Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(9):1231-1241. doi:10.7150/ijms.6244 This issue Cite
Research Paper
Determining Timing of Hepatectomy for Colorectal Cancer with Distant Metastasis According to Imaging-Based Tumor Shrinkage Ratio
Surgical Oncology, Gifu University, Graduate School of Medicine
Received 2013-3-14; Accepted 2013-7-22; Published 2013-8-1
Abstract
Background: The optimal timing of surgical resection of liver metastasis remains controversial, and guidelines regarding the upper limits of operative indications have not yet been defined. Surgical indication for metastasis from colorectal cancer (CLM) based on results of preoperative chemotherapy and RNF8 was investigated. Methods: Differences in CLM size on CT were evaluated as shrinkage rate/day by dividing tumor shrinkage rates by the interval in days between CT. Levels of RNF8 of resected colorectal cancer and CLM frozen specimen were detected. Results: When the cut line for shrinkage rate at 12 weeks was set at 0.35%, disease-free survival was significantly better in patients with a shrinkage rate >0.35% vs. ≤0.35% (p=0.003). RNF8 expression was significantly higher in Tis (p=0.001). In liver metastasis, RNF8 expression level was significantly lower in patients with partial response to FOLFOX than with stable disease, (p=0.017). Conclusions: A strategy of FOLFOX administration for 12 weeks to patients with low RNF8 expression and hepatectomy planned after 4 weeks rest may be accepted as the best therapeutic option for treating CLM.
Keywords: RNF8, tumor shrinkage rate, colorectal cancer, liver metastasis, chemotherapy, surgical resection
INTRODUCTION
Surgical resection plays a central role in the treatment of liver metastasis from colorectal cancer (CRC) [1]. The expansion of multidisciplinary care and advances in surgical procedure and technique in the past decade has resulted in acceptance of simultaneous resection because of its safety and efficiency [2]. However, recently developed neoadjuvant chemotherapy, of 5-fluorouracil/folinic acid with irinotecan (FOLFIRI) or oxaliplatin (FOLFOX) has also been found to be beneficial not only for initially unresectable [3] but also even resectable synchronous metastases [4]. The optimal timing of surgical resection of liver metastasis remains controversial. When arguing the timing or procedure, simultaneous or staged, results of recent chemotherapeutic developments should be considered to achieve better patient prognosis [5, 6]. However, the regimen to be selected as first-line neoadjuvant chemotherapy has also remained inconclusive.
Chemotherapeutic agents, such as oxaliplatin as a key drug of FOLFOX, commonly induce inter-/intra-strand DNA cross-links leading to DNA double-strand breaks (DSBs) [7]. Among biomarkers, RNF8 is one of the well-known molecules playing a critical role in DSB repair. RNF8 is a 485-residue nuclear protein with an N-terminal fork head-associated (FHA) domain and a C-terminal RING-finger (RF) domain [8]. Defects of RNF8 increase genomic instability, thereby elevating the risk of tumorigenesis, and RNF8 was detected to be down regulated in certain cancer cells, suggesting a possibility as a tumor suppressor [9, 10]. In contrast, RNF8 was also indicated to have oncogenic action because of the enhancement for cancer development due to facilitation of telomere fusion [11]. In spite of these controversial concepts, RNF8 is critical to the evaluation of chemotherapeutic effect.
In the present study, we investigated the clinical timing of hepatectomy for liver metastases from CRC based on results of neoadjuvant chemotherapy and demonstrated in an experimental procedure the importance of RNF8 as a useful biomarker to predict the effect of chemotherapy.
PATIENTS and METHODS
Clinical study
We retrospectively examined 50 patients with metastatic liver tumors from CRC who consulted our institution from 2006 to 2010. The patients comprised 38 men with a mean age of 62.3±11.5 years. The tumors were detected only in liver in 22 patients, but the other patients, tumors were present in other organs such as lung. Synchronous liver metastases were found in 42 patients, and primary CRC/liver metastases were resected in 13 of 36 patients. Solitary tumor was detected was in 15 patients, and the total number of tumors detected was 147. The diameter of each tumor was defined by using contrast CT imaging with a 5-mm slice width. To evaluate chemotherapeutic effect, tumor size was determined by measuring the major axis of the five largest tumors, even if more than six tumors were detected. Differences in tumor size were evaluated as shrinkage rate per day (%/day) by dividing the percent shrinkage in tumor size by the interval in days between imaging sessions. The chemotherapeutic effect was also evaluated according to RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1 [12]. Written informed consent was obtained from all patients enrolled in this study.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
The expression of RNF8 was evaluated for pathologically proved for cancer in 113 patients, who underwent surgery from October 2007 to December 2009 in our institute. After surgical removal, the specimens were stored at -80°C. Approximately 125 mm3 of each frozen specimen were soaked in RNAlater-ICE (Invitrogen, San Diego, CA, USA) and ground up. RNA of resected tissue or cultured cells was isolated using a Qiagen RNeasy Mini Kit (Qiagen, Germantown, MD, USA). The cDNA was synthesized from 500 ng of the total RNA using SuperScript VILO cDNA Synthesis Kit (Invitrogen). Quantitative PCR analysis was performed with a Thermal Cycler Dice real-time system (Takara Bio Inc., Shiga, Japan) with SYBR-Premix Ex Taq II (Takara Bio Inc.). The following primer pairs were used: human RNF8 (sense: 5-TCTAGGCATGTTTCATCCCAACAA-3, antisense: 5-GACTGCCTGGATGG TCACTCAC-3) and human ACTB (sense: 5-TGGCACCCAGCACAATGAA-3, antisense: 5-CTAAGTCATAGTCCGCCTAGAAGCA-3). Each mRNA value was normalized to that of the housekeeping mRNA, beta-actin (ACTH), using the standard curve method. Each experiment was done in either duplicate or triplicate, and then the average was calculated, as described previously [13]. The expression of RNF8 mRNA was described by the ratio of normal colon mucosa (N) to cancer tissue (T), i.e., the N/T ratio.
Immunohistochemistry
Specimens were fixed in 10% formalin and embedded in paraffin by conventional techniques. Freshly cut 3-µm sections were deparaffinized, and the sections were incubated with polyclonal rabbit RNF8 antibody (Abcam, CITY, STATE, USA) and kept at 18°C for 4 hours. The slides were subsequently treated with appropriate secondary antibodies. For the secondary developing reagents, the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) was used. These experimental steps followed previously reported methods [14, 15]. The immunohistochemical results for RNF8 of liver metastatic sites were arbitrarily classified into four scores according to the intensity of immunoreactivity [13]. Each score was evaluated by three independent researchers for each sample, and the average value is shown.
MTT assay
Colon carcinoma cell lines LS174T, COLO320DM, and SW1417L were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cellular proliferation of colon cancer cell lines was evaluated by the 3-[4,5-dimethylthiazol-2-yl]-2,5-dephenyl tetrazolium bromide (MTT) method, as described previously [16]. Cells were cultured in 96-well plates for 24 h and treated with several concentrations (0.0001-100 μM) of oxaliplatin (Elplat, Yakult, Tokyo, Japan) for 6, 24, 48, and 72 h. After 3-h incubation with MTT solution, cell-associated sediment was dissolved by adding dimethylsulfoxide (DMSO; Wako, Osaka, Japan). The absorbance of the resulting solution was measured using a Microplate reader (BioRad, Tokyo, Japan) at a wavelength of 540 nm.
Western blotting
Cells (3.8×105) were lysed in RIPA buffer [150 mM NaC1, 1.0% NP4O, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris (pH 8.0)] containing phosphatase inhibitors (Sigma, LOCATION) and protease inhibitors (Sigma). Proteins from each sample were electrophoresed through a polyacrylamide-SDS gel (Ready Gels J; Bio-Rad) in buffer (10×Tris/glycine/SDS buffer; Bio-Rad), and blotted on PVDF membranes (Immobilon-P Transfer Membrane; Millipore, Billerica, MA, USA) in transfer buffer (10×Tris/glycine buffer; Bio-Rad). The interaction was detected with an enhanced chemiluminescence (ECL) system (Western Lighting Plus-ECL, PerkinElmer Inc., Waltham, MA, USA) after the addition of anti-mouse or anti-rabbit IgG (GE Healthcare, Buckinghamshire, England), as described previously [17-19].
Transfection and small interfering RNA experiments for RNF8
SW1417 cells were cultured in a medium without antibiotics for 24 h before transfection to produce 50%-70% confluence. Cells were transfected with a small interfering RNA (siRNA) oligonucleotide using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) to result in a final siRNA concentration of 40 nmol/l in serum-free Opti-MEM (Invitrogen) according to the manufacturer's instructions [20]. The medium was replaced with RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum. After an additional 48 h, the total RNA and proteins were extracted, and the expression level of the RNF8 mRNA and protein was analyzed by real-time RT-PCR and a Western blotting analysis, respectively. The siRNA oligonucleotides for RNF8 were purchased from Invitrogen.
Statistical analyses
All data are presented as means ± SD. Disease free survival (DFS) was calculated by the Kaplan-Meier method and the log- rank test was used to compare these curves. Student t-test was used for examination of expression of RNF8 mRNA and characteristics of patient. Wilcoxon signed-rank test was used for comparison of Liver metastasis and primary lesion. Moreover, Pearson product-moment correlation coefficient was used for expression of RNF8 mRNA and IC50 in cell line.
RESULTS
Evaluation of chemotherapeutic effect according to tumor shrinkage rate
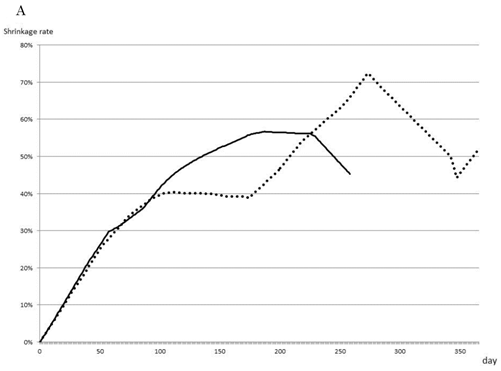
The rate of tumor shrinkage of whole cases was highest during the first 50 days from starting, and decreased gradually over 210 days after a plateau of 105 days (Fig. 1A). The effective regimen at the beginning of treatment maintained the tumor shrinkage rate (101 tumors) at 0.71±0.77% for the first 40 days and 0.61±0.75% for the next 100 days (Fig. 1B). In contrast, none of the effective regimens maintained tumor size without growth of the tumor during the first 30 days (data not shown). The chemotherapeutic effect of the treatment process is shown in Fig. 1C. The first-line treatment led to a tumor shrinkage speed of 0.4%/day over 270 days, but shifting to a second-line treatment stopped tumor growth during 160 days without any active actions. Tumor size was found to increase with third-line treatment.
As a selected first-line therapy, FOLFOX led to an average rate of tumor shrinkage of 0.4%/day during the first 100 days of therapy and maintained this rate until 210 days, with no differences observed between single use and combination with bevacizumab (Fig. 2A). The effects of second- and third-line therapy are shown in Fig. 2B, C. FOLFOX with bevacizumab resulted in a 0.14% shrinkage rate over 100 days, and FOLFIRI or FL, both with bevacizumab, maintained tumor size without growth. No other effective regimens were detected as second- or third-line therapies.
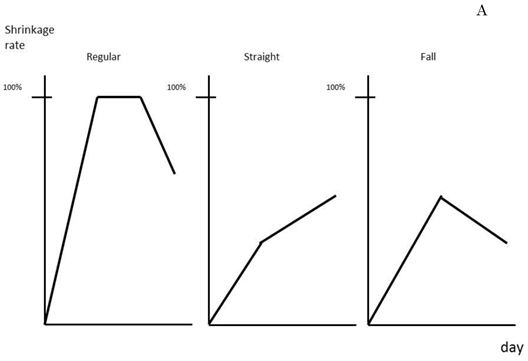
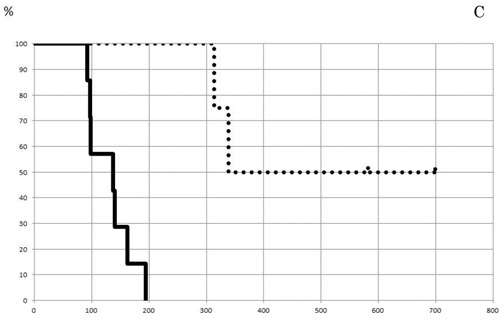
Average tumor size and number of tumors removed by hepatectomy cases were 43.7 mm and 3.2 tumors, respectively. Treatment effect in the patients was classified into three groups according to the shrinkage rate pattern: regular, straight, and falling, (Fig. 3A). Regular type showed high treatment effect at first followed by a gradual downslope after maintaining a plateau for a certain amount of time. The straight and falling types showed striking decrease of tumor size at first, but the pace then lowered slowly or clearly dropped, respectively. The length of disease-free survival (DFS) was found to be better in patients with the regular or straight pattern, as shown in Fig. 3B. The median lengths of DFS for the regular or straight pattern were 338±239 days and 140±37 days, respectively, both of which were better than that of the falling pattern, 97±28.6 days. When the shrinkage rate at 12 weeks was set at a cut line of 0.35%, DFS of patients above 0.35% was detected to be significantly better (p=0.003) than that of patients below the cut line (Fig. 3C). The median values of DSF of patients above and at or below 0.35% were calculated as 338±25 days and 137±27.5 days, respectively.
Expression of RNF8 in clinical cases and evaluation in cultured cells
The expression level of RNF8 was evaluated according to clinical factors. As shown in Table 1, there were no differences in other patient- or cancer-related factores, such as sex, age, tumore size, and serum level of tumor marker and the presence of lymph node or peritoneal metastasis. The level of RNF8 mRNA was higher in cancer tissue than in normal tissue in 64.8% of all cases. And the he N/T ratio was significantly lower in early Tis (Tis vs. others=0.33±0.21 vs. 2.86±3.86; p=0.001) and in negative synchronous distant metastases (negative vs. positive=2.40±0.32 vs. 3.73±5.45; p=0.04), including negative synchronous liver metastases (negative vs. positive=2.30±2.77 vs. 4.73±6.31; p=0.0069). From the comparison between primary tumor and metastatic tumor, immunohistochemically, RNF8 expression were found to decrease significantly (p=0.03) in sites of liver metastases (0.92±0.86) than primary tumor sites (1.69±1.25). In addition, RNF8 expression in liver metastasis was significantly lower in the patients with partial response to FOLFOX (0.41±0.09) than with standard disease (1.24±0.44) (p=0.017).
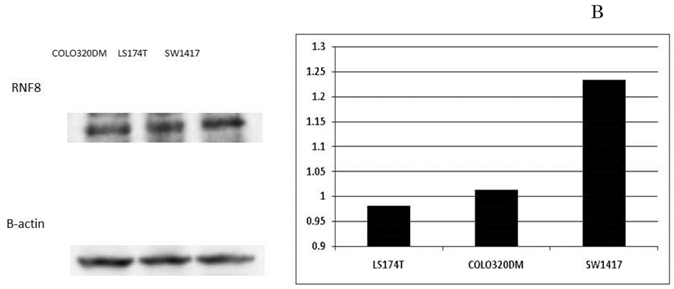
In the SW1417, COLO320DM, and LS174T colon cancer cell lines, the amounts of RNF8 mRNA expression were 5.79, 1.00 and 0.35, respectively. Western blotting showed the protein amounts of RNF8 (Fig. 4A), and they were evaluated for each value according to the ratio with β-actin to be 0.98, 1.01, and 1.23, respectively (Fig. 4B). In each cell line, IC50s of oxaliplatin were calculated as 0.91 µM, 1.48 µM, and 8.86 µM, respectively, and showed a significant positive correlation with RNF8 mRNA expression level (p=0.028). In the cell line with the highest expression, SW1417, blockage of RNF8 by the siRNA technique decreased the IC50 of oxaliplatin to 5.3% of control (Fig. 4C).
Patient characteristics

Effect of chemotherapy as evaluated by tumor shrinkage rate. The cumulative tumor shrinkage rate is shown for all patients (A). The effective chemotherapy-induced cumulative shrinkage ratio is described (B). The dotted line indicates first-line chemotherapy, the dashed line second-line therapy, and the solid line third-line therapy. Cumulative tumor shrinkage rates separated by different timings are shown (C).

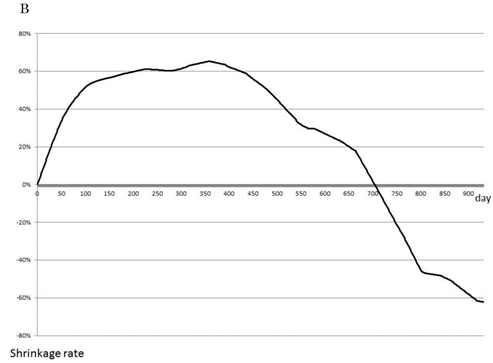

Most effective chemotherapy regimens for each timing. Shrinkage rates for Bev+FOLFOX (solid line) or FOLFOX (dotted line) as first-line therapy are compared (A). Shrinkage rates for Bev+FOLFOX (solid line) or FOLFIRI (dashed line) or Bev+FOLFIRI (dotted line) or FL (one-point chain line) or Bev+FL (two-point chain line) as second-line therapy are compared (B). Shrinkage rates for FOLFIRI (dashed line) or Bev+FOLFIRI (dotted line) or FL (one-point chain line) or CET+CPT11 (long dashed line) as third-line therapy are compared (C).


Prediction of patient prognosis. Shrinkage rate was separated into three patterns (A) and the prognosis of each pattern was evaluated (B). The solid line indicates Regular type, the dotted line Straight type, and the dashed line Falling type. (C) Tumor shrinkage rate at the 84th day (12th week) and disease-free survival period are shown. The disease-free survival rate in patients with a shrinkage rate of >0.35% (dotted line) was significantly higher than that in patients with a shrinkage rate of ≤0.35% (p=0.003%).

Expression of RNF8 in colorectal cancer cell lines. The expressions of RNF8 were different for cancer cell lines evaluated by RT-PCR (A) and Western blotting (B). In the cell line with the highest expression, SW1417, blockage of RNF8 by the siRNA technique significantly decreased the IC50 of oxaliplatin (C). The solid line indicates SW1417, and the dashed line indicates SW1417 siRNA. The experimental techniques are described in “Patients and Methods”.


DISCUSSION
Although recent developments in chemotherapy have changed the strategy for CRC with liver metastases, hepatectomy itself is still the main procedure expected to provide a complete cure. Under effective chemotherapeutic regimens, traditional surgical strategies in accordance with past concepts will be used decreasingly in the coming years. The present study primarily addressed the timing of hepatectomy and whether it should be performed first or staged with neoadjuvant chemotherapy involving FOLFOX or FOLFIRI based on the novel concepts of tumor shrinkage rate and RNF8 expression.
Tumor shrinkage rate in the present study was shown to be highest at the start of chemotherapy, but it decreased gradually after 100 days (14 or 15 weeks). According to a recent report, chemotherapeutic effect disappears after 4 months or more [21], supporting the present results. The tumor shrinkage rate also showed first-line chemotherapy to be the most effective and to be un-expectable though second to third line objectively. In the present study, the expediency of FOLFOX showed it to be the most favorable first-line chemotherapy. Evaluation of FOLFOX has shown it to increase the chance for hepatectomy by neoadjuvant treatment compared with FOLFIRI (22% vs. 9%) [22]. Evidence from a review of 3,278 patients in 23 studies of FOLFOX showed that radiological and pathological responses were observed in 64% and 45% of patients, respectively, and that neoadjuvant chemotherapy achieved an improvement in DFS [23]. Taken together, treatment with FOLFOX not exceeding 100 days might improve patient prognosis when selected as the first-line neoadjuvant chemotherapy. An oxaliplatin-based regimen was available as first-line therapy in the present study, and bevacizumab prolonged the term of certain effect but did not further decrease tumor size. Because bevacizumab is well-known to induce sinusoidal obstruction syndrome, the term of 8 or more weeks should be necessary before hepatectomy [24, 25]. The present study found the combination of FOLFOX with bevacizumab to be the best available regimen for second-line therapy, indicating that single therapy with FOLFOX is best for first-line therapy, whereas FOLFOX in combination with bevacizumab should be selected as second-line therapy. According to the guidelines of the National Comprehensive Cancer Network (NCCN), both FOLFOX with or without bevacizumab are recommended as first-line chemotherapy against CRC with liver metastasis. Combining bevacizumab with FOLFOX for CRC liver metastasis might prevent sinusoidal obstruction [26] and improve the pathologic response [24, 27]. However, the pros and cons of bevacizumab combination use are now being evaluated worldwide and remain controversial.
Determining favorable timing of hepatectomy during chemotherapy is another critical problem. The most preferable time for surgery during chemotherapy depends not only on the effect to damage cancer invasion but also negative influences caused by anticancer drugs [28]. Indeed, the complication rate from hepatectomy with certain neoadjuvant chemotherapies was suggested to increase if therapy includes more than six cycles [29]. In contrast, most favorable outcomes with FOLFOX and FOLFIRI were detected at 9 weeks [22] and 8 weeks [30], respectively. Clinical studies such as the EORTC (European Organization for Research and Treatment of Cancer)-40983 studies [4] also demonstrated optimal timing of surgery to be at 12 weeks. In the present study, the shrinkage rate at 12 weeks during chemotherapy was found to be a critical turning point for patient prognosis. To reduce postoperative complications, 4 weeks are necessary to wash out anticancer drugs [31]. Taken together with our finding that no tumor growth was detected in first 30 days of chemotherapy even if chemotherapy had no effect, we suggest that optimal timing of hepatectomy be based on the shrinkage rate at 12 weeks plus an additional 4-week rest period. Using tumor size as a response to chemotherapy, Adam et al. [32] classified the individual efficacy of chemotherapy in cases of CRC with liver metastasis into three groups: response type (>50% decrease in tumor size), stabilization type (<25% increase or <50% decrease), and progression type (>25% increase). Then, 5-year survival was much lower in patients with progression type compared with the response and stabilization types (8% vs. 37% and 30%, respectively; p<0.0001), and DFS was 3% compared with 21% and 20%, respectively (p=0.02), indicating that tumor progression before surgery is associated with a poor outcome, even after potentially curative hepatectomy. Because the 5-year overall survival rate for neoadjuvant chemotherapy was 71% and 15% in responders and non-responders, respectively, response to chemotherapy was suggested to be a significant prognostic factor after radical hepatectomy [33]. In the present study, even if chemotherapy was effective but was temporary in the initial short-term period (falling type), the prognosis was demonstrated to be worse. In contrast, if the shrinkage rate exceeded 0.35% at 12 weeks after the start of chemotherapy, a planned hepatectomy was suggested to lead to significantly favorable outcomes for DFS. The shrinkage value of 0.35% played a critical role in determining surgical indication. According to RESIST version 1.1 [12], partial response was estimated if the tumor size decreased by more than 30%. The critical term of 12 weeks (mean, 84 days) in the present study also created the indicator of 0.35% (30%/84 days = 0.357%/day) as a cutoff point leading to partial response due to chemotherapy. The shrinkage value of 0.35% might be a justifiable indicator to decide hepatectomy with high curability.
Genetic instability has been described in CRC [34]. RNF8 is a novel tumor suppressor and serves as a key in the repair of DSBs [35]. The present study revealed that RNF8 was associated with cancer progression, especially formation of distant metastasis. RNF8 has been shown to act as a tumor suppressor in mice by mediating repair of DNA DSBs, thereby preventing genomic instability [9, 36]. DSBs are the main mechanism of oxaliplatin-induced cell death [37] and are associated with oxaliplatin efficacy in advanced CRC [38]. Thus, RNF8 was suggested to be associated with the cell growth inhibitory effect of oxaliplatin. In the present study, correlation of RNF8 expression with the IC50 of oxaliplatin was detected in three different colon cancer cell lines. Blockage of RNF8 by siRNA techniques also increased the intensity of oxaliplatin, suggesting that RNF8 has a certain role in providing resistance to oxaliplatin-induced cell growth inhibition. A recent report showed the predictive factors for the effect of the FOLFOX regimen on CRC to be alkaline phosphatase [39] or mitochondrial transcription factor A [40]. In the present study, RNF8 was found to have the possibility of being added to these novel indicators.
As a conclusion, we believe that a strategy by which FOLFOX is administered to patients with low expression of RNF8 for 12 weeks and hepatectomy is planned after 4 weeks resting time will be accepted as the best therapeutic option for treating liver metastasis from CRC.
Abbreviations
CLM: metastasis from colorectal cancer
CRC: colorectal cancer
FOLFIRI: 5-fluorouracil/folinic acid with irinotecan
FOLFOX: 5-fluorouracil/folinic acid with oxaliplatin
DSBs: DNA double-strand breaks
FHA: forkhead-associate RING-finger
RF: RING-finger
RECIST: Response Evaluation Criteria in Solid Tumors
RT-PCR: Real-time reverse transcription polymerase chain reaction
MTT: 3-[4, 5-dimethylthiazol-2-yl]-2, 5-dephenyl tetrazolium bromide
siRNA: small interfering RNA
DFS: disease-free survival
NCCN: National Comprehensive Cancer Network
Competing Interests
The authors have declared that no competing interest exists.
References
1. Robinson S, Manas DM, Pedley I. et al. Systemic chemotherapy and its implications for resection of colorectal liver metastasis. SurgOncol. 2011;20:57-72
2. Sakamoto Y, Fujita S, Akasu T. et al. Is surgical resection justified for stage IV colorectal cancer patients having bilobar hepatic metastases?--an analysis of survival of 77 patients undergoing hepatectomy. J Surg Oncol. 2010;102:784-788
3. Tabernero J, Van Cutsem E, Díaz-Rubio E. et al. Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25:5225-5232
4. Nordlinger B, Sorbye H, Glimelius B. et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007-1016
5. Osada S, Imai H, Sasaki Y. et al. Strategy for synchronous and multiple liver metastasis. Hepatogastroenterology. 2012;59:198-203
6. Osada S, Imai H, Sasaki Y. et al. Surgical indications for multiple and synchronous and liver metastases from colorectal cancer. Hepatogastroenterology. 2012;113:198-203
7. Raymond E, Faivre S, Woynarowski JM. et al. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4-12
8. Ito K, Adachi S, Iwakami R. et al. N-Terminally extended human ubiquitin-conjugating enzymes (E2s) mediate the ubiquitination of RING-finger proteins, ARA54 and RNF8. Eur J Biochem. 2001;268:2725-2732
9. Li L, Halaby MJ, Hakem A. et al. Rnf8 deficiency impairs class switch recombination, spermatogenesis, and genomic integrity and predisposes for cancer. J Exp Med. 2010;207:983-997
10. Yoshioka T, Kimura M, Saio M. et al. Plk1 is negatively regulated by RNF8. Biochem Biophys Res Commun. 2011;410:57-61
11. Peuscher MH, Jacobs JJ. DNA-damage response and repair activities at uncapped telomeres depend on RNF8. Nat Cell Biol. 2011;13:1139-1145
12. Nishino M, Jackman DM, Hatabu H. et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. Am J Roentgenol. 2010;195:221-228
13. Matsui S, Osada S, Tomita H. et al. Clinical significance of aggressive hepatectomy for colorectal liver metastasis, evaluated from the HGF/c-Met pathway. Int J Oncol. 2010;37:289-297
14. Osada S, Saji S, Kuno T. Clinical significance of combination study of apoptotic factors and proliferating cell nuclear antigen in estimating the prognosis of hepatocellular carcinoma. J SurgOncol. 2004;85:48-54
15. Osada S, Saji S, Takahashi T. A case report of papilla Vater carcinoma showing positive expression of thymidine phosphorylase. Hepatogastroenterology. 2004;51:375-377
16. Osada S, Imai H, Tomita H. et al. Vascular endothelial growth factor protects hepatoma cells against oxidative stress-induced cell death. J Gastroenterol Hepatol. 2006;21:988-993
17. Osada S, Tomita H, Tanaka Y. et al. The utility of vitamin K3 (menadione) against pancreatic cancer. Anticancer Res. 2008;28:45-50
18. Osada S, Carr BI. Mechanism of novel vitamin K analog induced growth inhibition in human hepatoma cell line. J Hepatol. 2001;34:676-82
19. Osada S, Saji S, Osada K. Critical role of extracellular signal-regulated kinase phosphorylation on menadione (vitamin K3) induced growth inhibition. Cancer. 2001;91:1156-1165
20. Osada S, Matsui S, Komori S. et al. Effect of hepatocyte growth factor on progression of liver metastasis in colorectal cancer. Hepatogastroenterology. 2010;57:76-80
21. White RR, Schwartz LH. et al. Assessing the optimal duration of chemotherapy in patients with colorectal liver metastases. J SurgOncol. 2008;97:601-604
22. de Gramont A, Figer A, Seymour M. et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947
23. Chua TC, Saxena A, Liauw W. et al. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann Surg Oncol. 2010;17:492-501
24. Ribero D, Wang H, Donadon M. et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer. 2007;110:2761-2767
25. Aussilhou B, Dokmak S, Faivre S. et al. Preoperative liver hypertrophy induced by portal flow occlusion before major hepatic resection for colorectal metastases can be impaired by bevacizumab. Ann Surg Oncol. 2009;16:1553-1559
26. Klinger M, Eipeldauer S, Hacker S. et al. Bevacizumab protects against sinusoidal obstruction syndrome and does not increase response rate in neoadjuvant XELOX/FOLFOX therapy of colorectal cancer liver metastases. Eur J SurgOncol. 2009;35:515-520
27. Klinger M, Tamandl D, Eipeldauer S. et al. Bevacizumab improves pathological response of colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann Surg Oncol. 2010;17:2059-2065
28. Kemeny N. Presurgical chemotherapy in patients being considered for liver resection. Oncologist. 2007;12:825-839
29. Nakano H, Oussoultzoglou E, Rosso E. et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118-124
30. Tournigand C, Andre T, Achille E. et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237
31. Karoui M, Penna C, Amin-Hashem M. et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1-7
32. Adam R, Pascal G, Castaing D. et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052-1061
33. Chiappa A, Bertani E, Makuuchi M. et al. Neoadjuvant chemotherapy followed by hepatectomy for primarily resectable colorectal cancer liver metastases. Hepatogastroenterology. 2009;56:829-34
34. Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643-649
35. Mailand N, Bekker-Jensen S, Faustrup H. et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell. 2007;131:887-900
36. Santos MA, Huen MS, Jankovic M. et al. Class switching and meiotic defects in mice lacking the E3 ubiquitin ligase RNF8. J Exp Med. 2010;207:973-981
37. Chiu SJ, Chao JI, Lee YJ. et al. Regulation of gamma-H2AX and securin contribute to apoptosis by oxaliplatin via a p38 mitogen-activated protein kinase-dependent pathway in human colorectal cancer cells Toxicol. Lett. 2008;179:63-70
38. Kweekel DM, Antonini NF, Nortier JW. et al. Explorative study to identify novel candidate genes related to oxaliplatin efficacy and toxicity using a DNA repair array. Br J Cancer. 2009;101:357-362
39. Maisano R, Azzarello D, Del Medico P. et al. Alkaline phosphatase levels as a prognostic factor in metastatic colorectal cancer treated with the FOLFOX 4 regimen: a monoinstitutional retrospective study. Tumori. 2011;97:39-42
40. Yoshida Y, Hasegawa J, Nezu R. et al. Clinical usefulness of mitochondrial transcription factor A expression as a predictive marker in colorectal cancer patients treated with FOLFOX. Cancer Sci. 2011;102:578-582
Author contact
![]() Corresponding author: Dr. Shinji Osada, MD. 1-1, yanagido, gifu city, 501-1194, JAPAN. TEL: +81-58230-6233. FAX: +81-58230-1074. E-mail: stingac.jp
Corresponding author: Dr. Shinji Osada, MD. 1-1, yanagido, gifu city, 501-1194, JAPAN. TEL: +81-58230-6233. FAX: +81-58230-1074. E-mail: stingac.jp

 Global reach, higher impact
Global reach, higher impact