 h post-transfection, among which Mu1 had the best effective inhibition.
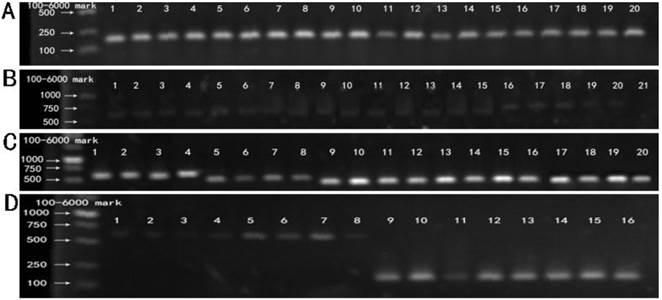
RT-PCR products of five recombinant plasmids. M: 100-6000 bp DNA Marker. (A) Lane 1-20 showed the RT-PCR results of BxPC-3 cells tranfected with five recombinant plasmids (Mu0, Mu1, Mu2, Mu3,Mu4) at 12 h, 24 h, 36 h, 48 h respectively, using the primer of β-actin. (B) Lane 1-20 showed the RT-PCR results of BxPC-3 cells tranfected with five recombinant plasmids (Mu0, Mu1, Mu2, Mu3, Mu4) at 12 h, 24 h, 36 h, 48 h respectively, using the primer of hIL-18 full length. Lane 21 is negative control. (C) Lane1-20 showed the RT-PCR results of BxPC-3 cells tranfected with five recombinant plasmids (Mu0, Mu1, Mu2, Mu3, Mu4) at 12 h, 24 h, 36 h, 48 h respectively, using the original primers. (D) Control. Lane 1-4 and lane 5-8 showed RT-PCR results of BxPC-3 cells tranfected with pEGFP-C1 and untreated BxPC-3 cells cultivated at 12 h, 24 h, 36 h, 48 h respectively, using the primer of hIL-18 full length. Lane 9-12 and lane 13-16 showed RT-PCR results of BxPC-3 cells with the same treatment (according to Lane 1-4 and 5-8) using the primer of β-actin.
3. Results
3.1 MTT assays of BxPC-3 cells transfected with five IL-18 recombinant plasmid
IL-18 has been shown to have significant antitumor activity in several preclinical animal models. Phase I clinical trials of recombinant human IL-18 have demonstrated that it can be safely administered to patients with advanced cancer (16). MTT assay is a conventional experiment for cells proliferation. MTT results showed that the BxPC-3 cells with five recombinant plasmids all had the most effective inhibition at 24 h post-transfection, and Mu1 had the best effective inhibition. Differences between Mu1, Mu2 and the pEGFP-C1 control group were significant (P<0.05). But Mu0, Mu3, Mu4 compared to the pEGFP-C1 control group had no significant difference (P>0.05) (Figure 2).
3.2 RT-PCR products of five recombinant plasmids
RT-PCR results of BxPC-3 cells transfected with five recombinant plasmid and pEGFP-C1, respectively. Fig.3A showed the cDNA of five recombinant plasmids (Mu0, Mu1, Mu2, Mu3, Mu4), pEGFP-C1 and untreated BxPC-3 cells were amplified with primers of β-actin, which was confirmed that RNA extraction and reverse transcription into cDNA was successful. Fig.3B and 3D showed the hIL-18 cDNA were amplified from five recombinant plasmids (Mu0, Mu1, Mu2, Mu3, Mu4), pEGFP-C1 and untreated BxPC-3 cells using the primer of hIL-18 full length, but the bands were light expect Mu0, Which indicated that pro-IL-18 was low expression in BxPC-3 cells transfected. The cDNA of five recombinant plasmids (Mu0, Mu1, Mu2, Mu3, Mu4) were amplified successfully using the original primers from Fig.3C, which demonstrated that five recombinant plasmids were transfected into BxPC-3 cells successfully and expressed.
3.3 Analysis of hIL-18 expression in BxPC-3 cells by ELISA
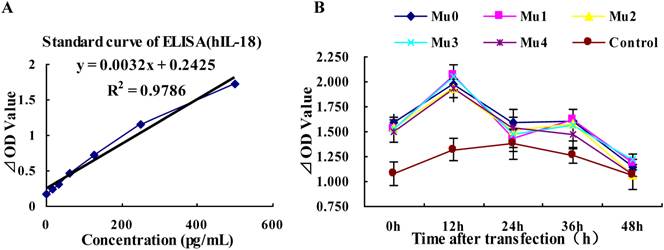
Differences in the DNA sequences of IL-18 may lead to altered IL-18 production, the antigenity of hIL-18 maybe change after it mino acid peptide cutting. The results showed that the expression of hIL-18 in BxPC-3 cells with five recombinant plasmids rapid rise at peak value after12 h transfected, and appeared small-crest at 36h post-transfection, but the expression of hIL-18 was decreased gradually between 36h and 48h post-transfection. Comparing with the control group (pEGFP-C1), Mu0, Mu1, Mu2, Mu3, and Mu4 had the significant difference (P<0.05) (Figure 4 and table 2).
Analysis of hIL-18 expression in BxPC-3 cells (by hIL-18 ELISA kit). (A) The standard curve of hIL-18 ELISA kit. (B) The different expression levels of hIL-18 in the cultivation supernatant of BxPC-3 cells after post-transfection of five recombinant plasmids at 0, 12, 24, 36, 48 h.
hIL18 ELISA data analysis
| Sample | OD (Mean ±SD) | P value | ||||
|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 36 h | 48 h | ||
| Mu0 | 1.524±0.091 | 1.973±0.104 | 1.593±0.075 | 1.400±0.124 | 1.147±0.060 | <0.01 |
| Mu1 | 1.458±0.111 | 2.060±0.105 | 1.470±0.165 | 1.431±0.110 | 1.179±0.061 | <0.05 |
| Mu2 | 1.472±0.089 | 1.928±0.139 | 1.532±0.135 | 1.440±0.064 | 1.066±0.017 | <0.05 |
| Mu3 | 1.447±0.127 | 2.048±0.130 | 1.589±0.168 | 1.315±0.156 | 1.227±0.055 | <0.05 |
| Mu4 | 1.431±0.111 | 1.936±0.093 | 1.575±0.191 | 1.292±0.127 | 1.083±0.032 | <0.05 |
| Control | 1.004±0.124 | 1.245±0.113 | 1.379±0.150 | 1.124±0.073 | 1.071±0.147 | |
OD: Optical density as 492 wavelength
IFN-γ ELISA data analysis
| Sample | OD (Mean ±SD) | P value | ||||
|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 36 h | 48 h | ||
| Mu0 | 2.633±0.114 | 2.528±0.046 | 2.684±0.174 | 2.673±0.021 | 2.585±0.217 | <0.01 |
| Mu1 | 2.633±0.049 | 2.543±0.041 | 2.631±0.085 | 2.659±0.044 | 2.642±0.026 | <0.01 |
| Mu2 | 2.676±0.088 | 2.505±0.173 | 2.620±0.022 | 2.655±0.114 | 2.626±0.042 | <0.01 |
| Mu3 | 2.688±0.068 | 2.490±0.053 | 2.623±0.020 | 2.692±0.073 | 2.550±0.126 | <0.01 |
| Mu4 | 2.655±0.039 | 2.445±0.022 | 2.640±0.006 | 2.605±0.111 | 2.628±0.018 | <0.01 |
| C1 | 1.887±0.072 | 1.965±0.064 | 1.965±0.057 | 2.114±0.079 | 2.405±0.081 | >0.05 |
| Control | 1.930±0.171 | 2.168±0.165 | 2.111±0.100 | 2.485±0.173 | 2.489±0.241 | |
OD: Optical density as 492 wavelength
Analysis of IFN-γ expression in BxPC-3 cells (by IFN-γ ELISA kit). (A) The standard curve of IFN-γ ELISA kit. (B) The different expression levels of IFN-γ in the cultivation supernatant of BxPC-3 cells after post-transfection of five recombinant plasmids at 0 h, 12 h, 24 h, 36 h, 48 h.
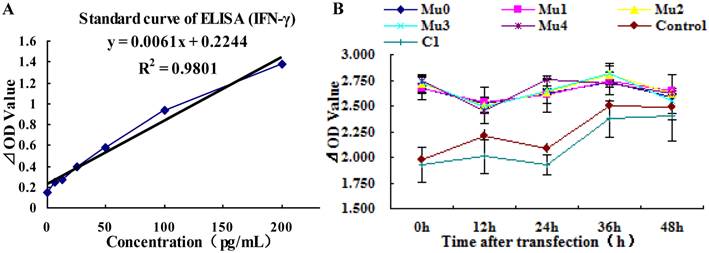
3.4 Analysis of IFN-γ induction in BxPC-3 cells by ELISA
Predominantly biologic effects of IL-18 include activation of monocytes, NK cells, and production of IFN-γ as well as other cytokines in vivo (16), the expression of IFN-γ in BxPC-3 cells were increased with five recombinant plasmids after 12h transfected, but the expression of IFN-γ was decreased slowly soon. The expression of IFN-γ was more steady on observation points, only small-fluctuation after high expression. Comparing with the control group (untreated BxPC-3 cells), Mu0, Mu1, Mu2, Mu3, and Mu4 had the significant difference (P<0.01) (Table 3). The difference between the pEGFP-C1 group and control group was not significant (P>0.05) (Figure 5 and Table 3).
3.5 Analysis of IL-2 induction in BxPC-3 cells by ELISA
The result of hIL-2 ELISA data analysis showed that Mu0, Mu1, Mu2, Mu3, and Mu4 had no difference comparing with the control group (untreated BxPC-3 cells), which pointed that pancreatic cancer BxPC-3 cells line may not express hIL-2 (P>0.05) (Results not showed).
3.6 Expression and localization of recombinant hIL-18-EGFP
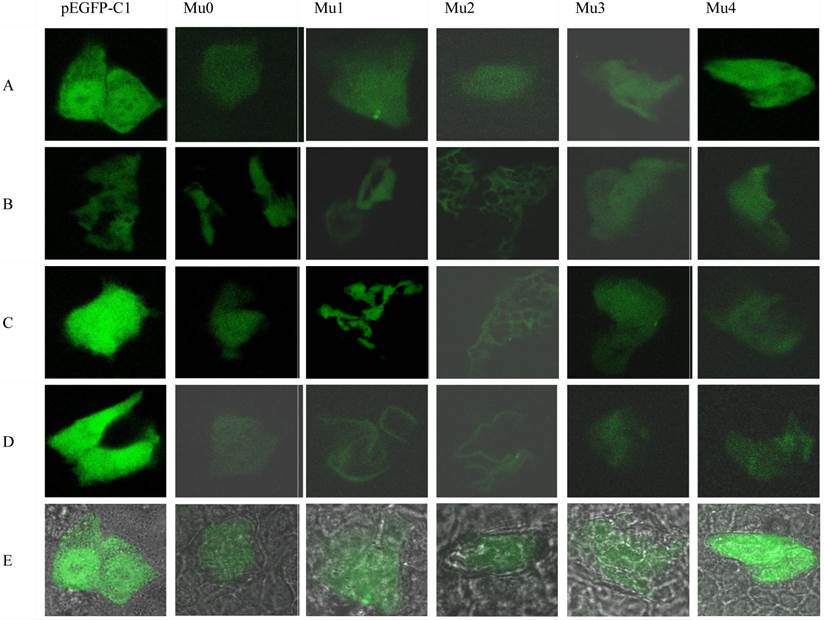
To further investigate the intracellular distribution of recombinant hIL-18-EGFP hIL-18 Mu0, Mu1, Mu2, Mu3, Mu4 and pEGFP-C1 were transfected into BxPC-3 cells. The fused proteins were expressed and observed by laser confocal microscopy 510 (Figure 6). The fluorescence intensity of transfected with pEGFP-C1 was continuously bright after transfected 12h to 48h. The transfected cells presented a diffusing and uniform fluorescence throughout the cytoplasm and nucleus 12 h post-transfection, fluorescence intensity of other cells are decreasing after transfected with Mu 0, Mu 1, Mu 2, Mu 3, Mu 4 12h to 48h, and the fluorescence of the hIL-18 Mu1 and Mu 2 appeared targeted to the membranous region of the BxPC-3 cells.
4. Discussion
Bioactive IL-18 induces IFN-γ production, Fas Ligand (FasL) expression, and inhibits angiogenesis, raising the issue of anti-tumor effects of a tumor-derived cytokine in pancreatic carcinoma cells [17]. In this work, we cut the N-terminal 36 amino acid peptide (form of Mu1, presumed mature protein gene), N-terminal 56 amino acid peptide (form of Mu2), C-terminal 20 amino acid peptide (form of Mu3), and middle 20 amino acid peptide (form of Mu4) of hIL-18 cDNA respectively, which were inserted into pEGFP-C1. The recombinant plasmids were transfected into BxPC-3 cells for proliferation inhibition, MTT results showed that both Mu1 and Mu2 groups showed significant (P<0.05) compared with pEGFP-C1 transfected control cells. It was proved that mature form of IL-18 with functional activity in antitumor [18], so the growth inhibition of Mu1 in BxPC-3cells is understandable, but the growth inhibition of Mu2 suggested that the inhibition activity is related with cut forepart and integrate middle and later amino acid peptide of IL-18. Interestingly, as Fig. 6 showed, the fluorescence of the Mu1 and Mu 2 appeared targeted to the membranous region of the BxPC-3 cells after transfected 24h to 48h, these results suggest that the N-terminal forepart amino acid peptide of hIL-18 presumed mature protein probably exposed by cutting, and combine to some membrane proteins of BxPC-3 cells, the same situation was reported in chicken IL-18 [19]. Maybe there are relationship between inhibition activity and the intracellular distributions of IL-18, which perhaps indicated that truncated former amino acid peptide of hIL-18 lead to intracellular distributions variations of IL-18, and bring about growth inhibition differences in BxPC-3 cells.
Localization of enhanced green fluorescent protein (EGFP)-tagged hIL-18 recombinant plasmids inBxPC-3 cells. Confocal micrographs of transfected BxPC-3 cells expressing EGFP-tagged hIL-18 recombinant plasmid at 12 h post-transfection (A)24 h post-transfection, (B)36 h post-transfection, (C)48 h post-transfection, (D)and composite photo(E)respectively. Images were taken in the plane where maximum fluorescence appeared. Transfected cells presented uniform fluorescence throughout the cytoplasm and the nucleus 12 h post-transfection, and the fluorescence intensity of transfected cells with pEGFP-C1 are durable(A-D), fluorescence intensity of other cells are decreasing. But from 24 h and 36 h post-transfection, the fluorescence of the hIL-18 Mu1 and Mu2 appeared targeted to the membranous region of the BxPC-3 cells. Magnification, 200×.
The results of RT-PCR products and expression level of hIL-18 in BxPC-3 cells revealed that five recombinant plasmids were transfected into BxPC-3 cells successfully, and all mutants of IL-18 were active in IL-18 induction (P<0.05), although quantity of induced IL-18 were decreased rapidly, autocrine expression of IL-18 in BxPC-3 cells is transient. The antigenicity of IL-18 were not changed by our cutting of N-terminal, middle and C-terminal amino acid peptide, it display that antigenic determinant of IL-18 perhaps is multiple locus or multiple repeated.
IL-18 is constitutively produced by dendritic cells and several cells line [17], autocrine IL-18 induced high level IFN-γ. The secretion of IFN-γ in BxPC-3 cells by IL-18 mutants was significant increased (P<0.01), and there was no statistical difference in the expression level of transfected pEGFP-C1 and untreated BxPC-3 cells (P>0.05). The secretion of IFN-γ was not changed with hIL-18 mutants, maybe the determinant of IFN-γ secretion was not cleaved by our truncated. IL-18 level in BxPC-3 cells with IL-18 mutants seems dose not corresponding to MTT results, the reason is not clear. The expression of IL-2 with recombinant plasmids was no statistical difference comparing with BxPC-3 cells (P>0.05), it pointed that IL-2 neither secreted by BxPC-3 cells, no induced by increasing of IL-18 and IFN-γ in BxPC-3 cells.
IL-18 is proposed as novel adjuvant cancer therapy, but the role of the proinflammatory IL-18 in cancer progression remains controversial; our results showed that variations in the DNA sequences of IL-18 may change IL-18 distribution in vitro, but not changed activity for IFN-γ and IL-18 secretion in BxPC-3 cells. A lot of reports suggest that careful preclinical studies are needed to determine the proper application of IL-18 in cancer therapy, there are a lot of works for various domain function of IL-18, make an intensive study for better comprehension and application in future.
Acknowledgements
This research was supported by the national Natural Science Foundation of China (NO. 30800218), the Natural Science Foundation of Zhejiang Province (NO.LY2H16007, Y2080506, Y3080255), the Key Project of Zhejiang province education office (NO.Z200908281).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Okamura H, Tsutsi H, Komatsu T. et al. Cloning of a new cytokine that induces IFN- production by T cells. Nature. 1995;378:88-91
2. Park S, Cheon S, Cho D. The dual effects of IL-18 in tumor progression. Cell Mol Immunol. 2007;4:329-35
3. Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1 are differentially regulated in humanblood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA. 1999;96:2256-61
4. Reading PC, Whitney PG, Barr DP. et al. IL-18, but not IL-12, regulates NK cell activity following intranasal herpes simplex virus type 1 infection. J Immunol. 2007;179:3214-21
5. Marshall DJ, Rudnick KA, McCarthy SG. et al. IL-18 enhances Th1 immunity and tumor protection of a DNA vaccine. Vaccine. 2006;24:244-53
6. Liu B, Novick D, Kim SH. et al. Production of a biologically active human interleukin 18 requires its prior synthesis as PRO-IL-18. Cytokine. 2000;12:1519-25
7. Puren AJ, Fantuzzi G, Gu Y. et al. Interleukin-18 (IFN gamma-inducing factor) induces IL-8 and IL-1beta via TNF alpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998;101:711-9
8. Tsuboi K, Miyazaki T, Nakajima M. et al. Serum interleukin-12 and interleukin-18 levels as a tumor marker in patients with esophageal carcinoma. Cancer Lett. 2004;24:207-14
9. Dwivedi S, Goel A, Natu SM. et al. Diagnostic and prognostic significance of prostate specific antigen and serum interleukin 18 and 10 in patients with locally advanced prostate cancer: a prospective study. Asian Pac J Cancer Prev. 2011;12:1843-8
10. Vidal-Vanaclocha F, Mendoza L, Telleria N. et al. Clinical and experimental approaches to the pathophysiology of interleukin-18 in cancer progression. Cancer Metastasis Rev. 2006;25:417-34
11. Mühl H, Pfeilschifter J. Interleukin-18 bioactivity: a novel target for immunopharmacological anti-inflammatory intervention. Eur J Pharmacol. 2004;500:63-71
12. Park S, Cheon S, Cho D. The Dual Effects of Interleukin-18 in Tumor Progression. Cell Mol Immunol. 2007;4:329-35
13. Kang HS, Jin SJ, Myung CS. et al. Delivery of interleukin-18 gene to lung cancer cells using cationic emulsion. J Drug Target. 2009;17:19-28
14. Liu Y, Lin N, Huang L. et al. Genetic Polymorphisms of the Interleukin-18 Gene and Risk of Prostate Cancer. DNA Cell Biol. 2007;26:613-8
15. Ohki EC, Tilkins ML, Ciccarone UC. et al. Improving the transfection efficiency of post-mitotic neurons. J Neurosci Methods. 2001;112:95-9
16. Srivastava S, Salim N, Robertson MJ. Interleukin-18: biology and role in the immunotherapy of cancer. Curr Med Chem. 2010;17:3353-7
17. Carbone A, Rodeck U, Mauri FA. et al. Human pancreatic carcinoma cells secrete bioactive interleukin-18 after treatment with 5-fluorouracil: implications for anti-tumor immune response. Cancer Biol Ther. 2005;4:231-41
18. Hwang KS, Cho WK, Yoo J. et al. Adenovirus-mediated interleukin-18 mutant in vivo gene transfer inhibits tumor growth through the induction of T cell immunity and activation of natural killer cell cytotoxicity. Cancer Gene Ther. 2004;11:397-407
19. Xu J, Deng TL, Li L. et al. Nitric oxide inducing function and intracellular movement of chicken interleukin-18 in cultured cells. Acta Biochim Biophys Sin (Shanghai). 2005;37:688-93
Author contact
![]() Corresponding author: Jian Xu, Life Science College, Zhejiang Chinese medical university, Hangzhou 310053, China. Tel: +86-571-86633001, E-mail: xujianmdcom
Corresponding author: Jian Xu, Life Science College, Zhejiang Chinese medical university, Hangzhou 310053, China. Tel: +86-571-86633001, E-mail: xujianmdcom

 Global reach, higher impact
Global reach, higher impact