3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(12):1720-1726. doi:10.7150/ijms.6651 This issue Cite
Research Paper
Prevention of Pleural Adhesions by Bioactive Polypeptides - A Pilot Study
Department of Surgery, Clinical Sciences Lund University, Lund, Sweden.
Received 2013-5-8; Accepted 2013-9-2; Published 2013-10-8
Abstract
Objective: Postoperative pleural adhesions lead to major problems in repeated thoracic surgery. To date, no antiadhesive product has been proven clinically effective. Previous studies of differently charged polypeptides, poly-L-lysine (PL) and poly-L-glutamate (PG) have shown promising results reducing postoperative abdominal adhesions in experimental settings. This pilot study examined the possible pleural adhesion prevention by using the PL+PG concept after pleural surgery and its possible effect on key parameters; plasmin activator inhibitor-1 (PAI-1) and tissue growth factor beta 1 (TGFb) in the fibrinolytic process.
Methods: A total of 22 male rats were used in the study, one control group (n=10) and one experimental group (n=12). All animals underwent primary pleural surgery, the controls receiving saline in the pleural cavity and the experimental group the PL+PG solution administered by spray. The animals were evaluated on day 7. Macroscopic appearance of adhesions was evaluated by a scoring system. Histology slides of the adhesions and pleural biopsies for evaluation of PAI-1 and TGFb1 were taken on day 7.
Results: A significant reduction of adhesions in the PL+PG group (p<0.05) was noted at day 7 both regarding the length and severity of adhesions. There were no significant differences in the concentration of PAI-1 and TGFb1 when comparing the two groups.
Conclusions: PL+PG may be used to prevent pleural adhesions. The process of fibrinolysis, and fibrosis was though not affected after PLPG administration.
Keywords: postoperative, pleural adhesions, polylysine, polyglutamate, PAI-1, TGFb1.
INTRODUCTION
The leading causes of adhesions emanating from serous organs such as pleura, the pericardium and peritoneum are various types of injuries, such as surgery and infections [1-3]. Desquamation of pleural adhesions can induce hemorrhage. Multiple operative procedures in the thoracic cavity have become increasingly common for treatment of secondary malignancies and pneumothorax [4]. Postoperative adhesions after the first surgical intervention makes the operation more challenging, constituting a potential risk of e.g. prolonging the duration of surgery and increase the risk of harming vital structures [5]. Even if VATS (video assisted thoracic surgery) is used 54% of the patients develop pleural adhesions after surgery, thereby stating that the problem with pleural adhesions still remains [5, 6].
Various types of agents have been tested in order to prohibit the development of postoperative pleural and pericardial adhesions with promising results [7-10], but none is widely used in clinical practice. There are some experimentally and clinically used antiadhesive agents for the prevention of postoperative intraabdominal adhesions, such as Seprafilm™ and Statofilm, but none have yet proven as an ideal solution in the thoracic cavity [11-13].
Previous animal studies have focused on combining the oppositely charged polypeptides poly-L-lysine (PL) and poly-L-glutamate (PG) to form a neutral biodegradable matrix at the site of peritoneal injury in order to prevent postoperative abdominal adhesions [14, 15]. The results have been promising with no negative aspects noted on immunology, bleeding or inflammation/healing [15, 16].
In this pilot study, we investigated if the polypeptides (PL+PG) administered by spray might prevent the development of pleural adhesions. Furthermore, concentrations of PAI-1 and active TGFb1 in the pleural adhesions, were measured, since these are known to be important parameters/factors involved in the formation of adhesions.
MATERIALS AND METHODS
Animals
A total of 22 male Sprague Dawley rats (Charles River, Sulzfeld, Germany) weighing approximately 250g each were used for the induction of pleural adhesions. The animals were kept under standardized conditions and had free access to water and pellets. The study was approved by the local ethical committee at Lund University (Lund, Sweden) and the animals received the best animal care in compliance with the guidelines of the Swedish Government and Lund University, Sweden.
Chemicals
The chemicals, poly-L-lysine (PL) MW>30kDa and poly-L-glutamate (PG) MW 15-50kD (Sigma Aldrich™, St. Louis, Missouri, USA), were freshly prepared at the day of the experiment with 2.54% glycerol and water in order to create an osmotic balanced solution to a final concentration of 0.5% (5mg/ml). They were put in separate bottle atomizers (Aptar Pharma, Crystal Lake, Illinois, USA) that administrated 0.025 ml volume with one spray dose. Four spray doses were used equivalent to 0.1ml.
Model
The animals were blindly and randomly selected to receive either PLPG (N=12, the experiment group) or receive saline (N=10, the control group, table 1).
The rats were anesthetized with 50mg/kg Ketalar™ (Parker Davis™ Detroit, Michigan, USA) and Xylazine 6mg/kg (Rompun™ Vet, Bayer AB, Gothenburg, Sweden) by intramuscular injections.
An incision over the trachea was made and the neck muscles were bluntly dissected. A thin catheter (16G plastic catheter) was introduced into the trachea as a tracheotomy; this technique has been described in previous studies [17, 18]. The catheter was connected to a ventilator providing 90 respirator counts and 10 ml/kg of tidal volume per minute.
A left anterolateral thoracotomy (2cm) between the fourth and fifth costae was performed during sterile conditions and a standardized pleural adhesion-creating model was performed, previously described by Takagi et al. [11]. In brief, the pleural adhesions were created by the electro-cauterized incision of the thorax wall. The experimental group received PL (0.5%, 0.100 ml) followed by PG (0.5%, 0.100 ml) at a distance of 2cm from the incised area by a spray atomizer treating both visceral and parietal pleura. Following treatment, immediate closure of the external thoracic muscles using running sutures was performed (5-0 Prolene™ Ethicon, Somerville, NJ, USA). The control group received 0.9% NaCl (0.100 ml). Prior to skin closure the animals received 0.5ml of bupivacaine injected at the wound site as local anaesthetic. The animals received subcutaneous saline (0.9%, 5 ml) for resuscitation and buprenorphine for postoperative pain control. All animals were weighed in conjunction with every operative procedure.
Evaluation
The pleural adhesions were evaluated 7 days after the primary surgery. All animals were weighed prior to (day 0) and after (day 7) surgery. The severity of adhesions was determined with a six-graded scale according to Oncel et al [19] (Table 1). The length of the adhesions was graded according to Takagi et al [11] (Table 2).
Evaluation of severity of adhesion according to Oncel et al (Ref 19).
| Grade 0 | No adhesions |
|---|---|
| Grade 1 | Loose filmy adhesions that can be separated by traction |
| Grade 2 | Adhesions requiring blunt dissection for separation |
| Grade 3 | Adhesions requiring sharp dissection for separation |
| Grade 4 | Serosal injury |
| Grade 5 | Tissue injury |
Evaluation of the length of the adhesions according to Takagi et al (Ref 11).
| Grade 0 | No adhesions |
|---|---|
| Grade 1 | <25% of intercostal incision line |
| Grade 2 | 25%-50% of intercostal incision line |
| Grade 3 | 50%-75% of intercostal incision line |
| Grade 4 | 75%-100% of intercostals line |
Biopsies from the adhesions were taken at day 7, immediately allocated to tubes and snap frozen to -80°C. Tissue samples were homogenized in acetate buffer in order to efficiently extract the proteins. Active proteins were normalized to total protein content [20]. PAI-1 and active TGFb1 levels were determined using ELISA techniques, all according to the manufacturer's instructions.
The visceral pleura histology biopsies were taken in the middle of the pleura incision. These biopsy samples were taken for evaluation at day 7 for preparing paraffin embedded specimens, and stained for collagen with Massons-Trichrome in order to visualize fibrosis. After evaluation, the animals were euthanized by an overdose of Ketalar™ and Rompun™ according to AVMA guidelines on euthanasia 2007 [21] whilst still kept under anaesthesia.
Methods of adhesion evaluation in experiment and control group.
| Day 0 | Day 7 | |
|---|---|---|
| Experimental group/ PLPG | Adhesion creative surgery | - Evaluation of adhesions Oncel et al and Takagi et al |
| - Evaluation of TGFb1 and PAI-1 in biopsies of pleural adhesions | ||
| - Histology of pleural adhesions | ||
| Control group/ NaCl | Adhesion creative surgery | - Evaluation of adhesions Oncel et al and Takagi et al |
| - Evaluation of TGFb1 and PAI-1 in biopsies of pleural adhesions | ||
| - Histology of pleural adhesions |
Statistical analysis
Results describing the length and severity of adhesions, as well as results of PAI-1 and TGFb were shown as median +/- interquartile. Mann Whitney U test was used due to the nonparametric distribution and p<0.05 was considered significant. The statistical analyses were performed using SPSS (SPSS® v19.0, SPSS Inc., Chicago, Ill., USA).
RESULTS
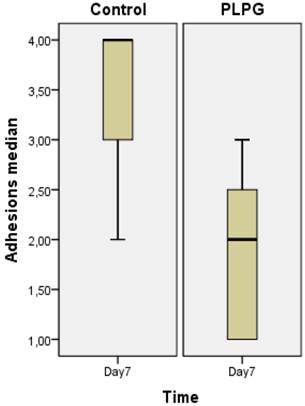
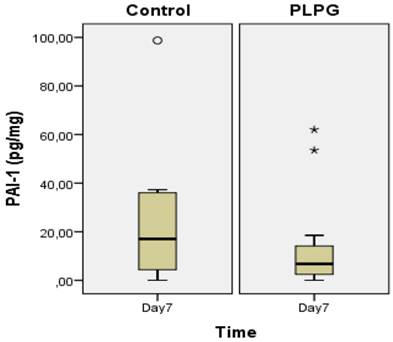
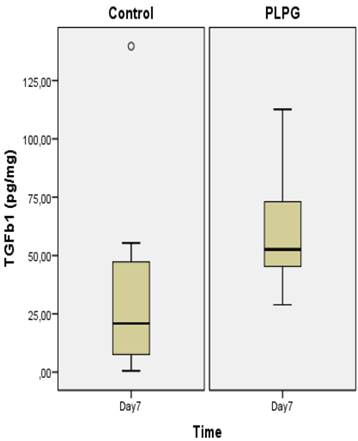
Almost all animals in the control group showed severe and long adhesions on gross examination (Figure 1). No animals exhibited the worst severity (grade 5). In the PLPG treatment group, adhesions were less severe and shorter on macroscopic examination, but no animals in the treated group had any adhesions. The median length of adhesions according to Takagi et al was 2 (+/- 1) in the treated animals and 4(+/-2) in the control group at day 7. This reached statistical significance (p<0.05; Figure 2). A significant lower severity in quality of the adhesions according to Oncel et al was also detected at day 7 (p<0.05; Figure 3). No significant differences were seen in PAI-1 (p=0.322) or TGFb1 levels (p= 0.378) between PLPG and control animals at day 7 (Figures 4 and 5).
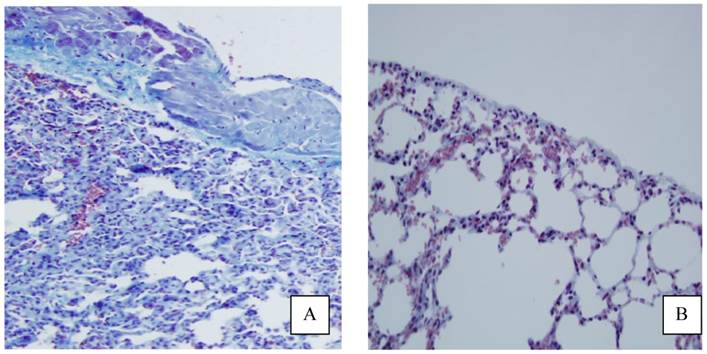
Histology slides showed macroscopically less fibrosis in the pleura of animals treated with PLPG than seen in the control group (Figure 6), but this was not quantified.
The weight of the animals did not differ between groups before surgery (day 0) or before evaluation procedure (day 7).
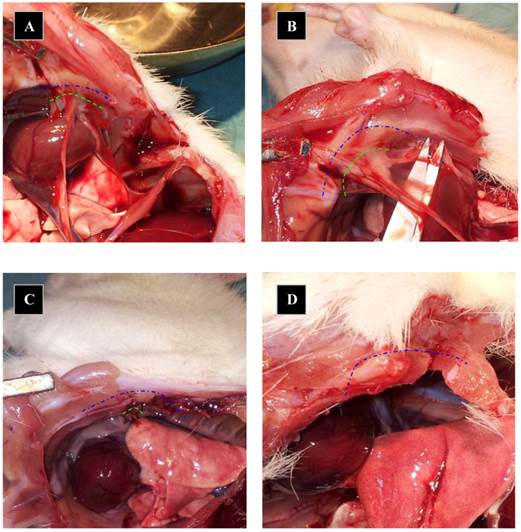
Pictures of macroscopical appearance. The pleura after 7 days. Adhesions length marked as green dotted line when evaluated at day 7 and blue dotted line mark the total length of pleural cauterized incision at day 0. Animals in A and B received NaCl after pleural adhesions were created and animals from picture C and D received PLPG. Dotted lines depict thoracotomy incision.
Severity of adhesions. Severity of adhesions according to Oncel et al (Ref 19) expressed as median +/- interquartile range measured in the control and experimental groups. A significant decrease in the quality of adhesions was seen in the PLPG (p<0.05) group at day 7 as compared to the controls.
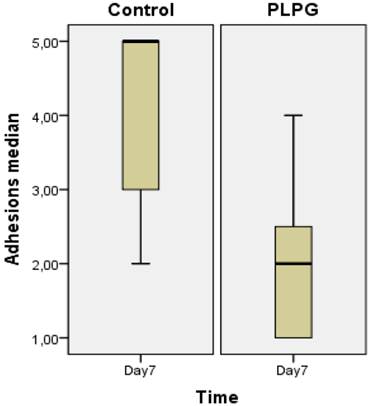
Length of adhesions. The length of adhesions was graded according to Takagi et al (Ref 11) and expressed as median+/- interquartile range measured in the control and experimental groups. Significantly less adhesion were seen in the PLPG (p<0.05) group at day 7 as compared to the controls.
Results of PAI-1 in biopsies. Levels of PAI-1 (pg/mg) in pleural adhesions at day 7 in the control group as compared to the PLPG group expressed as median +/- interquartile range. No significant difference was seen between the groups, (p=0.322). º and * show outliers.
Results of TGFb1 in biopsies. Levels of TGFb1 (pg/mg) in pleural adhesions at day 7 in the control group as compared to the PLPG group expressed as median +/- interquartile range. No significant difference was seen between the groups, (p= 0.378). º show outliers.
Appearance of pleural fibrosis on lung side with and without treatment. Histology slides stained for collagen with Massons-Trichrome showing more pronounced fibrosis in the control (A) group as was seen in the PLPG group (B).
DISCUSSION
In this pilot study we have demonstrated that the administration of differently charged polypeptides (PLPG) significantly diminished the severity of pleural adhesions and reduced the length, as well as the numbers of adhesions in a standardized rat model on pleural adhesions.
Previous studies have shown that the combination of PL and PG forms a neutrally charged biodegradable polymer that seals off the injured peritoneum, thereby decreasing development of adhesions [15]. Since the peritoneum and pleura share the same features when it comes to embryological origin, function and pattern for resolving after an injury we therefore hypothesized that the polymer complex of PLPG would react similarly when administered on a pleural injured surface as when delivered on a peritoneal injury [22-24].
Several previous studies have been made regarding the prevention of pleural adhesions [25-27]. Most of these have used biologic barriers as the principal anti-adhesive mechanisms. One of those studies has used carboxymethylcellulose combined with hyaluronic acid into a gel form (CMC+HA) [28]. This agent has proven promising results concerning the prevention of peritoneal adhesions. However, when it comes to prevent pleural adhesions the results have been somewhat contradictory [29, 30]. One recent study with a short polycation actually showed significantly shorter adhesions as compared to the CMC+HA when evaluated 28 days after pleural surgery [11]. Other studies have used HA and yet others collagen with positive results on pleural adhesion prevention [7, 31].
There have not yet been any reports comparing the anti-adhesion effect of PLPG with other anti-adhesive agents preventing pleural or abdominal adhesions. Thus one can only speculate regarding the differences between various groups in such a study. One study has been published indicating that CMC+HA (Seprafilm®) might increase the risk of granuloma generation in some cases, which may be hazardous regarding the use against pleural adhesions [32].
Another important aspect when using anti-adhesives in pleura is that the device should be easy to administer. We speculate that using a spray when administering anti-adhesives onto pleura e.g. PLPG is easier to handle than a non-spray device e.g. Interceed™ (Oxidized regenerated cellulose) or Seprafilm™ (CMC+HA bio films)
The electrostatic interaction between the outer bilipid cell surface membrane and various poly-L-lysines have previously been reported to be very rapid [33] and we speculated that the PL in our study might have interacted in the same way with the mesothelial cell on the pleura after administration. We further hypothesized that the administered PLPG would work as a biological barrier with sealant functions that would diminish the deposition of pleural adhesions after injury. The PL polymer was first administered as the positive cat ion adhering to the damaged pleura. The negative anion PG was then administered seconds thereafter interacting with PL producing a neutrally charged matrix, which thereby sealed the damaged area acting as a barrier to prevent postoperative adhesions.
The critical time for adhesions to develop is between 4 to 6 days and one previous study have shown that PLPG complex resides on the peritoneum more than one week after administration [34]. After 28 days the PLPG complex is degraded from the peritoneal site [15].
We choose to keep the polypeptides in a solution since we wanted to saturate the surface that was abraded on the pleura using a spray bottle that was easy and convenient to use in all angles. The spray bottle had lucent walls and the volume and amount of the dissolved polypeptides that was distributed at the pleural injured surface was the same at all times administered. Prior to the study we tested the spray bottle on to a paper and noticed that the aerosols of the spray reached a large area despite a short distance from the paper (20mm) (data not shown).
We deliberately lowered the administered volume of NaCl and PLPG (0.1ml) since rat pleura is sensitive to high volumes (1 ml was too high) despite this, the results were significant. One previous study has shown that the dose response effect of PLPG regarding abdominal adhesions is as low as 1.6mg/kg. In this study the dose was 2 mg/kg. PLPG has not shown any toxic effects itself in several previous experiments [14-16, 34-37]. If administered alone, PL exerts toxicity when not combined with PG. The LD50 dose has been established to be 40mg/kg when PL was administered alone [35]. In this study all animals fared well receiving the combination of PL and PG. However, future LD50 studies will have to be made in order to fully unravel if PL has any potential toxicity used in thorax and its dose response relationship regarding adhesions versus volume and dose.
Previous studies have shown that other biobarriers may be limited in their capacity to prevent adhesions if they are not spread enough on the peritoneum [38, 39]. For this reason, we chose to administer the PLPG with spray in order to enable a larger pleural surface to be saturated by the polypeptides.
A crucial and potent mechanism of creating pleural adhesions is the presence of foreign particles that potentially may form granuloma, which may end up in adhesions [40, 41]. One of our previous peritoneal studies have shown that the PLPG complex is completely absorbed in the submesothelial space. This study was carried out using fluorescence and electron microscopy that could trace the PLPG complex after several days being incorporated in the submesothelial space most likely ingested by the unaffected macrophages. [15] From this, we hypothesized, that PLPG would be ideal, leaving no foreign particles behind and hence no potential late effects with pleural adhesions formation.
The resolving process of the pericardium and pleura is similar to when the peritoneum resolves after injury [23, 42, 43]. The pleura and the pericardium are serous organs with supportive and protective functions. Both are lined with a single layer of mesothelial cells supported on a basal membrane that in turn rests on a rich vascular supplied connective tissue which also contains dormant inactivated cells with supportive and protective functions. Mesothelial cell detachment is an immediate consequence of pleural, pericardial and peritoneal injury [2, 44]. The injured pleura secrete various substances from adjacent and remote activated cells (mesothelial cells, macrophages, fibroblasts, among others) in a time dynamic manner [45-47]. Among the substances involved are important modulators of the serine proteases plasminogen activator inhibitor (PAI-1), as well as the fibrosis transforming growth factor beta (TGFb1). These substances (and others) have the capability of reducing fibrinolysis via inhibition of the activation of the serine protease (and other proteases) plasmin and also through other mechanisms [48]. The reduced fibrinolysis results in more abundant fibrin remnants that serve as a scaffold for the adhesion development through replacement of the fibrin residues by native collagen.
In this study some minor differences were seen in concentrations of PAI-1 and TGFb1 between the PLPG and controls. The small variations in concentrations of PAI-1 and TGFb1 might be explained by the differences in the quality of adhesions. However, we hypothesized, that the PLPG complex did not cause the small differences observed. In previous papers examining peritoneal adhesions no differences between treated and untreated animals were shown regarding levels of PAI-1 and TGFb1 [36, 37].
Conclusion
In this pilot study we have shown that PLPG diminishes the formation of pleural adhesions and that the resolving process of pleura does not seem to be affected. We also found that the PLPG dissolved in fluid was easy to administer and spray over the pleura as an aerosol.
Acknowledgements
This study was in parts financed by The Zoegas foundation in Helsingborg Sweden.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ogata Y, Matono K, Hayashi A, Takamor S, Miwa K, Sasatomi T, Ishibashi N, Shida S, Shirouzu K. Repeat pulmonary resection for isolated recurrent lung metastases yields results comparable to those after first pulmonary resection in colorectal cancer. World J Surg. 2005;29:363-8
2. Nkere UU, Whawell SA, Sarraf CE, Schofield JB, Thompson JN, Taylor KM. Pericardial trauma and adhesions in relation to reoperative cardiac surgery. Thorac Cardiovasc Surg. 1995;43:338-46
3. Brokelman WJ, Holmdahl L, Janssen IM, Falk P, Bergstrom M, Klinkenbijl JH, Reijnen MM. Decreased peritoneal tissue plasminogen activator during prolonged laparoscopic surgery. J Surg Res. 2009;151:89-93
4. Shah SA, Haddad R, Al-Sukhni W, Kim RD, Greig PD, Grant DR, Taylor BR, Langer B, Gallinger S, Wei AC. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg. 2006;202:468-75
5. Dobell AR, Jain AK. Catastrophic hemorrhage during redo sternotomy. Ann Thorac Surg. 1984;37:273-8
6. Tanaka K, Hida Y, Kaga K, Kato H, Iizuka M, Cho Y, Kondo S. Video-assisted thoracoscopic surgery lowers the incidence of adhesion to the chest wall but not to the mediastinal and interlobar pleurae. Surgical laparoscopy, endoscopy & percutaneous techniques. 2010;20:46-8
7. Seeger JM, Kaelin LD, Staples EM, Yaacobi Y, Bailey JC, Normann S, Burns JW, Goldberg EP. Prevention of postoperative pericardial adhesions using tissue-protective solutions. J Surg Res. 1997;68:63-6
8. Hsin MK, Yim AP. Management of complications of minimally invasive thoracic surgery. Respirology. 2010;15:6-18
9. Duncan DA, Yaacobi Y, Goldberg EP, Mines M, O'Brien D, Congdon F, Carmichael MJ. Prevention of postoperative pericardial adhesions with hydrophilic polymer solutions. J Surg Res. 1988;45:44-9
10. Mitchell JD, Lee R, Hodakowski GT, Neya K, Harringer W, Valeri CR, Vlahakes GJ. Prevention of postoperative pericardial adhesions with a hyaluronic acid coating solution. Experimental safety and efficacy studies. J Thorac Cardiovasc Surg. 1994;107:1481-8
11. Takagi K, Tsuchiya T, Araki M, Yamasaki N, Nagayasu T, Hyon SH, Nakajima N. Novel biodegradable powder for preventing postoperative pleural adhesion. J Surg Res. 2013;179:e13-9
12. Lalountas M, Ballas KD, Michalakis A, Psarras K, Asteriou C, Giakoustidis DE, Nikolaidou C, Venizelos I, Pavlidis TE, Sakantamis AK. Postoperative adhesion prevention using a statin-containing cellulose film in an experimental model. Br J Surg. 2012;99:423-9
13. Lalountas MA, Ballas KD, Skouras C, Asteriou C, Kontoulis T, Pissas D, Triantafyllou A, Sakantamis AK. Preventing intraperitoneal adhesions with atorvastatin and sodium hyaluronate/carboxymethylcellulose: a comparative study in rats. Am J Surg. 2010;200:118-23
14. Nehez L, Tingstedt B, Axelsson J, Andersson R. Differently charged polypeptides in the prevention of post-surgical peritoneal adhesions. Scand J Gastroenterol. 2007;42:519-23
15. Nehez L, Tingstedt B, Vodros D, Axelsson J, Lindman B, Andersson R. Novel treatment in peritoneal adhesion prevention: protection by polypeptides. Scand J Gastroenterol. 2006;41:1110-7
16. Tingstedt B, Nehez L, Lindman B, Andersson R. Efficacy of bioactive polypeptides on bleeding and intra-abdominal adhesions. Eur Surg Res. 2007;39:35-40
17. Patrick G, Stirling C. Measurement of mucociliary clearance from the trachea of conscious and anesthetized rats. J Appl Physiol. 1977;42:451-5
18. Takahashi S, Patrick G. Long-term retention of 133Ba in the rat trachea following local administration as barium sulfate particles. Radiation research. 1987;110:321-8
19. Oncel M, Remzi FH, Senagore AJ, Connor JT, Fazio VW. Comparison of a novel liquid (Adcon-P) and a sodium hyaluronate and carboxymethylcellulose membrane (Seprafilm) in postsurgical adhesion formation in a murine model. Dis Colon Rectum. 2003;46:187-91
20. Holmdahl L, Eriksson E, Risberg B. Measurement of fibrinolytic components in human tissue. Scand J Clin Lab Invest. 1997;57:445-51
21. http://www.avma.org/issues/animal_welfare/euthanasia.pdf
22. Michailova KN, Usunoff KG. Serosal membranes (pleura, pericardium, peritoneum). Normal structure, development and experimental pathology. Advances in anatomy, embryology, and cell biology. 2006;183:1-144
23. Michailova KN. Ultrastructural observations on the human visceral pleura. European journal of morphology. 1997;35:125-35
24. Michailova KN. The serous membranes in the cat. Electron microscopic observations. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 1996;178:413-24
25. Peyton CC, Keys T, Tomblyn S, Burmeister D, Beumer JH, Holleran JL, Sirintrapun J, Washburn S, Hodges SJ. Halofuginone infused keratin hydrogel attenuates adhesions in a rodent cecal abrasion model. J Surg Res. 2012;178:545-52
26. Hill-West JL, Chowdhury SM, Sawhney AS, Pathak CP, Dunn RC, Hubbell JA. Prevention of postoperative adhesions in the rat by in situ photopolymerization of bioresorbable hydrogel barriers. Obstet Gynecol. 1994;83:59-64
27. Naito Y, Shin'oka T, Hibino N, Matsumura G, Kurosawa H. A novel method to reduce pericardial adhesion: a combination technique with hyaluronic acid biocompatible membrane. J Thorac Cardiovasc Surg. 2008;135:850-6
28. Ballore L, Orru F, Nicolini F, Contini SA, Galletti G, Gherli T. [Experimental results of the use of hyaluronic acid based materials (CV Seprafilm and CV Sepracoat) in postoperative pericardial adhesions]. Acta bio-medica de L'Ateneo parmense: organo della Societa di medicina e scienze naturali di Parma. 2000;71:159-66
29. Bel A, Kachatryan L, Bruneval P, Peyrard S, Gagnieu C, Fabiani JN, Menasche P. A new absorbable collagen membrane to reduce adhesions in cardiac surgery. Interactive cardiovascular and thoracic surgery. 2010;10:213-6
30. Noishiki Y, Shintani N. Anti-adhesive membrane for pleural cavity. Artificial organs. 2010;34:224-9
31. Mitchell JD, Lee R, Neya K, Vlahakes GJ. Reduction in experimental pericardial adhesions using a hyaluronic acid bioabsorbable membrane. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 1994;8:149-52
32. Klingler PJ, Floch NR, Seelig MH, Branton SA, Wolfe JT, Metzger PP. Seprafilm-induced peritoneal inflammation: a previously unknown complication. Report of a case. Dis Colon Rectum. 1999;42:1639-43
33. Hategan A, Sengupta K, Kahn S, Sackmann E, Discher DE. Topographical pattern dynamics in passive adhesion of cell membranes. Biophys J. 2004;87:3547-60
34. Nehez L, Vodros D, Axelsson J, Tingstedt B, Lindman B, Andersson R. Prevention of postoperative peritoneal adhesions: effects of lysozyme, polylysine and polyglutamate versus hyaluronic acid. Scand J Gastroenterol. 2005;40:1118-23
35. Isaksson K, Akerberg D, Andersson R, Tingstedt B. Toxicity and dose response of intra-abdominally administered poly-L-alpha-lysine and poly-L-glutamate for postoperative adhesion protection. Eur Surg Res. 2010;44:17-22
36. Åkerberg D Grunditz C, Posaric- Bauden M, Isaksson K, Andersson R, Tingstedt B. The Influence on abdominal adhesions and inflammation in rabbits after exposure to differently charged polypeptides. JBiSE. 2012;5:432-38
37. Åkerberg D Isaksson K, Posaric- Bauden M, Andersson R, Tingstedt B. Effects of polylysine and polyglutamate on inflammation and the normal process of peritoneal healing after surgery. J Tissue Sci Eng. 2012;3:117-23
38. Lim R, Morrill JM, Lynch RC, Reed KL, Gower AC, Leeman SE, Stucchi AF, Becker JM. Practical limitations of bioresorbable membranes in the prevention of intra-abdominal adhesions. J Gastrointest Surg. 2009;13:35-41 discussion -2
39. Sheldon HK, Gainsbury ML, Cassidy MR, Chu DI, Stucchi AF, Becker JM. A sprayable hyaluronate/carboxymethylcellulose adhesion barrier exhibits regional adhesion reduction efficacy and does not impair intestinal healing. J Gastrointest Surg. 2012;16:325-33
40. Ferrer J, Montes JF, Villarino MA, Light RW, Garcia-Valero J. Influence of particle size on extrapleural talc dissemination after talc slurry pleurodesis. Chest. 2002;122:1018-27
41. Fraticelli A, Robaglia-Schlupp A, Riera H, Monjanel-Mouterde S, Cau P, Astoul P. Distribution of calibrated talc after intrapleural administration: an experimental study in rats. Chest. 2002;122:1737-41
42. Li J. Ultrastructural study on the pleural stomata in human. Functional and developmental morphology. 1993;3:277-80
43. Michailova KN. A combined electron microscopic investigation of the peritoneal mesothelium in the rat. European journal of morphology. 1995;33:265-77
44. Hurle A, de la Vega M, Feijoo JJ, Ray VG, Abad C, Ponce G, Perez-Arellano JL. Effect of physical protection on the mesothelial integrity of the pericardium. Ann Thorac Surg. 1997;63:1091-4
45. Cheng D, Lee YC, Rogers JT, Perkett EA, Moyers JP, Rodriguez RM, Light RW. Vascular endothelial growth factor level correlates with transforming growth factor-beta isoform levels in pleural effusions. Chest. 2000;118:1747-53
46. Baumann MH, Heinrich K, Sahn SA, Green C, Harley R, Strange C. Electron microscopic analysis of the normal and the activated pleural macrophage. Exp Lung Res. 1993;19:731-42
47. Baumann MH, Strange C, Sahn SA, Kinasewitz GT. Pleural macrophages differentially alter pleural mesothelial cell glycosaminoglycan production. Exp Lung Res. 1996;22:101-11
48. Idell S, Zwieb C, Kumar A, Koenig KB, Johnson AR. Pathways of fibrin turnover of human pleural mesothelial cells in vitro. Am J Respir Cell Mol Biol. 1992;7:414-26
Author contact
![]() Corresponding author: Bobby Tingstedt, MD, PhD, Associate Professor. Department of Surgery, Skåne University Hospital at Lund, Clinical Sciences Lund, Lund University, SE-221 85 Lund, Sweden. Tel: +46-46171434 Email: Bobby.tingstedtlu.se.
Corresponding author: Bobby Tingstedt, MD, PhD, Associate Professor. Department of Surgery, Skåne University Hospital at Lund, Clinical Sciences Lund, Lund University, SE-221 85 Lund, Sweden. Tel: +46-46171434 Email: Bobby.tingstedtlu.se.

 Global reach, higher impact
Global reach, higher impact