3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(12):1665-1673. doi:10.7150/ijms.6727 This issue Cite
Research Paper
Dose Adjustment Strategy of Cyclosporine A in Renal Transplant Patients: Evaluation of Anthropometric Parameters for Dose Adjustment and C0 vs. C2 Monitoring in Japan, 2001-2010
1. Department of Hospital Pharmacy, Kyoto Prefectural University of Medicine
2. Department of Clinical Pharmacokinetics, Faculty of Pharmaceutical Sciences, Kobe Gakuin University
3. Department of Transplantation and Regenerative Surgery, Kyoto Prefectural University of Medicine
Received 2013-5-20; Accepted 2013-8-30; Published 2013-9-23
Abstract
The optimal use and monitoring of cyclosporine A (CyA) have remained unclear and the current strategy of CyA treatment requires frequent dose adjustment following an empirical initial dosage adjusted for total body weight (TBW). The primary aim of this study was to evaluate age and anthropometric parameters as predictors for dose adjustment of CyA; and the secondary aim was to compare the usefulness of the concentration at predose (C0) and 2-hour postdose (C2) monitoring. An open-label, non-randomized, retrospective study was performed in 81 renal transplant patients in Japan during 2001-2010. The relationships between the area under the blood concentration-time curve (AUC0-9) of CyA and its C0 or C2 level were assessed with a linear regression analysis model. In addition to age, 7 anthropometric parameters were tested as predictors for AUC0-9 of CyA: TBW, height (HT), body mass index (BMI), body surface area (BSA), ideal body weight (IBW), lean body weight (LBW), and fat free mass (FFM). Correlations between AUC0-9 of CyA and these parameters were also analyzed with a linear regression model. The rank order of the correlation coefficient was C0 > C2 (C0; r=0.6273, C2; r=0.5562). The linear regression analyses between AUC0-9 of CyA and candidate parameters indicated their potential usefulness from the following rank order: IBW > FFM > HT > BSA > LBW > TBW > BMI > Age. In conclusion, after oral administration, C2 monitoring has a large variation and could be at high risk for overdosing. Therefore, after oral dosing of CyA, it was not considered to be a useful approach for single monitoring, but should rather be used with C0 monitoring. The regression analyses between AUC0-9 of CyA and anthropometric parameters indicated that IBW was potentially the superior predictor for dose adjustment of CyA in an empiric strategy using TBW (IBW; r=0.5181, TBW; r=0.3192); however, this finding seems to lack the pharmacokinetic rationale and thus warrants further basic and clinical investigations.
Keywords: cyclosporine, dose adjustment, ideal body weight, C0 monitoring, C2 monitoring
INTRODUCTION
Cyclosporine A (CyA) is a lipophilic cyclic undecapeptide originally derived from the filamentous fungus Tolypocladium inflatum.1 CyA has been used as a potent immunosuppressive agent for the treatment of autoimmune diseases and a number of organ transplantations, such as kidney, liver, heart, and bone marrow,2-7 resulting in a significant improvement in clinical outcome. Despite its effectiveness, there are several problems and limitations in the treatment of CyA: these include transplant rejection,8 opportunistic infection,9 and renal toxicity.10
In medicine, most of drugs for adult patients are administered at a flat-fixed dose. Only the dosage of some drugs such as aminoglycosides, phenytoin, and sympathicomimetics are based on the total body weight (TBW) of the patients. In oncology, the dosage of most antitumor agents is adjusted for body surface area (BSA) of the patients.11 Dose adjustments of these drugs are required for their effectiveness and safety; the narrow therapeutic range of a drug from a maximum effect to a minimum toxicity demands a strict dosage. Therefore, to avoid adverse effects and to ensure its effectiveness, dosage individualization and therapeutic drug monitoring (TDM) mainly based on the plasma concentrations are required. CyA also has a narrow therapeutic range and requires TDM. However, CyA is extensively affected by many factors such as transplant organ, time after transplantation, presence of various disease states, concurrent use of drugs, race, age, and gender,12,13 resulting in a large individual variability. Therefore, the current strategy of CyA treatment demands frequent dose adjustment for TDM following empirical initial dosage adjusted for TBW, which would not be considered optimal.
It was commonly considered that the area under the whole blood concentration-time curve (AUC0-9) of CyA could be closely related to clinical outcome. However, TDM based on AUC0-9 required serial measurements of CyA blood concentrations at multiple time points after administration. With regard to the burden for patients, the prediction of AUC0-9 from a single-point monitoring is desired and the CyA blood level at predose (trough level: C0) has been widely used in clinical practice; recently, monitoring the blood level at 2-hour postdose (C2) is recommended.14 However, some authors state that the efficacy of C2 monitoring is uncertain and continue to support C0 monitoring.15,16
Although various strategies of TDM for CyA have been discussed and developed for more than 20 years, the optimal use and monitoring of CyA have remained unclear and frequent dose adjustment is needed. The primary aim of this retrospective study was to evaluate age, gender and anthropometric parameters as a predictor for dose adjustment of CyA and the secondary aim was to compare the usefulness of C0 and C2 monitoring. Therefore, we performed an open-label, non-randomized, retrospective study in 81 renal transplant patients in Japan during 2001-2010.
MATERIALS AND METHODS
Patients
This retrospective study focused on 81 adult (over age 20) patients who received a living-related renal transplantation and were treated with CyA at the University Hospital, Kyoto Prefectural University of Medicine, from July 2001 to February 2010. The exclusion criterion was either clinical diagnosis of transplant rejection, nephrotoxicity or cytomegalovirus infection. The characteristics of the study population are summarized in Table 1. In all subjects, immunosuppressive therapy was performed in the same way; main concomitant drugs for immunosuppressive therapy were nifedipine for renal hypertension and lafutidine for the prevention of steroid-induced gastric ulcer. Anti-hyperlipemia drugs including statin were not used in our institution, because hyperlipemia after transplantation was almost caused by corticosteroid medication and was reversible.
The study was approved by the Ethics Committee of Kyoto Prefectural University of Medicine, and informed consent was obtained from all subjects.
Equations of anthropometric parameters
| Anthropometric parameters | Unit | Equation | |
|---|---|---|---|
| Body mass index (BMI) | kg/m2 | = TBW / HT(m)2 | |
| Body surface area (BSA) | m2 | = TBW0.425 * HT(cm)0.725 * 0.007184 | |
| Ideal body weight (IBW) | kg | = 45.4 + 0.89 * (HT(cm) - 152.4) + 4.5 | for male |
| = 45.4 + 0.89 * (HT(cm) - 152.4) | for female | ||
| Lean body weight (LBW) | kg | = 1.1 * TBW - 0.0128 * BMI * TBW | for male |
| = 1.07 * TBW - 0.0148 * BMI * TBW | for female | ||
| Fat free mass (FFM) | kg | = 0.285 * TBW + 12.1 * HT(m)2 | for male |
| = 0.287 * TBW + 9.74 * HT(m)2 | for female |
TBW, total body weight; HT, height.
Immunosuppressive Regimen of CyA
The immunosuppressive regimen of CyA developed by the Department of Transplantation and Regenerative Surgery, Kyoto Prefectural University of Medicine, is as follows: for living-related renal transplantation, the initial dosage of CyA (Neoral®, approximately 12 mg/kg/day; Novartis Pharmaceuticals, East Hanover, NJ) was administered orally for 2 days before transplantation. CyA (Sandimmun®, 4 mg/kg/day; Novartis Pharmaceuticals, East Hanover, NJ) was administered by continuous intravenous infusion on the day of transplantation, and thence, followed by oral administration of CyA (Neoral®, approximately 12 mg/day/kg, twice daily after meals). The dosage of CyA was adjusted according to the daily/alternate-day blood trough level (C0) and weekly AUC0-9 of CyA on days 7, 14, 21 and 28. Target C0 level and AUC0-9 of CyA ranged from 200 to 300 ng/mL and from 5000 to 6000 ng•hr/mL, respectively. On the day of transplantation, methylprednisolone was given at a dosage of 500 mg. Subsequently, patients received prednisolone at 50 mg/day on days 0-3, 40 mg/day on days 4-11 and then the dosage was gradually reduced each week (30, 25, 20, 15 and then 10 mg/day orally twice daily after meals). On day 21, azathioprine (1-1.5 mg/kg/day) or mycophenolate mofetil (20-25 mg/kg/day) was added to the regimen.
Whole Blood Sampling and Pharmacokinetic Study of CyA
Whole blood samples for C0 level of CyA were obtained daily before dosing until 14 days after transplantation, and thence, were obtained every other day. In addition, to ensure the outcome of CyA treatment in our institution, pharmacokinetic studies of CyA were performed 2 days before and on days 7, 14 and 28 after transplantation, which consisted of a series of whole blood samples at 0, 1, 2, 3, 4, 6 and 9 hr after CyA administration. The blood concentration of CyA was measured by the antibody-conjugated magnetic immunoassay (ACMIA) method using an Dimension Xpand® system. The lower limit of detection in this method was 25 ng/mL. Inter- and intra-assay reproducibility were between 5-10 (C.V. %) according to results of International Proficiency Testing Scheme.
Pharmacokinetic Analysis of CyA
Non-compartmental pharmacokinetic analysis was applied to the pharmacokinetic study of CyA. The maximum whole blood concentration of CyA (Cmax) and the time when CyA concentration reached Cmax (tmax) were obtained as the measured values. The terminal elimination rate constant (λz) was determined by the linear regression of at least three data points from the terminal portion of the whole blood concentration profile. The terminal elimination half-life (t1/2) was determined by dividing ln2 by λz. The area under the whole blood concentration vs. time curve (AUC0-9) and the area under the first-moment curve to the last measured whole blood concentration (AUMC0-9) were calculated using the linear trapezoidal rule from 0 to 9 hr. The mean residence time (MRT) was calculated by dividing AUMC by AUC0-9. Absolute apparent total body clearance (CLtot(app,abs)) was calculated by dividing AUC0-9 by the actual dosage without any adjustment such as anthropometric parameters, and then the absolute apparent volume of distribution at steady state (Vdss(app,abs)) was calculated by MRT* CLtot(app,abs). All of the pharmacokinetic parameters including C0 and C2 levels obtained on day 28 were used in the following analysis, when these were considered to reach steady states.
Correlation between AUC0-9 and C0, C2 and Cmax of CyA
The relationships between AUC0-9 of CyA and its C0, C2 and Cmax level were assessed with a linear regression analysis model and the correlation coefficient was calculated. Prediction bias was estimated as the percent of mean prediction error (ME):
%ME = 1/n * ∑[(predicted value - observed value)/ observed value*100]
where n is the total number of samples, 81 patients in this study. Prediction precision was estimated as the percent of mean absolute prediction error (MAE):
%MAE = 1/n * ∑[|predicted value - observed value|/ observed value*100]
Smaller values for %ME and %MAE indicate less bias and greater precision. The regression model was evaluated as the percent of mean difference in %MAE (∆%MAE) in comparison to that using C0:
∆%MAE = %MAE(ƒ(C0)) - %MAE(ƒ(C2 or Cmax))
where %MAE(ƒ) represents the %MAE obtained by a regression model using C0, C2 or Cmax. If the value of ∆%MAE is positive, the regression model is superior to that using C0. In contrast, if negative, the regression model is inferior. In addition, the 95% confidence interval (95% C.I.) of ∆%MAE does not include 0, the superiority/inferiority of a regression model was judged to be statistically significant.
Anthropometric Parameters and Correlation of AUC0-9 of CyA
In addition to age, 7 anthropometric parameters were tested for a predictor for AUC0-9 of CyA: total body weight (TBW), height (HT), body mass index (BMI), body surface area (BSA), ideal body weight (IBW), lean body weight (LBW), and fat free mass (FFM). The equations to calculate these parameters except for TBW and HT are summarized in Table 1. Correlations between AUC0-9 of CyA and these parameters were analyzed with a linear regression model in which the adjusted dosages for these parameters were independent variables (dose/parameter) and the correlation coefficient was calculated. Prediction bias, precise and regression model were similarly evaluated using %ME, %MAE and ∆%MAE, where ∆%MAE is redefined as follow:
∆%MAE = %MAE(ƒ(dose/TBW)) - %MAE(ƒ(dose/parameter))
The effect of gender on CyA pharmacokinetic parameters including AUC0-9 were analyzed by Student's unpaired t-test.
All the values were presented as mean ± S.D. Analyses in this study were conducted using the StatView software package version 5.0 (SAS Institute Inc., Cary, North Carolina).
RESULTS
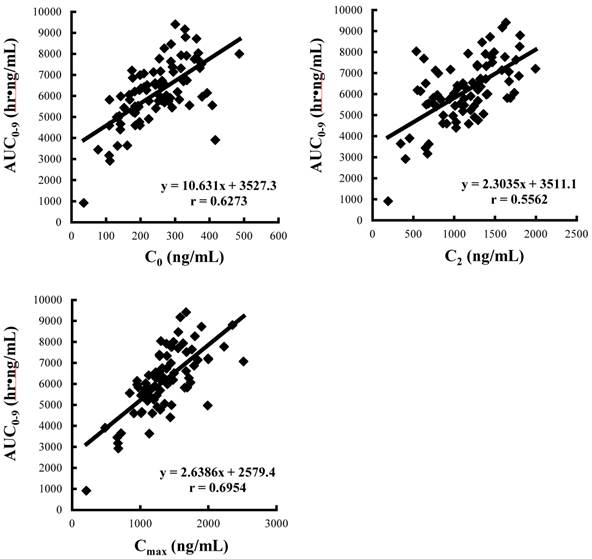
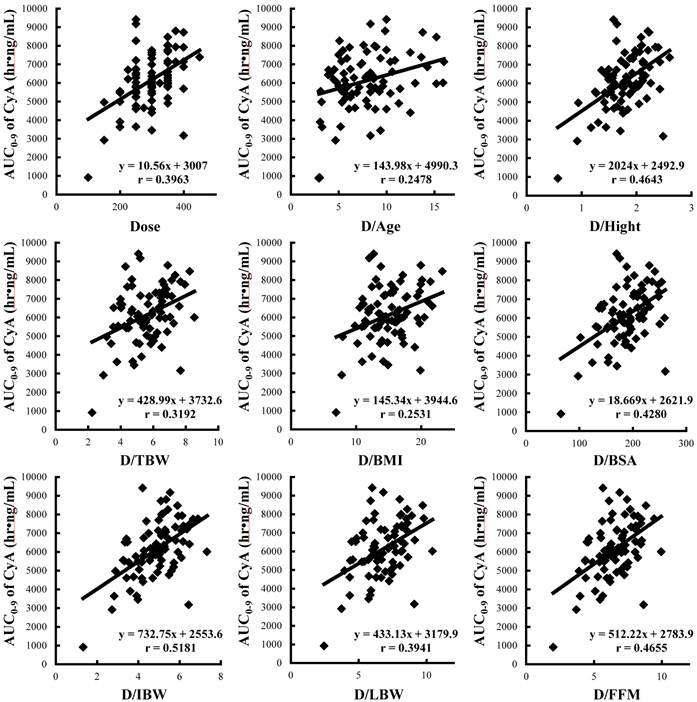
Demographic and anthropometric parameters of subjects are summarized in Table 2. There were significant differences in all anthropometric parameters between genders. Whole blood concentration profiles of CyA on day 28 were individually described in Figure 1 and the pharmacokinetic parameters are summarized in Table 3. Significant differences between genders were observed in dose, blood C2 level, CLtot(app,abs) and Vdss(app,abs). Figure 2 shows the correlations between AUC0-9 and C0, C2 and Cmax of CyA and correlation coefficients, %ME, %MAE and Δ%MAE are represented in Table 4. The rank order of correlation coefficients was Cmax > C0 > C2 and the same order was obtained for %ME and %MAE. However, there was no statistical superiority of C0 over C2 observed. Figure 3 shows correlations between AUC0-9 and absolute dose and adjusted dose for age and anthropometric parameters, and then correlation coefficient, %ME, %MAE and Δ%MAE are shown in Table 5. The rank order of correlation coefficient was dose/IBW > dose/FFM > dose/HT > dose/BSA > absolute dose > dose/LBW > dose/TBW > dose/BMI > dose/Age. The rank order of %ME and %MAE was the same, except that the rank of dose/FFM and dose/HT was reversed. However, there was no statistical superiority/inferiority over dose/TBW.
Demographic and anthropometric parameters of subjects
| Male | Female | ||
|---|---|---|---|
| (n=50) | (n=31) | ||
| Age | (year) | 43.3±13.8 | 38.5±12.5 |
| [23:70] | [22:64] | ||
| HT | (cm) | 168.4±5.9 | 158.0±6.8* |
| [153.5:179.4] | [141.4:173.0] | ||
| TBW | (kg) | 59.0±9.6 | 46.1±7.5* |
| [37.8:93.0] | [32.8:63.0] | ||
| BMI | (kg/m2) | 20.8±2.9 | 18.4±2.3* |
| [14.4:28.9] | [14.4:22.5] | ||
| BSA | (m2) | 1.67±0.14 | 1.43±0.13* |
| [1.29:2.12] | [1.15:1.71] | ||
| IBW | (kg) | 64.1±5.3 | 50.4±6.0* |
| [50.9:73.9] | [35.6:63.7] | ||
| LBW | (kg) | 48.9±5.9 | 36.6±4.7* |
| [33.8:67.9] | [27.1:46.4] | ||
| FFM | (kg) | 51.2±4.4 | 37.6±3.8* |
| [39.3:65.4] | [28.9:45.3] |
HT, height; TBW, total body weight; BMI, body mass index; BSA, body surface area; IBW, ideal body weight; LBW, lean body weight; FFM, fat free mass.
Each value represents the mean ± S.D. Square bracket indicates the range.
*, p<0.01 against male.
Pharmacokinetic parameters of CyA in renal transplant patients on day 28 after transplantation
| All subjects | male | Female | ||
|---|---|---|---|---|
| (n=81) | (n=50) | (n=31) | ||
| Dose | (mg) | 296.0±65.0 | 311.5±65.3 | 269.4±53.9** |
| C0 | (ng/mL) | 245.1±88.4 | 251.1±97.7 | 235.4±73.1 |
| C2 | (ng/mL) | 1138.1±391.8 | 1060.4±398.9 | 1263.3±358.2* |
| Cmax | (ng/mL) | 1346.7±402.5 | 1319.7±437.9 | 1390.2±339.9 |
| Tmax | (hr) | 2.38±0.93 | 2.50±1.02 | 2.19±0.75 |
| t1/2 | (hr) | 3.30±1.06 | 3.43±1.24 | 3.08±0.64 |
| MRT | (hr) | 5.60±1.50 | 5.82±1.78 | 5.25±0.75 |
| CLtot(app,abs) | (L/hr) | 41.81±13.6 | 45.0±15.6 | 36.7±7.6** |
| Vdss(app,abs) | (L) | 228.6±74.8 | 251.1±80.0 | 192.4±49.5** |
| AUC0-9 | (hr·ng/mL) | 6132.7±1494.1 | 5980.9±1559.9 | 6316.0±1341.8 |
C0, whole blood concentration at predose; C2, whole blood concentration at 2-hour postdose; Cmax, maximum whole blood concentration; Tmax, time when CyA concentration reached Cmax; t1/2, terminal elimination half-life; MRT, mean residence time; CLtot(app,abs), absolute apparent total clearance; Vdss(app,abs), absolute apparent volume of distribution at steady state.
Each value represents the mean ± S.D.
*, p<0.01, **, p<0.01, significant difference against male.
Correlation coefficients, %ME, %MAE and Δ%MAE between AUC0-9 and C0, C2 or Cmax of CyA
| Independent variables | Correlation coefficient | ME (%) | MAE (%) | ∆MAE (%)† | [95% C.I.]†† |
|---|---|---|---|---|---|
| C0 | 0.6326 | 6.87 | 18.86 | N.D. | N.D. |
| C2 | 0.6078 | 7.02 | 19.85 | -0.995 | [-4.55:2.57] |
| Cmax | 0.7108 | 5.37 | 16.66 | 2.199 | [-2.16:6.56] |
ME, mean prediction error.
MAE, mean absolute prediction error.
N.D., not determined.
†, ΔMAE = MAE(ƒ(C0)) - MAE(ƒ(C2 or Cmax)).
††, 95% C.I., 95% confidential interval of ΔMAE.
Correlation coefficients, %ME, %MAE and Δ%MAE between AUC0-9 and absolute dose or adjusted dose by age or anthropometric parameters
| Independent variables | Correlation coefficient | ME (%) | MAE (%) | ∆MAE (%)† | [95% C.I.]†† |
|---|---|---|---|---|---|
| Dose | 0.3963 | 8.32 | 21.64 | 1.36 | [-1.11:3.83] |
| Dose/Age | 0.2478 | 10.42 | 24.82 | -1.82 | [-4.50:0.85] |
| Dose/HT | 0.4643 | 7.61 | 20.57 | 2.43 | [-0.89:5.74] |
| Dose/TBW | 0.3192 | 9.42 | 23.00 | N.D. | N.D. |
| Dose/BMI | 0.2531 | 9.86 | 23.75 | -0.75 | [-1.86:0.35] |
| Dose/BSA | 0.4280 | 7.99 | 20.99 | 2.00 | [-0.55:4.55] |
| Dose/IBW | 0.5181 | 7.27 | 19.57 | 3.43 | [-0.07:6.92] |
| Dose/LBW | 0.3941 | 8.60 | 21.58 | 1.29 | [-0.05:2.62] |
| Dose/FFM | 0.4655 | 7.84 | 20.58 | 2.42 | [-0.20:5.03] |
HT, height; TBW, total body weight; BMI, body mass index; BSA, body surface area; IBW, ideal body weight; LBW, lean body weight; FFM, fat free mass.
ME, mean prediction error.
MAE, mean absolute prediction error.
N.D., not determined.
†, ΔMAE = MAE(ƒ(Dose/TBW)) - MAE(ƒ(Dose/parameter)).
††, 95% C.I., 95% confidential interval of ΔMAE.
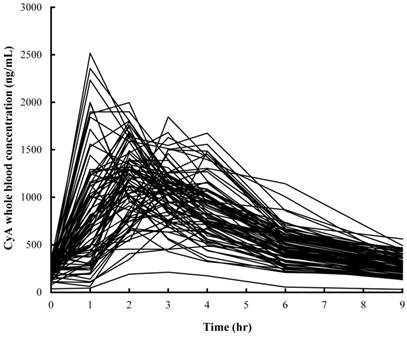
Individual whole blood concentration of CyA vs. time curve in 81 patients on day 28 after renal transplantation
Correlations between AUC0-9 and C0, C2 and Cmax of CyA. Data was obtained from the pharmacokinetics study on day 28 after renal transplantation (n=81).
Correlations between AUC0-9 and absolute dose and adjusted dose for age and anthropometric parameters in renal transplant patients (n=81). HT: height, TBW: total body weight, BMI: body mass index, BSA: body surface area, IBW: ideal body weight, LBW: lean body weight, FFM: fat free mass.
DISCUSSION
In 1999, Halloran et al reported that the blood level at C2 showed the greatest predictive correlation with CyA systemic exposure,17 and the monitoring of C2 level has become the current gold standard for TDM of CyA therapy. The immunosuppressive effect of CyA is based on the inhibition of calcineurin phosphatase activity. It was reported that the inhibition activity of CyA was closely related to blood concentration profile and that the degree of variation in individual immune response was decreased at high blood levels of CyA, approximately > 700 ng/mL, whereas a marked variability (mean % CV, 84.0%) was observed around the estimated half-maximal effective concentration (EC50), 200 ng/mL.18,19 Therefore, a monitoring targeted for Cmax is considered to be useful, not only as a predictor of AUC0-9 but also for pharmacodynamic monitoring, and thus its strategy would be a rational. On the other hand, after oral administration, a large variation in individual Tmax could be unavoidable. The limitation of C2 monitoring could come from the difference between C2 and Cmax. In this study, the C2 of 43 patients were different from individual Cmax: their Tmax were not at 2 hr. In addition, the mean percent of underestimation, which was defined by (Cmax-C2)/C2*100, was 25.5% (maximum, 168.5%; data not shown), which could potentially lead unexpected overdosing. Therefore, there are potential limitations as a predictor of AUC and a risk of overdosing of CyA in using C2 monitoring. Although C2 monitoring is thought to be a theoretically novel approach, it was practically considered not to be a useful approach for single monitoring even after oral dosing of Neoral®, which is an oral microemulsion formulation of CyA and might reduce the intra- and inter-individual variability in CyA absorption. Thus, C2 monitoring should be used with C0 monitoring, which has less variability. In contrast, after infusion of CyA, a single monitoring targeted for Cmax should be recommended because the end of infusion theoretically equals Tmax.20
There have been many reports that focused on the effect of body size parameters on drug disposition and dosage adjustment, especially in severely obese patients. In a similar approach to these investigations, we assessed the demographic and anthropometric parameters of patients as a candidate for the predictor of pharmacokinetics and dose adjustment of CyA: gender, age, HT, TBW, BMI, BSA, IBW, LBW, and FFM.
In this retrospective study, the demographic and anthropometric parameters of subject, except for age were significantly different between genders (Table 2). Likewise, there were significant differences in pharmacokinetic parameters of CyA between genders: the absolute apparent total body clearance (CLtot(app,abs)) and absolute apparent volume of distribution at steady state (Vdss(app,abs)) - without any adjustment including TBW, expressed in absolute terms (L/hr and L, respectively). The differences in pharmacokinetic parameters between genders were considered to be derived from the difference in body size between genders. In addition, no significant difference was observed in dose- and body size-independent parameters, Tmax, t1/2 and MRT. Therefore, it was concluded that the effect of gender on the pharmacokinetics of CyA was relatively small and negligible in this study.
The linear regression analyses between AUC0-9 of CyA and dose adjusted for candidate parameters (dose/parameter) indicated their potential usefulness as shown in the following rank order: IBW > FFM > HT > BSA > absolute dose > LBW > TBW > BMI > Age (Table 5). BMI is the worldwide metric recommended by WHO to classify obesity and has often been used in pharmacokinetic studies; however, it failed to demonstrate usefulness as a predictor, possible due to the fact that adipose tissue is not distinguished from lean mass.21,22 BSA was initially developed by Du Bois et al.23 Although it seems to be controversial, it was successfully reported that BSA was significantly related to the drug disposition of some antitumor agents and was closely correlated with liver volume.24,25 It is well known that there is a regional and racial difference in the calculations of BSA. In Japan, the original formula, Fujimoto formula, has been widely used. However, a recent investigation of BSA calculation in Japanese recommended the DuBois formula rather than the Fujimoto one.26 Therefore; the former was used in this study. In addition, in clinical practice, BSA is generally capped to a value of 2 m2. This limitation was applicable to only one patient in this study (observed value, 2.12 m2), but it did not produce any substantial change in the regression analysis, so it was ignored in this study. The concept of LBW is closely related to that of FFM, which is the weight devoid of most adipose tissue, and these two terms are often used interchangeably.27 The linear regression analyses in this study indicated the IBW as the best predictor for AUC0-9 and drug dosage of CyA, although no statistical superiority was observed over the empiric predictor, TBW. It means that at any body weight and any body composition, patients whose HTs are the same should receive the same dosage.
The concept of IBW was initially derived from insurance data, which represented a large quantity of evidence that related size to mortality. Subsequently, an empirical equation to estimate IBW was developed by Devine28 in 1974 and is the most common reference cited in the pharmacokinetic studies. Considering its derivation as a predictor, however, IBW seems to have less pharmacokinetic evidence than any other anthropometric parameters in this study.
In this study, there was no significant correlation between t1/2 and dose-normalized AUC0-9 (AUC0-9/dose) of CyA (correlation coefficient: r=-0.0072, p=0.9486, data not shown). In addition, CyA is a low-hepatic extraction drug and highly binds to plasma proteins, which means that the change of hepatic blood flow does not affect the metabolism of CyA. Therefore, it was considered that after oral dosing, the alteration of CyA metabolism including the variability in hepatic blood flow would not play a dominant role in its AUC0-9, and thus the volume of distribution was thought to be a modulator of AUC0-9; with the presumption that the drug absorption should be constant among this population. Indeed, Vdss(app,abs) was closely correlated with dose-normalized AUC0-9 in this study (correlation coefficient: r=-0.8900, p<0.0001, data not shown). It was well known that CyA is distributed in adipose tissue due to its lipophilic property.29,30 The distribution volume of such a lipophilic drug is generally considered to be correlated to TBW and BMI, for which adipose tissue is related to lean mass. In contrast, for IBW, FFM, LBW and BSA, adipose tissue is compensated for and these parameters are considered to be correlated to the distribution volume of hydrophilic drugs.31,32 These findings were in conflict with the results obtained in this study and IBW seems to lack the rationale for a predictor of CyA. However, our results were supported by previous clinical reports: Flechner et al. investigated the impact of TBW on CyA pharmacokinetics between obese and non-obese patients and reported that there was a significant difference in TBW, but not in Vdss(app,abs) adjusted for IBW. They concluded that in the early transplant period, CyA should be given to obese patients based on IBW.33 Likewise; Yee et al. also reported that after intravenous infusion, CLtot and Vdss of CyA were not significantly different between obese and non-obese patients when adjusted for IBW. They concluded that the maintenance dosage of CyA should probably be given on the basis of IBW rather than TBW.34,35 In addition, García-Sáiz et al. reported that the trough CyA concentration-dose ratio (CDR) was not correlated with %IBW calculated by TBW/IBW*100, to which the distribution volume of hydrophilic drugs was generally correlated.36 Grevel et al. reported that the height of patients correlated with CLtot after infusion of CyA, whereas TBW, BSA and %IBW were not correlated to CLtot.37 However, no report provided a plausible mechanistic explanation for the fact that the distribution volume of CyA is independent of body weight and composition, regardless of its distribution characteristics. Indeed, their clinical reports did not have enough evidence to explain the mechanism; a similar problem found in this study. There is one possible explanation; since CyA has a high affinity to albumin and low density lipoprotein and distributes to erythrocytes, their qualitative and quantitative changes could have a great potential to modulate the distribution balance between adipose and lean tissue, resulting in TBW-independent drug disposition.
CONCLUSION
In conclusion, although C2 monitoring targeted for Cmax has several advantages, after oral administration, a large variation in individual Tmax could be unavoidable and thought to be at a high risk for overdosing of CyA. Therefore, after oral dosing, it was not practically considered to be a useful approach for single monitoring and should be used together with C0 monitoring.
The regression analyses between AUC0-9 of CyA and the anthropometric parameters indicated that IBW was potentially the superior predictor for dose adjustment of CyA compared to the a priori strategy using TBW; however, this finding seems to lack the pharmacokinetic rationale and thus warrants further basic and clinical investigations.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Borel JF, Feurer C, Magnée C. et al. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977;32:1017-1025
2. Martino R, Sureda A, Ayats R. et al. Successful treatment of chronic autoimmune neutropenia with cyclosporin A. Haematologica. 1994;79:66-69
3. Tilney NL, Milford EL, Araujo JL. et al. Experience with cyclosporine and steroids in clinical renal transplantation. Ann Surg. 1984;200:605-613
4. Demetris AJ, Lasky S, Van Thiel DH. et al. Pathology of hepatic transplantation: A review of 62 adult allograft recipients immunosuppressed with a cyclosporine/steroid regimen. Am J Pathol. 1985;118:151-161
5. Keogh A, Spratt P, McCosker C. et al. Ketoconazole to reduce the need for cyclosporine after cardiac transplantation. N Engl J Med. 1995;333:628-633
6. Bacigalupo A, Van Lint MT, Occhini D. et al. Increased risk of leukemia relapse with high-dose cyclosporine A after allogeneic marrow transplantation for acute leukemia. Blood. 1991;77:1423-1428
7. Hamwi A, Salomon A, Steinbrugger R. et al. Cyclosporine metabolism in patients after kidney, bone marrow, heart-lung, and liver transplantation in the early and late posttransplant periods. Am J Clin Pathol. 2000;114:536-543
8. Mueller PW, Delaney V, MacNeil ML. et al. Indicators of acute renal-transplant rejection in patients treated with cyclosporine. Clin Chem. 1990;36:759-764
9. Minami M, Ohta M, Ohkura T. et al. Cytomegalovirus infection in severe ulcerative colitis patients undergoing continuous intravenous cyclosporine treatment in Japan. World J Gastroenterol. 2007;13:754-760
10. Kennedy MS, Deeg HJ, Siegel M. et al. Acute renal toxicity with combined use of amphotericin B and cyclosporine after marrow transplantation. Transplantation. 1983;35:211-215
11. Smorenburg CH, Sparreboom A, Bontenbal M. et al. Randomized cross-over evaluation of body-surface area-based dosing versus flat-fixed dosing of paclitaxel. J Clin Oncol. 2003;21:197-202
12. Wacke R, Rohde B, Engel G. et al. Comparison of several approaches of therapeutic drug monitoring of cyclosporin A based on individual pharmacokinetics. Eur J Clin Pharmacol. 2000;56:43-48
13. Gören S, Karahoca A, Onat FY. et al. Prediction of cyclosporine A blood levels: an application of the adaptive-network-based fuzzy inference system (ANFIS) in assisting drug therapy. Eur J Clin Pharmacol. 2008;64:807-814
14. Levy GA. C2 monitoring strategy for optimising cyclosporin immunosuppression from the Neoral formulation. BioDrugs. 2001;15:279-290
15. Billaud EM. C2 versus C0 cyclosporine monitoring: still not the end. Transplantation. 2005;80:542
16. Knight SR, Morris PJ. The clinical benefits of cyclosporine C2-level monitoring: a systematic review. Transplantation. 2007;83:1525-1535
17. Halloran PF, Helms LM, Kung L. et al. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation. 1999;68:1356-1361
18. Furukawa T, Kurasaki-Ida T, Masuko M. et al. Pharmacokinetic and pharmacodynamic analysis of cyclosporine A (CsA) to find the best single time point for the monitoring and adjusting of CsA dose using twice-daily 3-h intravenous infusions in allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2010;92:144-151
19. Fukudo M, Yano I, Masuda S. et al. Pharmacodynamic analysis of tacrolimus and cyclosporine in living-donor liver transplant patients. Clin Pharmacol Ther. 2005;78:168-181
20. Duncan N, Arrazi J, Nagra S. et al. Prediction of intravenous cyclosporine area under the concentration-time curve after allogeneic stem cell transplantation. Ther Drug Monit. 2010;32:353-358
21. He YL, Sabo R, Campestrini J. et al. The effect of age, gender, and body mass index on the pharmacokinetics and pharmacodynamics of vildagliptin in healthy volunteers. Br J Clin Pharmacol. 2008;65:338-346
22. Hijiya N, Panetta JC, Zhou Y. et al. Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood. 2006;108:3997-4002
23. Du Bois D, Du Bois EF. Clinical calorimetry. Tenth paper. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863
24. Smorenburg CH, Sparreboom A, Bontenbal M. et al. Randomized cross-over evaluation of body-surface area-based dosing versus flat-fixed dosing of paclitaxel. J Clin Oncol. 2003;21:197-202
25. Urata K, Kawasaki S, Matsunami H. et al. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321
26. Kouno T, Katsumata N, Mukai H. et al. Standardization of the body surface area (BSA) formula to calculate the dose of anticancer agents in Japan. Jpn J Clin Oncol. 2003;33:309-313
27. Janmahasatian S, Duffull SB, Ash S. et al. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051-1065
28. Devine BJ. Gentamicin therapy. Drug Intell Clin Pharm. 1974;8:650-655
29. Wagner O, Schreier E, Heitz F. et al. Tissue distribution, disposition, and metabolism of cyclosporine in rats. Drug Metab Dispos. 1987;15:377-383
30. Kawai R, Mathew D, Tanaka C. et al. Physiologically based pharmacokinetics of cyclosporine A: extension to tissue distribution kinetics in rats and scale-up to human. J Pharmacol Exp Ther. 1998;287:457-468
31. Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119-133
32. Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71-87
33. Flechner SM, Kolbeinsson ME, Tam J. et al. The impact of body weight on cyclosporine pharmacokinetics in renal transplant recipients. Transplantation. 1989;47:806-810
34. Yee GC, Lennon TP, Gmur DJ. et al. Effect of obesity on cyclosporine disposition. Transplantation. 1988;45:649-651
35. Yee GC, McGuire TR, Gmur DJ. et al. Blood cyclosporine pharmacokinetics in patients undergoing marrow transplantation. Influence of age, obesity, and hematocrit. Transplantation. 1988;46:399-402
36. García-Sáiz M, López-Gil A, Alfonso I. et al. Factors influencing cyclosporine blood concentration-dose ratio. Ann Pharmacother. 2002;36:193-199
37. Grevel J, Reynolds KL, Rutzky LP. et al. Influence of demographic factors on cyclosporine pharmacokinetics in adult uremic patients. J Clin Pharmacol. 1989;29:261-266
Author contact
![]() Corresponding author: Nobuyuki Sugioka, PhD. Department of Clinical Pharmacokinetics, Faculty of Pharmaceutical Sciences, Kobe Gakuin University, 1-1-3 Minatojima, Chuo-ku, Kobe 650-8586, Japan. Tel.: +81-78-974-4441, fax: +81-78-974-4441. E-mail: nsugiokakobegakuin.ac.jp
Corresponding author: Nobuyuki Sugioka, PhD. Department of Clinical Pharmacokinetics, Faculty of Pharmaceutical Sciences, Kobe Gakuin University, 1-1-3 Minatojima, Chuo-ku, Kobe 650-8586, Japan. Tel.: +81-78-974-4441, fax: +81-78-974-4441. E-mail: nsugiokakobegakuin.ac.jp

 Global reach, higher impact
Global reach, higher impact