3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(9):1157-1165. doi:10.7150/ijms.5638 This issue Cite
Research Paper
Designer Cytokine Hyper Interleukin 11 (H11) is a Megakaryopoietic Factor
1. Department of Diagnostics and Cancer Immunology, Greater Poland Cancer Centre, Poznan, 61-866, Poland;
2. Chair of Medical Biotechnology, Poznan University of Medical Sciences, Poznan, 61-866, Poland.
Received 2012-12-2; Accepted 2013-5-28; Published 2013-7-9
Abstract
Interleukin-11 (IL-11) displays megakaryopoietic activity. We constructed super-cytokine Hyper- IL11 (H11) by linking soluble IL-11 receptor α (sIL-11Rα) with IL-11, which directly targets β-receptor (gp130) signal transducing subunit. The effects of H11 on hematopoiesis with a focus on megakaryopoiesis were studied. The expansion, differentiation and type of colony formation of cord blood progenitor Lin-CD34+ cells were analyzed.
H11 was more effective than recombinant human IL-11 (rhIL-11) in enhancement of the Lin-CD34+ cells expansion and differentiation into megakaryocytes (Mk). It induced higher expression of CD41a and CD61 antigens, resulting in a substantially larger population of CD34-CD41ahighCD61high cells. H11 treatment led to increased number of small and mainly medium megakaryocyte colony formation (Mk-CFU). Moreover, it induced the formation of a small number of large colonies, which were not observed following rhIL-11 treatment. Significantly higher number of H11 derived Mk colonies released platelets-like particles (PLP). Furthermore, H11 was considerably more potent than rhIL-11 in promoting differentiation of Lin-CD43+ cells toward erythrocytes.
Our results indicate that H11 is more effective than rhIL-11 in enhancing expansion of early progenitors and directing them to megakaryocyte and erythroid cells and in inducing maturation of Mk. Thus, H11 may prove beneficial for thrombocytopenia treatment and/or an ex vivo expansion of megakaryocytes.
Keywords: interleukin 11, hyper cytokine, megakaryopoiesis, stem cells, platelets, thrombocytopenia.
Introduction
Thrombocytopenia is a common adverse reaction associated with intensive myelosuppressive therapy in cancer patients. Moreover, it is a typical symptom of other disorders such as idiopathic thrombocytopenia purpura (ITP), myelodysplastic syndrome (MDS), acquired immunodeficiency syndrome (AIDS) or chronic liver disease [1]. In oncology thrombocytopenia significantly increases the risk of bleeding and limits therapeutic dose intensity and causes dose delay, what finally compromises the treatment outcome. The current thrombocytopenia standard treatment is the platelet transfusion. However, the procedure is associated with number of potential risks such as transfusion reactions, transmission of infection, alloimmunization or platelet refractoriness [2]. Platelet transfusion is also expensive and limited by supply. Moreover, novel cancer therapies and growing number of aged patients may further increase the incidence of the thrombocytopenia. Although platelet transfusion can provide a temporary benefit, it does not cure thrombocytopenia. Thus, the intensive search for novel therapeutic strategies including new thrombopoietic agents is required.
Three therapeutic approaches for the treatment of thrombocytopenia have attracted the attention of scientists. One was focused on the stimulation of endogenous megakaryocytes to increase in vivo platelet production. In turn, two others were focused on expanding in vitro hematopoietic stem cells (HSC) in order to: (i) co- infuse them with unmanipulated HSC or (ii) to produce functional platelets [3]. The first of in vitro strategies reasoned that final differentiation and thrombopoiesis would occur in vivo and would shorten the period of thrombocytopenia. The second one would deliver functional platelets ready for infusion. The in vitro expansion technique may be used to obtain Mk by culturing CD34+ cells derived from bone marrow (BM), cord blood (CB) and peripheral blood (PB) [3]. Independent on strategy, a proper cell factor or combination of factors which would increase platelet number is needed.
Thrombopoietin (TPO) is the most powerful Mks/platelets agent [4]. Mice deficient in TPO or its receptor show dramatic (85%) decrease in circulating platelets as well as bone marrow and splenic Mks [5, 6]. The Mks in these mice are however, structurally and functionally normal, and are sufficient to prevent spontaneous bleeding [5-7]. This suggests that other than TPO regulators of megakaryopoiesis may exist and may be able to sustain homeostasis. It is known that TPO affects megakaryopoiesis at different stages by cooperation with variety of pleiotropic hematopoietic growth factors. At early phases TPO co-acts with Flt3 ligand, c-kit ligand and IL-3 to stimulate the proliferation of primitive hematopoietic stem cells (HSC) [8, 9]. Addition of differentiation-inducing agents such as IL-6 or IL-11 results in megakaryocyte maturation [10, 11]. IL-11 acts synergistically with IL-3, TPO and stem cell factor (SCF) to increase the number and enhance maturation of megakaryocytic progenitors [12]. It induces maturation of early Mks by increasing Mk size and ploidy [13]. Unlike TPO, IL-11 is not expressed constitutively and is not detectable in normal serum. The IL-11 expression is induced by IL-1 and transforming growth factor beta (TGFβ) and IL-11 has been found in sera of patients with thrombocytopenia. These data indicate a physiological role of IL-11 in regulating the normal platelet number and function in hematopoietic stress and injury [14]. IL-11 is the only agent approved by Food and Drug Administration (FDA) in USA to prevent severe thrombocytopenia and reduce the platelet transfusions following myelosuppressive chemotherapy for non-myeloid malignancies.
We have constructed a novel designer cytokine Hyper IL-11 (H11), which is composed of full length of soluble IL-11 receptor (sIL-11R) and IL-11 [15, 16]. The fusion proteins are expected to be more stable and are needed in lower effective dose than separate components in supporting bioactivity. The purified recombinant H11 was active in three different in vitro bio-assays: human hepatoma cells-HepG2-assay, B9 (hybridoma cells) bio-assay, and Ba/F3-gp130 bio-assay. H11 displayed much higher activity than IL-11 alone [15]. Moreover, H11 demonstrated immune-adjuvant properties in the therapeutic whole cell cancer vaccine formulation in in vivo orthotopic renal cell cancer model [17].
Accordingly, in these studies we have evaluated the H11 potential for stimulation of hematopoiesis with a focus on megakaryopoiesis in vitro. Since IL-11 is an active megakaryopoietic agent and H11 is more active in various biological systems than IL-11, we hypothesize that H11 may enhance megakaryopoiesis, what potentially may be used for the enhancement of endogenous platelet production and/or the stimulation of Mk expansion ex vivo for therapeutic use.
Materials and methods
Isolation of human hematopoietic stem/progenitor cells Lin-CD34+
Umbilical cord blood (CB) was collected from full-term deliveries, after obtaining informed consent from the mothers. CB collection was approved by the Bioethical Committee of Poznan University of Medical Sciences. Fresh heparinised cord blood samples obtained from four individuals were pooled, diluted with PBS (1:4 blood:PBS) and whole mononuclear cells were isolated by Ficoll-Hypaque (Sigma, St. Louis, MO) density gradient centrifugation. The mononuclear cell layer was collected, washed twice with PBS and then Lin- stem and progenitor cells were isolated using the Lineage Cell Depletion Kit human (Miltenyi Biotec Inc., Auburn, CA). Shortly, the mononuclear cells resuspended in PBS containing 2% FBS and 1 mM EDTA were stained with Biotin-Antibody Cocktail (anti-CD2, CD3, CD11b, CD14, CD15, CD16, CD19, CD56, CD123, CD235a) and mixed with Anti-Biotin Microbeads. Next the labeled cells were separated using LS columns (Miltenyi Biotec Inc., Auburn, CA) according to the manufacturer's directions. Subsequently, Lin-CD34+ cells were isolated using the CD34 MicroBead Kit human (Miltenyi Biotec Inc., Auburn, CA). Lin- cells were centrifuged, resuspended in PBS containing 2% FBS and 1 mM EDTA, stained with CD34 Microbeads in the presence of antibody to human Fc receptor to minimize nonspecific binding and then Lin-CD34+ cells were separated using LS columns.
Liquid Cell Cultures
Lin-CD34+ cells were cultured in the serum free medium X-Vivo10 (Lonza, Verviers, Belgium) at 37ºC, in 5% CO2, with 95% humidity. To induce cell expansion and differentiation, Lin-CD34+ cells (5x104 per well) were cultured in 48-well plates in the presence of rhSCF and rhIL-6 (control) plus cytokines such as rhTPO, rhIL-11 or H11. The cytokines were used in the following concentrations: rhSCF 50 ng/ml (PreproTech Inc. Rocky Hill, NJ), rhIL-6 20 ng/ml (PreproTech Inc. Rocky Hill, NJ), rhTPO 50 ng/ml (PreproTech Inc. Rocky Hill, NJ), rhIL-11 50 ng/ml (Sigma, St. Louis, MO), H11 50 ng/ml. For each cytokine cocktail, the cultures were carried out in triplicates. At specified time points cell number, viability and phenotype were evaluated. Viability of cells was analyzed using trypan blue exclusion test.
Flow cytometry
The differentiation of Lin-CD34+ cells was evaluated by flow cytometry. At specified time points, cells were harvested, washed twice with PBS and then stained for 30 min at 4ºC with different combinations of the following murine monoclonal antibodies: fluorescein isothiocyanate (FITC)-labeled anti CD34, phycoerythrin (PE)-labeled anti-CD61, phycoerythrin (PE)-labeled anti-CD41a from BD Biosciences Pharmingen San Jose, CA and PE-Texas Red (ECD)-labeled anti-CD41a, phycoerythrin-Cy5 (PE-Cy5)-labeled anti-CD14, phycoerythrin (PE)-labeled anti-CD15, phycoerythrin-Cy7 (PE-Cy7)-labeled anti-CD235a, and phycoerythrin (PE)-labeled anti-CD61 from Coulter Beckman Marseille, France. Anti-CD15 antibody was used to evaluate granulopoiesis, anti-CD14 for monocytopoiesis, anti-CD41a and anti-CD61 antibodies for megakaryopoiesis, anti-CD235a for erythropoiesis. After labeling cells were washed with PBS and analyzed using Navios Flow Cytometer (Coulter Beckman, Marseille, France) or FACSCanto flow cytometer (BD Biosciences Pharmingen, San Jose, CA) and FlowJo (v8.7) and FACSDiva (v6.1.2) softwares. Isotype controls consisted of the respective murine monoclonal IgG1 or IgM antibodies (BD Biosciences Pharmingen, San Jose, CA).
Megakaryocyte colony assay
The colony forming unit megakaryocyte (CFU-Mk) assay was performed using MegaCult-C system (StemCell Technologies, Vancouver, Canada) according to the manufacturer's instructions. To the 1ml of MegaCult-C medium were added, 25 μl of 1.1 x 105 Lin-CD34+ cell suspension, specified cytokines, 13.2 μl of low-density lipoproteins (LDL) (5mg/ml) and 0.6 ml cold collagen solution (3mg/ml). Then 0.75 ml of the final culture mixture was dispensed into each of two wells of a double chamber slide. MegaCult-C medium was first supplemented with rhIL-6 (10ng/ml) and rhIL-3 (10ng/ml) (ImmunoTools, Friesoythe, Germany) and then rhTPO (50ng/ml), rhIL-11 (50 ng/ml) or H11 (50 ng/ml) were added. Cells were cultured at 37ºC, in 5% CO2/95% air atmosphere of 95% humidity for 10 days. Human CFU-Mks were detected by immunohistochemistry and then the colonies were analyzed using light microscope. Three categories of colonies were identified and subdivided by colony size, small (3 to 20 cells per colony), medium (21 to 49 cells per colony), or large (>50 cells per colony). Moreover, the same colonies were analyzed in terms of releasing platelet-like fragments (PLP). According to MetaCult manual, as the cells within the Mk-CFU mature, there might be observed PLP surrounding the colony [18]. PLPs occur in the form of strands and particles surrounding the colonies, which are CD41a/CD61 positive based on immunohistochemical staining. Accordingly the colonies were divided on colonies with non or singular (up to 10) platelet-like fragments and colonies with clearly visible platelet-like particles (from 10 up to hundreds) surrounding the colony (named PLP rich).
Immunohistochemistry
The immunohistochemistry was performed by using MegaCult-C Staining Kit (StemCell Technologies, Vancouver, Canada) according to the manufacturer protocol. The cells grown in MegaCult-C system were dehydrated and fixed in 1:3 methanol:acetone solution for 20 min and air-dried for 15 min. Next, the cultures were rehydrated by applying 0.05 M Tris/NaCl buffer, pH 7.6 (washing buffer) for 20 min, blocked with 5% human serum in washing buffer, and stained with the primary (mouse anti-CD41a/CD61) or the control antibody (mouse anti-TNF) for 30 min. After washing, biotin-conjugated goat anti-mouse IgG antibody was applied to each slide for 30 min, washed and then avidin alkaline phosphatase conjugate was added. After next washing, the alkaline phosphatase substrate solution was applied, washed again, counterstained with Evans Blue, washed and air-dried.
Statistics
To test for significant differences between control, IL-11 and H11 treated groups one-way ANOVA was used. In the case of significance ANOVA (p<0.05) post hoc tests with Bonferroni correction were performed. Differences were considered significant when p < 0.05.
Results
Isolation and analysis of CB Lin-CD34+ cells
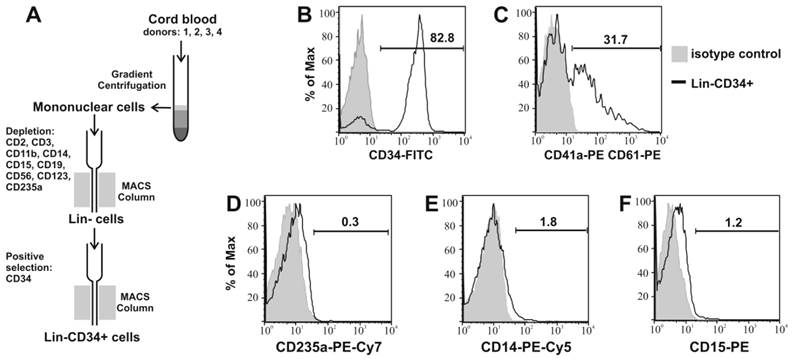
Lin-CD34+ cells were isolated from CB by negative and then positive selection as shown in Figure 1A. 83.6% (±1.5) of the obtained cells expressed CD34 antigen (Table 1). 35.9% (±9) of cells were CD41a/CD61 positive and around 30% of this population were both CD34 and CD41a/CD61 positive. The mean percentage of CD235a positive cells was 1.1%, while CD14+ and CD15+ 3% and 1.6%, respectively (Fig. 1B, C, D, E, F).
Increase in cell number after stimulation with cytokines
Isolated cells were cultured for two weeks in X-Vivo 10 medium supplemented with rhSCF and rhIL-6 (control) plus in the presence of cytokines such as rhTPO, rhIL-11 or H11 promoting growth and differentiation of stem and progenitor cells. The number and phenotype of cultured cells were analyzed at the specified days.
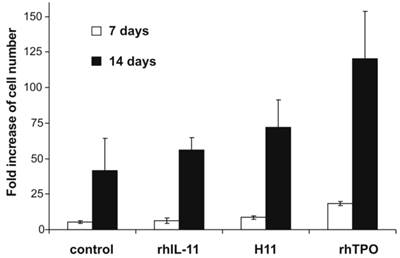
Viability and cell number was measured on days 7 and 14. At both time points for all combinations of cytokines, the cell viability was higher than 90%. Adding H11 to the medium resulted in 8.5 (± 1.5) - fold increase in the number of cells on day 7 and 72 (± 19.3) - fold increase on day 14 (Fig.2). In the presence of H11 the increase in cell number was lower than in the presence of rhTPO but compared to the control cells it was 2-fold higher. Furthermore, the increase in cell number induced by H11 was 1.3 times higher than in the presence of rhIL-11.
Isolation and characterization of CB Lin-CD34+ cells. (A) schematic graph of isolation process of Lin-CD34+ cells from CB. Obtained Lin-CD34+ cells were analyzed by flow cytometry for expression of CD34+ (B), CD41a/61 (C), CD235a (D), CD14 (E) and CD15 (F) antigens. The representative data of four times repeated isolation are shown.
Phenotypic analysis of cells. Lin-CD34+ CB cells were isolated and cultured in X-Vivo 10 medium supplemented with rhSCF and rhIL-6 (control) plus rhTPO, rhIL-11 or H11. At day 0 and 14 cells were analyzed for the expression of CD34, CD41a/CD61, CD235a, CD14, CD15 antigens by flow cytometry. The mean percentage and (± SD) of four independent experiments for each cytokine combination is shown.
| Initial | Control | rhIL-11 | H11 | rhTPO | ||
|---|---|---|---|---|---|---|
| day 0 | day 14 | |||||
| Total: | ||||||
| CD34+ | 83.6(1.5) | 4.1(1.4) | 4.5(1) | 4.1(1.5) | 6.2(3.3) | |
| CD235a+ | 1.1* | 11.5(9.5) | 16.3(11) | 30.8(14) | 4.9(4.5) | |
| CD15+ | 1.6* | 28(7.8) | 27.9(5.8) | 26.7(5.8) | 19.5(8.5) | |
| CD14+ | 3* | 13.3(3) | 14.7(4.3) | 14.8(3.8) | 11.7(8.2) | |
| CD41a+/CD61+ | 35.9(9) | 42.2(11.8) | 39.8(14.6) | 51.4(9.7) | 63.2(11.4) | |
| Subpopulations | ||||||
| CD34+CD41a+CD61+ | 29.7(4.7) | 2.3(1.2) | 2.3(0.9) | 2.6(1.4) | 3.4(2.8) | |
| CD34-CD41a+CD61+ | 5.3(3.1) | 37.7(10) | 34.8(12) | 41.2(6.9) | 42.4(8.4) | |
| CD34-CD41ahighCD61high | 0.9(0.6) | 2.2(2.8) | 2.7(2.8) | 7.55(5.2) | 17.5(2.9) | |
* mean percentage of two independent experiments.
Increase in cell number after stimulation with H11. 5x104 Lin-CD34+ cells were cultured in X-Vivo 10 medium supplemented with rhSCF and rhIL-6 (control) and additionally in the presence of rhTPO, rhIL-11 or H11. All cytokines were used at concentration of 50 ng/ml, except of rhIL-6 which was used at 20 ng/ml. On day 7 and 14 cells were counted. Results are presented as fold increase of cell number relative to the number of cells seeded. The means and ± standard deviation of three independent experiments per cytokine combination are given.
Effect of cytokines on the cell differentiation
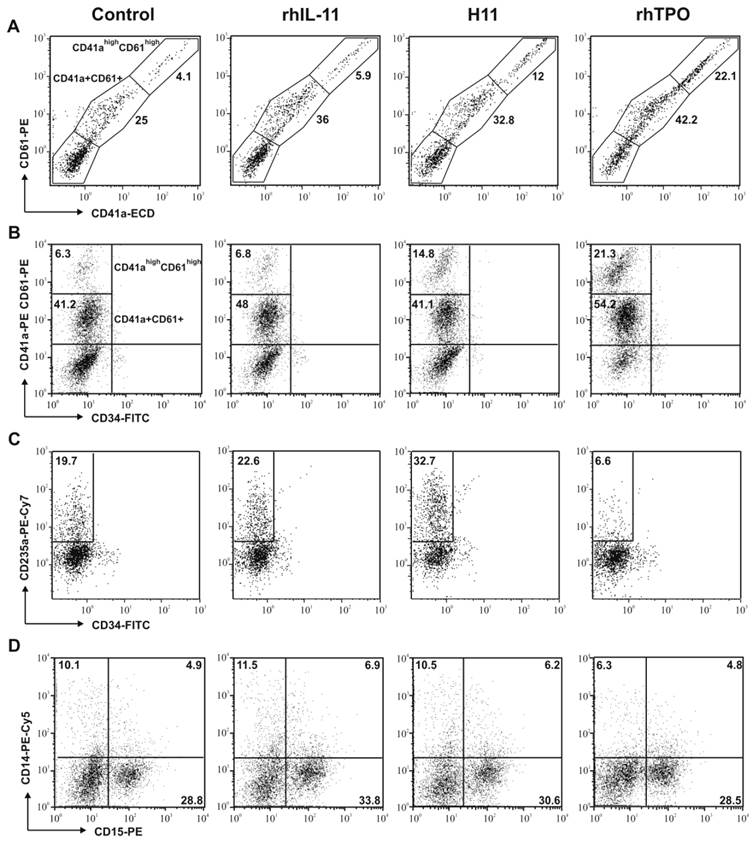
During liquid culture in the presence of rhSCF, rhIL-6 (control) with addition of rhTPO, rhIL-11 or H11 the differentiation of cells towards megakaryocytes, erythrocytes, granulocytes and monocytes was observed with loss of expression of CD34 antigen (Table 1, Fig. 3).
Anti-CD41a and anti-CD61 antibodies were used to evaluate magakaryopoiesis. CD41 is composed of two subunits (120 kDa a, and 23 kDa b) that interact with CD61 to form a functional receptor for fibrinogen, fibronectin and von Willebrand factor. Expression of both markers (CD41a and CD61) increases during maturation of cells toward megakaryocytes.
Two subpopulations of megakaryocytes were distinguished and characterized: (i) of low expression of both markers (CD41a+CD61+) and (ii) of high expression of both markers (CD41ahighCD61high) (Fig. 3A). The expression of CD41 and CD61 was found to be similar in both subpopulations (Fig.3A). Thus we analyzed both markers simultaneously.
Addition of H11 to the culture medium increased the number of megakaryocytic cells when compared to control and rhIL-11 (Table 1, Fig. 3B,). After 14 days, the number of CD34 negative and CD41a/CD61positive cells was the highest in H11 and rhTPO stimulated cultures, 48.8% and 59.9%, respectively. Moreover, when compared with control, the subpopulation of CD34-CD41ahighCD61high cells was 1.2-fold more abundant in cultures stimulated with rhIL-11, 3.4-fold with H11 and 8-fold with rhTPO (Table 1).
Moreover, both rhIL-11 and H11 promoted differentiation of Lin-CD34+ hematopoietic precursors toward erythroid cells, however the effect of H11 was more pronounced (Fig. 3C). After 14 days of culture the mean percentage of CD235a+ cells was 30.8% (±14) in H11, 16.3%(±11) in rhIL-11 and 4.9%(4.5) in TPO stimulated cultures, whereas 11.5%(±9.5) in control. The difference between rhIL-11 and H11 stimulation was about 1.9-fold (Table 1).
Based on the analysis of CD14 expression, no difference was seen in the differentiation toward monocytic lineage between control and cytokines stimulated cells (Table 1, Fig. 3D). Moreover, the analysis of CD15+ expression indicated no difference between control, rhIL-11, and H11 treated cells in terms of granulopoiesis (Table 1, Fig. 3D).
Effect of cytokines on the megakaryocyte colony formation and platelets-like particles (PLP) production
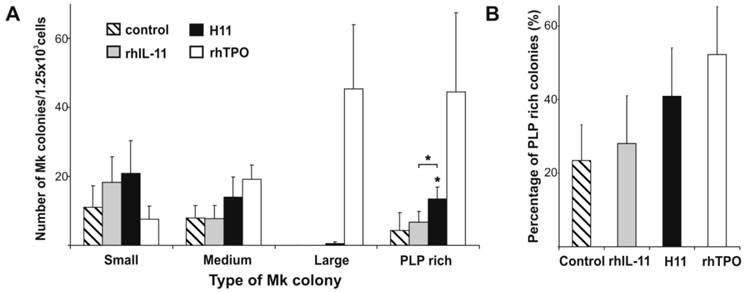
Adding both rhIL-11 or H11 to the MegaCult-C culture increased the number of Mk colonies as compared to control (rhIL-6 and rhIL-3 alone), however, a difference in colony distribution was observed (Fig. 4 A). rhIL-11 activated cells of low proliferative capacities resulted in higher number of small colonies, while frequency of medium and large size colonies were comparable to the control (rhIL-11 vs control: 18.3(±7.3) vs 11(±6.2), 7.8(±3.7) vs 8(±3.6), and 0 vs 0 for small, medium, and large colonies respectively). H11 increased not only a number of small Mk colonies, but also of a medium ones (20.8(±9.5) and 14(±5.8) respectively). Moreover, a few large colonies appeared upon H11 stimulation. TPO displayed different colonogenic pattern. Incubation with TPO resulted in lower number of small colonies (7.7 (±3.7)), while it increased the number of medium and especially large colonies (19.2 (±4.1) and 45.3 (±18.6), respectively) as compared with the control (Fig. 4 A). Large Mk colonies that have grown upon TPO stimulation were more abundant and compact than these upon H11 activation.
Figure 5 shows that Mk colonies differed morphologically in terms of releasing PLP; some colonies did not indicate the symptoms of thrombopoiesis-like process (Fig. 5 A and B), while others did (Fig 5 C and D). The appearance of PLP was independent on colony size; it was observed for small, medium and large colonies.
Both rhIL-11 and H11 increased the number of PLP rich colonies compared with control (stimulated only with rhIL-6 and rhIL-3) (Fig. 4 A) however, the effect of H11 was significant. The difference between IL-11 and H11 stimulation was significant as well. The mean number of total PLP rich colonies stimulated by H11 was 13.5(±3.3), while by rhIL-11 and control were 6.7 (±3.1) and 4.3 (±5.1), respectively. rhTPO gave rise the highest mean number of colonies undergoing thrombopoiesis-like process (44.5 (±22.9)). The percentages of PLP rich colonies were 23.4(±9.7), 28(±12.9), 40.9(±13.1) and 52.3(±13) under stimulation of control medium alone, and in the presence of rhIL-11, H11 or rhTPO, respectively (Fig. 4 B).
Effect of H11 on the differentiation of CB Lin-CD34+ cells. Cells were seeded in X-Vivo 10 medium supplemented with rhSCF and rhIL-6 (control) plus rhTPO, rhIL-11 or H11. After 14 days of culture cells were harvested and the expression of CD41a and CD61 (A), CD34 and CD41a/61 (B), CD34 and CD235a (C), CD14 and CD15 (D) antigens was analyzed by flow cytometry. The most pronounced results of at least three times repeated experiment are shown.
H11 impact on frequency and kind of megakaryocytic colonies. Lin-CD34+ cells purified from CB were assessed for their capacity to stimulate Mk colonies formation when cultured in collagen and MegaCult-C medium containing rhIL-6, rhIL-3 (control) plus rhIL-11, H11 or rhTPO. Colonies were evaluated by immunohistochemistry (anti-CD41a/CD61) and counted under light microscopy. Results represent: A. the number of small, medium, large and platelets-like particles (PLP) rich colonies, and B. the percentage of PLP rich colonies. The means and ± standard deviations of three independent experiments per cytokine combination are shown. * p<0.05.
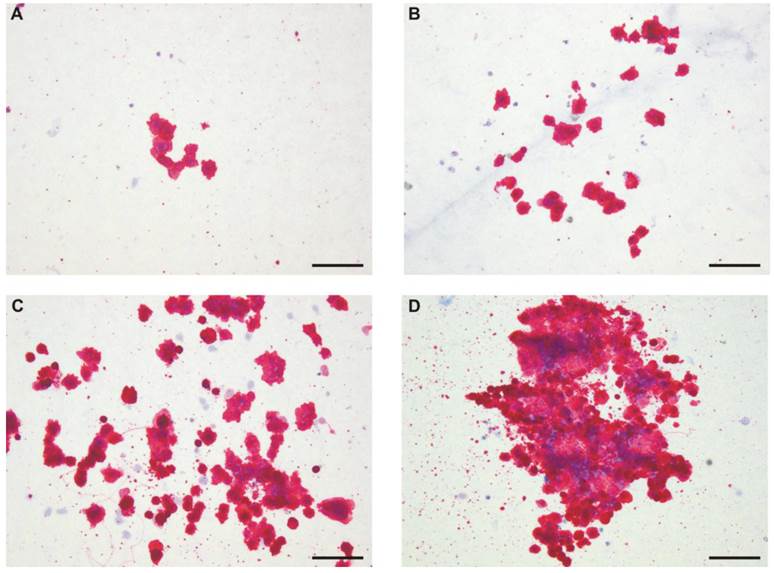
The morphology of megakaryocytic colonies. Representative pictures of small (A), medium (B) and of large (C and D) Mk-colonies are shown. Lin-CD34+ cells were cultured for 10 days in a semisolid medium supplemented with rhIL-6 and rhIL-3 (control) (A) plus rhIL-11 (B), H11 (C) or rhTPO (D). Mk cells were detected by immunohistochemistry using anti-CD41a/CD61 antibody. Pictures were taken under light microscopy (magnification 10X). Colonies cultured in the presence of H11 and TPO are examples of PLP rich colonies. Scale bar represents 100µm. The experiment was repeated three times.
Discussion
Since IL-11 enhances megakaryocytopoiesis [11, 13], the designer molecule based on IL-11 but more potent e.g. H11 may be useful for proper and enhanced megakaryocyte development. However, H11 activity must be carefully assessed in various in vitro systems. H11 is a fusion protein composed of IL-11 and its shIL-11Rα. The activity of IL-11 is strictly dependent on the cell surface expression of both IL-11R and gp130, while H11 needs only gp130 - a signal transducing subunit. H11 may affect (induce or impede) cells that normally do not respond to IL-11. Thus, the studies of H11 activity on hematopoiesis/megakaryopoiesis were needed.
Here, we compared the ability of IL-11 and H11 to expand the hematopoietic progenitor cells obtained from cord blood. The cells were enriched twice: first, the lineage-committed cells were removed and then CD34+ cells were selected. Since, Lineage Cell Depletion Kit did not contain any Mk marker, the high population of CD41/CD61 positive cells was obtained. It was in agreement with Proulx et al. study, where similar progenitor enrichment system was used [19]. Since previous in vitro studies indicated that IL-11 as single factor had no effect on megakaryopoiesis [20, 21], the influence of IL-11 and H11 on expansion and differentiation of Lin-CD34+ cells were evaluated in the presence of rhSCF and rhIL-6 or rhIL-3 and rhIL-6. Moreover, rhTPO stimulated cells were analyzed for comparative reasons.
H11 was more active than IL-11 in terms of increasing of total number of nucleated cells and enhancement of the Lin-CD34+ cell differentiation into megakaryocytes. After 14 days of culture two subpopulations of CD41a/CD61 positive cells were observed (of low and high both markers expression). Comparing with rhIL-11, hypercytokine H11 induced higher expression of CD41a and CD61 antigens, resulting in considerably larger subpopulation of CD34-CD41ahighCD61high cells. Our results were consistent with study of Matsumura-Takeda et al., where two megakaryocytic subpopulations based on CD41 expression were observed [22]. The expression of CD41 and CD61 was similar in each subpopulation and cells with higher expression of CD41a and CD61 were considered to be more mature Mk [22].
Moreover, stimulation with H11 resulted in the significantly higher number of PLP rich colonies compared with rhIL-11. It may indicate that H11 more than IL-11 affected the maturation of Mk or platelets releasing process. In Mk-CFU assay the H11 had greater potential for megakaryocyte colony formation than rhIL-11. In contrary to the rhTPO, which preferentially induced growth of large Mk colonies, combination of rhIL-11 with rhIL-3 and rhIL-6 resulted in increased number of small Mk colonies. However, H11 not only induced formation of higher number of small colonies but also medium size, and additionally single large colonies appeared. Above assays are very indicative since the colony size is correlated with hierarchal model of hematopoiesis in which progenitors lose their proliferative potential as they divide and mature toward more differentiated progenitors (larger colonies derive from more primitive progenitor). Accordingly, the rhIL-11 induced more differentiated progenitors towards Mk cells than rhTPO. H11 also induced progenitors committed to Mk lineage, but most likely at the earlier stages than rhIL-11.
Besides the effects of IL-11 and H11 on megakaryopoiesis, the studies demonstrated that both factors induce also erythropoiesis, while the effect of H11 was much more pronounced. H11 stronger than rhIL-11 promoted differentiation of hematopoietic precursors toward erythroid cells what resulted in the higher expression of CD235a. The involvement of IL-11 in erythropoiesis was described previously [23, 24]. According to proposed model of the hierarchy of the stem cell commitment to the Mk lineage, there are common megakaryocytic/erythroid progenitors (MkEP). MkEPs eventually produce subclasses of even more lineage-restricted Mk and erythroid progenitor cells [25].
The dose response curve of H11 needs to be investigated. Probably the effect of H11 may still be increased. At the present study the same concentrations of rhIL-11 and H11 were used (50ng/ml). Since the molecular weight of rhIL-11 and H11 differs (20kDa and 58,8kDa, respectively), three times more rhIL-11 molecules were available for gp130 activation. However, the action of rhIL-11 strongly depends on the access of membrane IL-11Rα, contrary to H11. The low-level expression of IL-11Rα was observed on cells committed to Mk lineage with exception of platelets which do not express α receptor [20, 26].
Presented studies indicate that H11 is fully active hematopoietic factor and may be explored as megakaryopoietic factor. It not only surpassed the activity of IL-11 like maturation of Mk (since H11 induced higher expression of CD41a and CD61 antigens, resulting in a substantially larger population of CD34-CD41ahighCD61high cells and it also led to increased number of Mk colonies released PLP), but probably also acted on more primitive hematopoietic cells (since upon H11 stimulation more erythroid committed cells were observed).
Interleukin 11 exerts pleiotropic activities. It exhibits effects not only on hematopoietic system, but it also acts on various cell types like epithelial cells, osteoclasts, endothelial cells, B-cell, lymphocyte, macrophage [27]. IL-11 has been shown to induce acute phase proteins, modulate antigen-antibody responses, stimulate the growth of certain lymphocytes, promote neuronal development, inhibit adipogenesis, increase bone absorption and participate in placentation [27, 28]. As mentioned above, the activity of IL-11 is strictly dependent on the cell surface expression of both IL-11R and gp130, while H11 needs only gp130. Since Hyper-IL-11 could stimulate all cells in the body, a strategy to properly target the designer cytokine needs to be examined as well. However, even if H11 would display unacceptable toxicity in vivo, it would not exclude utility of H11 for an ex vivo expansion of megakaryocyte precursor cells.
Acknowledgements
The study was supported by grants from Greater Poland Cancer Centre (no 1/2010) and from The National Science Centre, Krakow, Poland (2011/01/B/NZ4/05796). We thank Malgorzata Klobus for excellent technical assistance.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Peeters K, Stassen JM, Collen D, Van Geet C, Freson K. Emerging treatments for thrombocytopenia: increasing platelet production. Drug Discov Today. 2008;13:798-806
2. Vadhan-Raj S. Management of chemotherapy-induced thrombocytopenia: current status of thrombopoietic agents. Semin Hematol. 2009;46:S26-32
3. Reems JA, Pineault N, Sun S. In vitro megakaryocyte production and platelet biogenesis: state of the art. Transfus Med Rev. 2010;24:33-43
4. Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100:3457-69
5. Gurney AL, Carver-Moore K, de Sauvage FJ, Moore MW. Thrombocytopenia in c-mpl-deficient mice. Science. 1994;265:1445-7
6. de Sauvage FJ, Carver-Moore K, Luoh SM, Ryan A, Dowd M, Eaton DL. et al. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J Exp Med. 1996;183:651-6
7. Bunting S, Widmer R, Lipari T, Rangell L, Steinmetz H, Carver-Moore K. et al. Normal platelets and megakaryocytes are produced in vivo in the absence of thrombopoietin. Blood. 1997;90:3423-9
8. Ramsfjell V, Borge OJ, Veiby OP, Cardier J, Murphy MJ Jr, Lyman SD. et al. Thrombopoietin, but not erythropoietin, directly stimulates multilineage growth of primitive murine bone marrow progenitor cells in synergy with early acting cytokines: distinct interactions with the ligands for c-kit and FLT3. Blood. 1996;88:4481-92
9. Kobayashi M, Laver JH, Kato T, Miyazaki H, Ogawa M. Thrombopoietin supports proliferation of human primitive hematopoietic cells in synergy with steel factor and/or interleukin-3. Blood. 1996;88:429-36
10. Koike K, Nakahata T, Kubo T, Kikuchi T, Takagi M, Ishiguro A. et al. Interleukin-6 enhances murine megakaryocytopoiesis in serum-free culture. Blood. 1990;75:2286-91
11. Burstein SA, Mei RL, Henthorn J, Friese P, Turner K. Leukemia inhibitory factor and interleukin-11 promote maturation of murine and human megakaryocytes in vitro. J Cell Physiol. 1992;153:305-12
12. Neben S, Donaldson D, Sieff C, Mauch P, Bodine D, Ferrara J. et al. Synergistic effects of interleukin-11 with other growth factors on the expansion of murine hematopoietic progenitors and maintenance of stem cells in liquid culture. Exp Hematol. 1994;22:353-9
13. Teramura M, Kobayashi S, Hoshino S, Oshimi K, Mizoguchi H. Interleukin-11 enhances human megakaryocytopoiesis in vitro. Blood. 1992;79:327-31
14. Turner KJ, Neben S, Weich N, Schaub RG, Goldman SJ. The role of recombinant interleukin 11 in megakaryocytopoiesis. Stem Cells. 1996;14(Suppl 1):53-61
15. Dams-Kozlowska H, Gryska K, Kwiatkowska-Borowczyk E, Izycki D, Rose-John S, Mackiewicz A. A designer Hyper interleukin 11 (H11) is a biologically active cytokine. BMC Biotechnol. 2012;12:8
16. Mackiewicz A, Dams-Kozlowska H, Rose-John S. Chimeric soluble Hyper-IL-11 and use thereof. Patent No WO/2005/113591. 2005
17. Suchorska WM, Dams-Kozlowska H, Kazimierczak U, Wysocki PJ, Mackiewicz A. Hyper-interleukin-11 novel designer molecular adjuvant targeting gp130 for whole cell cancer vaccines. Expert Opin Biol Ther. 2011;11:1555-67
18. Stemcells Technologies. MegaCult-C assays for quantitation of human and mouse megakaryocytic progenitors; Version 3.2.1. Technical Manual Stemcells Technologies. 2010.
19. Proulx C, Boyer L, Hurnanen DR, Lemieux R. Preferential ex vivo expansion of megakaryocytes from human cord blood CD34+-enriched cells in the presence of thrombopoietin and limiting amounts of stem cell factor and Flt-3 ligand. J Hematother Stem Cell Res. 2003;12:179-88
20. Weich NS, Wang A, Fitzgerald M, Neben TY, Donaldson D, Giannotti J. et al. Recombinant human interleukin-11 directly promotes megakaryocytopoiesis in vitro. Blood. 1997;90:3893-902
21. Majka M, Ratajczak J, Villaire G, Kubiczek K, Marquez LA, Janowska-Wieczorek A. et al. Thrombopoietin, but not cytokines binding to gp130 protein-coupled receptors, activates MAPKp42/44, AKT, and STAT proteins in normal human CD34+ cells, megakaryocytes, and platelets. Exp Hematol. 2002;30:751-60
22. Matsumura-Takeda K, Sogo S, Isakari Y, Harada Y, Nishioka K, Kawakami T. et al. CD41+/CD45+ cells without acetylcholinesterase activity are immature and a major megakaryocytic population in murine bone marrow. Stem Cells. 2007;25:862-70
23. Rodriguez MH, Arnaud S, Blanchet JP. IL-11 directly stimulates murine and human erythroid burst formation in semisolid cultures. Exp Hematol. 1995;23:545-50
24. Quesniaux VF, Clark SC, Turner K, Fagg B. Interleukin-11 stimulates multiple phases of erythropoiesis in vitro. Blood. 1992;80:1218-23
25. Ciurea SO, Hoffman R. Cytokines for the treatment of thrombocytopenia. Semin Hematol. 2007;44:166-82
26. Weich NS, Fitzgerald M, Wang A, Calvetti J, Yetz-Aldape J, Neben S. et al. Recombinant human interleukin-11 synergizes with steel factor and interleukin-3 to promote directly the early stages of murine megakaryocyte development in vitro. Blood. 2000;95:503-9
27. Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, Dorner AJ. Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia. 1999;13:1307-15
28. Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood. 1997;89:3897-908
Author contact
![]() Corresponding author: Hanna Dams-Kozlowska, PhD. Department of Diagnostics and Cancer Immunology, Greater Poland Cancer Centre, 15 Garbary St. 61-866 Poznan, Poland. Tel.: +48 61 88 50 874 Fax.: +48 61 85 28 502 Email: hanna.dams-kozlowskapl.
Corresponding author: Hanna Dams-Kozlowska, PhD. Department of Diagnostics and Cancer Immunology, Greater Poland Cancer Centre, 15 Garbary St. 61-866 Poznan, Poland. Tel.: +48 61 88 50 874 Fax.: +48 61 85 28 502 Email: hanna.dams-kozlowskapl.

 Global reach, higher impact
Global reach, higher impact