3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(9):1099-1107. doi:10.7150/ijms.5924 This issue Cite
Research Paper
The Effect of Intraoperative Use of High-Dose Remifentanil on Postoperative Insulin Resistance and Muscle Protein Catabolism: A Randomized Controlled Study
1. School of Nutrition & Dietetics, Kanagawa University of Human Services, Kanagawa, Japan;
2. Department of Anesthesiology, Kanagawa Cancer Center, Kanagawa, Japan;
3. Department of Gastrointestinal Surgery, Kanagawa Cancer Center, Kanagawa, Japan.
Received 2013-1-21; Accepted 2013-6-17; Published 2013-7-4
Abstract
Objective: We investigated the effect of the intraoperative use of a high dose remifentanil on insulin resistance and muscle protein catabolism.
Design: Randomized controlled study.
Patients and Intervention: Thirty-seven patients undergoing elective gastrectomy were randomly assigned to 2 groups that received remifentanil at infusion rates of 0.1 μg·kg-1·min-1 (Group L) and 0.5 μg·kg-1·min-1 (Group H).
Main outcome measures: Primary efficacy parameters were changes in homeostasis model assessment as an index of insulin resistance (HOMA-IR) and 3-methylhistidine/creatinine (3-MH/Cr). HOMA-IR was used to evaluate insulin resistance, and 3-MH/Cr was used to evaluate the progress of muscle protein catabolism. Intraoperative stress hormones, insulin, and blood glucose were assessed as secondary endpoints.
Results: Eighteen patients in Group L and 19 in Group H were examined. HOMA-IR values varied within normal limits in both groups during surgery, exceeding normal limits at 12 h after surgery and being significantly elevated in Group L. There were no significant differences in the 3-MH/Cr values between the 2 groups at any time point. The stress hormones (adrenocorticotropic hormone, cortisol, and adrenaline) were significantly elevated in Group L at 60 min after the start of surgery and at the initiation of skin closure. There were no significant differences in insulin values, but blood glucose was significantly elevated in Group L at 60 min after the start of surgery and at the start of skin closure.
Conclusion: Use of high-dose remifentanil as intraoperative analgesia during elective gastrectomy reduced postoperative insulin resistance, although it did not reduce postoperative muscle protein catabolism.
Keywords: remifentanil, insulin resistance, muscle protein catabolism.
Introduction
In recent years, perioperative management methods for enhancing recovery after surgery and improving patient prognosis have been suggested on the basis of evidence from many studies [1]. Such management methods are known as “enhanced recovery after surgery programs.” In the programs, measures taken throughout the perioperative period to reduce postoperative insulin resistance are believed to have effects on the enhanced recovery after surgery [2, 3]. Although there are a number of indices that influence the capacity for postoperative recovery, poor intraoperative glucose control is a risk factor for the incidence of serious postoperative complications [4] and affects mortality [5]. Insulin resistance has been reported to exert a negative effect on postoperative patient outcomes under conditions associated with prolonged hospitalization [6] and postoperative wound infection [7]. Poor intraoperative blood glucose control likely results in stress-induced hyperglycemia due to surgical invasion, complicated with protein catabolism in vivo. As previous studies indicates, remifentanil-focused anaesthetic management improves blood glucose control by suppressing intraoperative stress hormone secretion and preventing hyperglycemia [8-10]. There have been no investigations, however, on the effect of remifentanil dosage on postoperative insulin resistance and muscle protein catabolism.
In this study, we investigated the hypothesis that intraoperative use of high-dose remifentanil may reduce perioperative insulin resistance and muscle protein catabolism by suppressing the stress-induced neuroendocrine response.
Methods
1. Ethics
Ethical approval for this study (Trial No. 26) was provided by the Institutional Review Board of Kanagawa Cancer Center, Japan on September 24, 2010, and the study was carried out in accordance with the 2004 Helsinki Declaration
2. Subjects
Subjects were patients scheduled to undergo laparotomic or laparoscopic elective gastrectomy (total gastrectomy or distal gastrectomy). They met all the following eligibility criteria. The criteria were as follows: Age ≥45 and <75 years, American Society of Anesthesiologists physical status (ASA-PS) grade I or II with body mass index under 35 Kg·m-2 , and written informed consent obtained prior to enrollment. Exclusion criteria were as follows: advanced cancer-related muscle protein catabolism prior to surgery [11], steroid administration, hemoglobin A1c >6.5% , intraoperative blood loss more than 500 mL, and insulin or glucose administrated intraoperatively.
Total number of subject enrollment was set to be 46, of which 23 subjects were for the low-dose 0.25 μg·kg-1·min-1 remifentanil anaesthesia group (Group L) and 23 subjects were for the high-dose 0.5 μg·kg-1·min-1 remifentanil anaesthesia group (Group H). As this study was an exploratory investigation, and because there is not the study that investigated relationship between the endpoint and remifentanil consumption in the past, the target sample size was based on the assumption that “blood glucose levels can constitute an endpoint, indirectly expressing comparable levels of analgesia.” Since blood glucose data from previous studies presented blood glucose levels of 118 mg·dL-1 for patients receiving 0.25 μg·kg-1·min-1 remifentanil and 92 mg·dL-1 for those receiving 1.0 μg·kg-1·min-1[10], average blood glucose levels in this study were set at 120 mg·dL-1 for Group L and 100 mg·dL-1 for Group H. With standard deviations of 20 mg·dL-1 for both groups, a 2-sided significance level of 0.05, and 90% power, it was estimated that the sample size required for a t-test was to be 44 (22 patients per group), and enrollment was therefore set at 46 patients (23 patients per group) to allow for cases of dropouts and withdrawals [12].
3. Experimental procedure
This study was designed as a randomized controlled trial. Subjects were registered using a patient allocation table by block randomization and allocated to either Group L or Group H.
The same preoperative management was given to the patients of both L and H groups [13]. Patients fasted after taking an evening meal at 18:00 the night before surgery and were allowed to drink an oral rehydration solution (ORS) up to 2 h before entering the operating room. ORS contains 2.5% carbohydrate and 50 mmol·L-1 sodium ions. Preoperative body fluid management with ORS is known as preoperative oral rehydration therapy (ORT) and is widely practiced in Japan instead of infusion therapy [14,15]. In our hospital, patients are asked to drink 1500 mL of ORS packaged in 500-mL plastic bottles (OS-1, Otsuka Pharmaceutical Factory, Inc., Tokushima, Japan) for preoperative fluid and electrolyte replacement.
After a patient entered the operating room, a 20G intravenous line was secured in the patient's left forearm and a glucose-free infusion of bicarbonated Ringer's solution was administered intravenously. An epidural catheter was then placed between the Th 7-11 vertebrae, and a test injection of 2.0 mL of 1% mepivacaine was administered.
After the induction of general anaesthesia by the intravenous administration of 0.5 μg·kg-1·min-1 remifentanil, 1 mg·kg-1 propofol, and 0.5-1.5 mg·kg-1 rocuronium, tracheal intubation was performed. When intubation was difficult and several attempts were required, it was so described in the patient report form. An arterial pressure line was placed in the radial artery of the left arm after induction of general anaesthesia.
Except for maintenance rate of remifentanil infusion, general anaesthesia was maintained similarly in the both groups. Epidural analgesia and intravenous fluids containing glucose were not used during surgery. Additional rocuronium was administered as a muscle relaxant to control adductor pollicis contraction to ≤10%. Propofol at around 2 μg·mL-1 or 4 mg·kg-1·h-1 was administered by target-controlled infusion (TCI) as a sedative. The dose of propofol was adjusted using a bispectral index (BIS) monitor (Nihon Kohden Corporation, Tokyo, Japan) with an index BIS value of 45-60. The dose of remifentanil after tracheal intubation was not predetermined, but was fixed 3 min before the operation started at 0.1 μg·kg-1·min-1 for Group L and 0.5 μg·kg-1·min-1 for Group H. Hemodynamic depression was treated by the administration of 8 mg ephedrine or 0.1 mg phenylephrine for decreased SBP, or the administration of 0.5 mg atropine for decreased HR. Stress response was treated by bolus administration of 1 μg·kg-1 remifentanil. If there was no response to this treatment, noradrenaline or β-blocker was used.
Remifentanil infusion was terminated after the completion of blood sampling at the start of skin closure, and continuous epidural analgesia was started as postoperative analgesia after intravenous administration of fentanyl 100 μg and flurbiprofen 50 mg, irrespective of body weight, in both groups. Continuous epidural analgesia was initiated with a bolus dose of 3 mL of 0.2% ropivacaine, followed by continuous administration (4 mL·h-1) of a mixture of 0.2% ropivacaine (280 mL) and 1,000 μg fentanyl via a disposable pump (Coopdeck Balloonjector, 300 mL, Daiken Medical Co., Ltd., Tokyo, Japan).
After the tracheal tube was removed, continuous administration of a maintenance infusion containing 7.5% glucose was performed at a rate of 60 mL·h-1 for 12 h following surgery. In addition, pain control was performed by bolus administration of epidural anaesthesia or non-steroidal anti-inflammatory drug (NSAID) suppositories as required.
4. Endpoints
The primary endpoint of this study was the assessment of insulin resistance and muscle protein catabolism at 12 h after surgery. Insulin resistance was assessed by homeostasis model assessment as an index of insulin resistance (HOMA-IR), calculated by the equation “[fasting insulin (μU·mL-1) × fasting blood glucose (mg·dL-1)/405].” Its standard value for the Japanese population is <1.6, and values of ≥2.5 indicate insulin resistance [16]. We also obtained HOMA-β (%), calculated by the equation “fasting insulin (μU·mL-1) × 360/[fasting blood glucose (mg·dL-1) - 63]” to assess the secretory function of pancreatic β-cells. This was calculated because it was necessary to know whether abnormal values for HOMA-IR and HOMA-β were due to insulin resistance or pancreatic β-cell dysfunction. HOMA-β is expressed as a percentage (the constants are set as such that its value is 100% when β-cells are functioning at maximum ) [17]. However, no standard value for the Japanese population has been established [17]. Muscle protein catabolism was assessed in terms of changes in blood 3-methylhistidine/creatinine (3-MH/Cr) [18] and transthyretin (TTR), a rapid turnover protein. Intraoperative stress hormones (adrenocorticotropic hormone, ACTH; cortisol; adrenaline, Ad; noradrenaline, NAd; and dopamine, DOA), insulin, and blood glucose were assessed as secondary endpoints.
HOMA-IR and 3-MH/Cr were measured before anaesthesia induction, 60 min after the start of surgery, at the start of skin closure, and 12 h after the end of surgery. TTR was measured before anaesthesia induction, 12 h after the end of surgery, and 7 days after surgery. Stress hormones, insulin, and blood glucose were measured before anaesthesia induction, 60 min after the start of surgery, and at the start of skin closure.
5. Statistical analysis
Values were expressed as mean ± standard deviation. Groups were compared using χ2 test for nominal scales, Wilcoxon paired rank sum test for ordinal scales, repeated-measures analysis of variance (ANOVA), and unpaired Student's t-test for continuous scales, with p < 0.05 regarded as significant.
Changes from the baseline (pre surgery) values were different between the groups by using the unpaired Student's t-test. Mean value was higher than at baseline (pre surgery) within the same group by using the repeated-measures of ANOVA, and subsequently, multiple comparisons were performed using the Tukey-Kramer HSD test. The statistical analysis software used was JMP©8.0.1 (SAS Institute, Tokyo, Japan).
Results
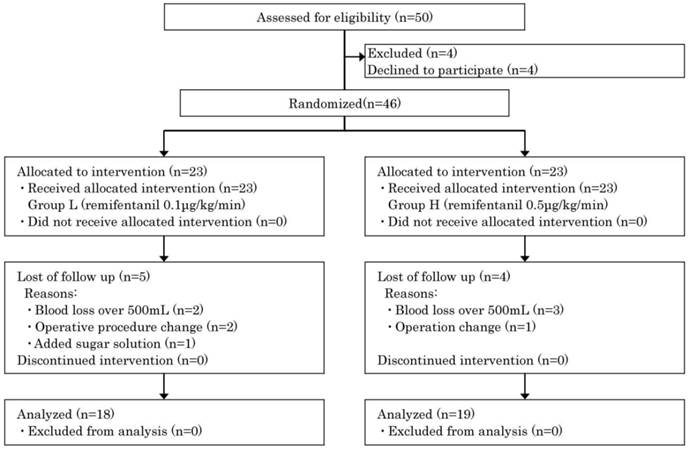
A total of 46 patients were enrolled, 23 in each group. Of these, the study could be completed as planned by 18 patients (78%) in Group L and 19 (83%) in Group H (Figure 1).
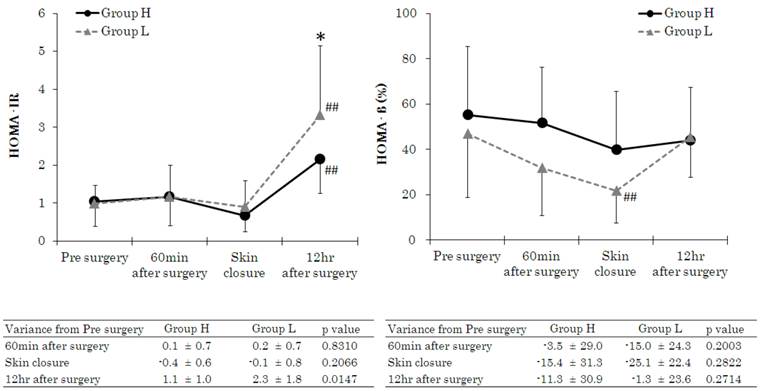
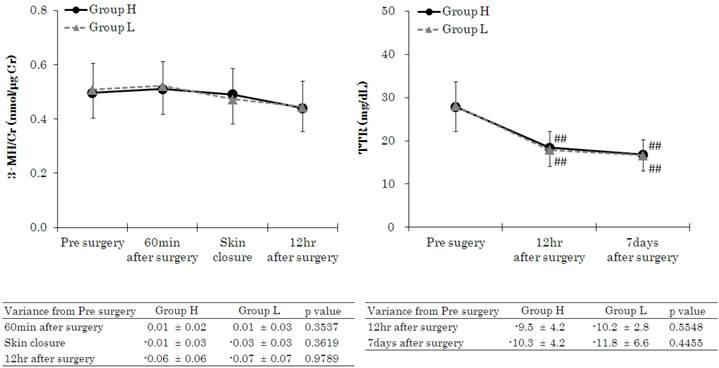
Patient characteristics and intraoperative variables are given in Table 1. There were no significant differences between the 2 groups in gender, age, height, body weight, ASA-PS, type of surgery, operation time, anaesthesia time, fluid volume, or blood loss. Intraoperative urine volume, however, was higher in Group H. Intraoperative drug consumption of remifentanil, propofol, and rocuronium was calculated by dividing total consumption by administration times and body weight. Remifentanil consumption was higher in Group H, whereas propofol and rocuronium were used to a greater extent in Group L. Figure 2 shows the HOMA-IR and HOMA-β measurements. HOMA-IR varied within normal limits before induction of anaesthesia, 60 min after the start of surgery, and at the start of skin closure, with no significant differences between the 2 groups. However, at 12 h after the end of surgery, mean values exceeded the standard range (<1.6) in both groups and HOMA-IR value was significantly higher in the Group L than in the Group H. HOMA-β values at 60 min after the start of surgery and skin closure were significantly lower in Group L than in the Group H, but significant difference was not noted. It was significantly lower at skin closure compared to baseline value in the Group L. Figure 3 shows 3-MH/Cr and TTR measurements. There were no significant differences in 3-MH/Cr and TTR measurements between the 2 groups at any time point. In both groups, although TTR level was within normal limits (22-44 mg·dL-1) before anaesthesia induction, it decreased below the standard value at 12 h after the end of surgery and at 7 days after surgery.
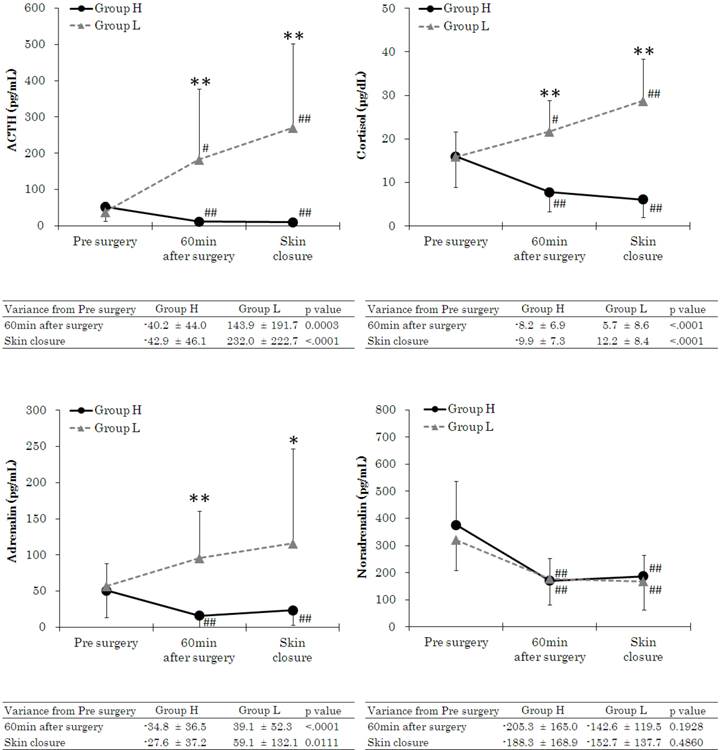
Figure 4 shows stress hormone measurements. The stress hormones ACTH, cortisol, and Ad were significantly elevated in Group L at 60 min after the start of surgery and at the start of skin closure.
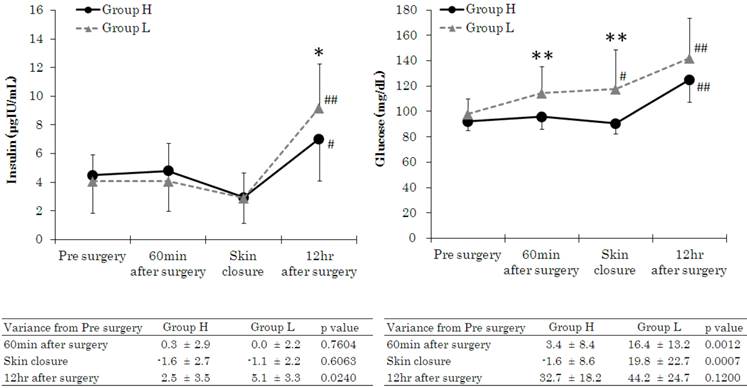
Figure 5 shows insulin and blood glucose measurements. There were no significant differences in insulin values between the 2 groups during surgery, but blood glucose was significantly elevated in Group L at 60 min after the start of surgery and at the start of skin closure.
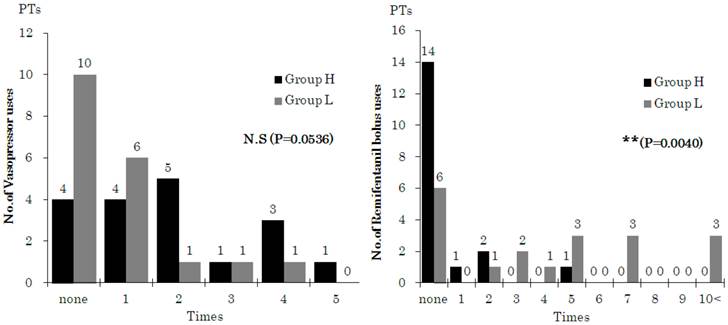
The frequency of management for intraoperative hemodynamic depression and stress responses is indicated in Figure 6. Vasopressors were used to treat hemodynamic depression more often in Group H, although this difference was not significant, and treatment for stress response due to surgical invasion was significantly frequent in Group L.
Patients were not given any medication or fluids in the recovery room other than those scheduled.
Patient characteristics and intraoperative variables.
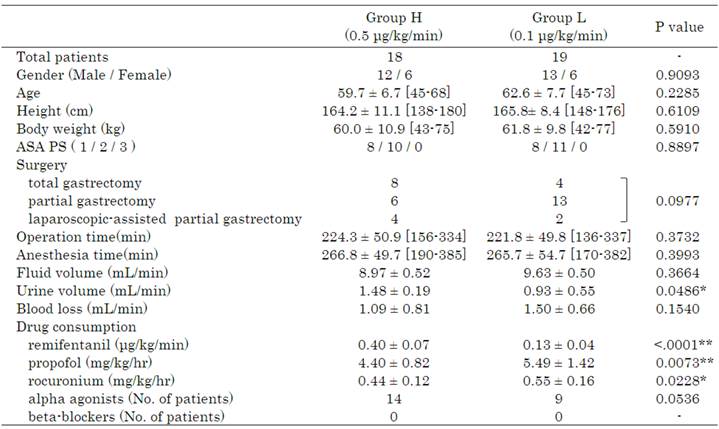
Urine volume was significantly higher in Group H than Group L. Drug consumption of propofol and rocuronium was significantly higher in Group L than Group H.
Flow diagram (progress steps of the trial: selection and randomization of elective gastrectomy patients to receive low- or high-dose remifentanil anaesthesia).
Changes in homeostasis model assessment as an index of insulin resistance (HOMA-IR) and homeostatic model assessment β-cell function (HOMA-β). Data are expressed as the mean ± standard deviation. Changes from the baseline (pre surgery) values were different (*p < 0.05, **p < 0.01) between the groups. Mean value was higher (#p < 0.05, ##p < 0.01) than at baseline (pre surgery) within the same group. Standard values: HOMA-IR, >2.5; HOMA-β, 40-60%.
Changes in 3-methylhistidine/creatinine (3-MH/Cr) and transthyretin (TTR). Data are expressed as the mean ± standard deviation. Changes from the baseline (pre surgery) values were different (*p < 0.05, **p < 0.01) between the groups. Mean value was higher (#p < 0.05, ##p < 0.01) than at baseline (pre surgery) within the group. Standard values: 3-MH/Cr, 0.13-0.53; TTR, 22-44 mg·dL-1.
Discussion
In this study, we investigated the effect of the intraoperative use of remifentanil at infusion rates of 0.1 μg·kg-1·min-1 (Group L) and 0.5 μg·kg-1·min-1 (Group H) on postoperative insulin resistance and muscle protein catabolism in patients undergoing elective gastrectomy. In addition, in this study, the patients did not consume carbohydrate fluids before surgery [1, 3], and we did not start epidural analgesia [1, 2,19] or administer glucose-containing fluids [20] during surgery. This was in order to assess the effect of different rates of remifentanil infusion alone on insulin resistance and muscle protein catabolism. In the study, we found that secretion of stress hormones during surgery was suppressed in Group H, and postoperative insulin resistance decreased at 12 h after the end of surgery. However, there was no significant difference between the 2 groups in the level of muscle protein catabolism. Secretion of stress hormones and blood glucose elevation were probably suppressed during surgery in Group H as a result of the use of high-dose remifentanil, as shown in previous studies [8-10]. Another finding was that urine volume was higher in Group H despite the absence of any difference between the 2 groups in fluid volume, which was consistent with the study of Myles et al [21].
HOMA-IR was significantly elevated in Group L at 12 h after the end of surgery. Generally, a highly reliable method such as the glucose clamp test, steady state plasma glucose technique, or minimal model method is chosen for assessing insulin resistance. In this study, however, we chose to use HOMA-IR for the following reasons: involvement of a large number of subjects, necessity to perform assessments with complicated postoperative procedures, and fact that HOMA-IR is recognized as highly reliable in patients with blood glucose ≤140 mg·dL-1[22]. HOMA-β was lower in Group L at 60 min after the start of surgery and at the start of skin closure, although significant difference was not recognized because of large values of standard deviation. These findings suggest that in Group L, the decline in insulin secretion by pancreatic β-cells was maintained during surgery and also suggest that although secretory function recovered after surgery, the response to insulin receptors in cells was poor. This was probably why there was no attenuation of postoperative insulin resistance in Group L. Since HOMA-IR is calculated from the amount of insulin excretion and the level of blood glucose, changes in HOMA-IR are not likely to occur during inoperative periods or at the time of unusual conditions when glucose is not loaded, whereas changes are very likely to occur in postoperative periods when glucose is loaded. In this study, no difference was noted in HOMA-IR values between the groups during and at the end of surgery, although significant difference was noted 12 hr later.
We also used blood 3-methylhistidine/creatinine (3-MH/Cr), rather than urine methylhistidine, as an index of muscle protein catabolism. This is because metabolic assessment of 3-MH/Cr is possible with blood in a shorter time than with urine [18]. We measured such 3-MH/Cr to assess muscle protein catabolism due to surgical invasion. We initially expected that the use of high-dose remifentanil would give rise to differences in stress-induced muscle protein catabolism. However, for all patients, values varied within normal limits (0.13-0.53 nmol·μg-1 Cr), although the values were close to the upper limit, and there was no significant difference between the 2 groups. 3-MH/Cr may have remained within normal limits in Group L due to the effect of the lipids in propofol which was used as a sedative. Propofol contains an amount of lipid equivalent to a 10% fat preparation. A large dose of propofol is required to guarantee a consistent level of sedation through its interaction with remifentanil, and as a result propofol consumption was significantly larger in Group L compared with that in Group H. It is therefore very likely that because Group L received a larger dose of fat than did Group H, muscle protein catabolism decreased and there was no rise in 3-MH/Cr.
Changes in adrenocorticotropic hormone (ACTH), cortisol, adrenaline (Ad), and noradrenaline (NAd). Data are expressed as the mean ± standard deviation. Changes from the baseline (pre surgery) values were different (*p < 0.05, **p < 0.01) between the groups. Mean value was higher (#p < 0.05, ##p < 0.01) than at baseline (pre surgery) within the group. Standard values: ACTH, 7.2-63.3 pg·mL-1; cortisol, 4.5-21.1 pg·mL-1; Ad <100 pg·mL-1; NAd, 140-450 pg·mL-1.
Changes in insulin and glucose. Data are expressed as the mean ± standard deviation. Changes from the baseline (pre surgery) values were different (*p < 0.05, **p < 0.01) between the groups. Mean value was higher (#p < 0.05, ##p < 0.01) than at baseline (pre surgery) within the group. Standard values: insulin, 2.2-12.4 pg·mL-1; glucose, 70-109 mg·dL-1.
Number of patients administrated a bolus dose of vasopressor or remifentanil (1μg/kg). Data are expressed as the number of patients (PTs). Number of patients with drug administration was different (*p < 0.05, **p < 0.01) between the groups (calculated using χ2 test).
The results of this study have shown that strong intraoperative analgesics may suppress the increase of stress hormone and blood glucose level during surgery, and thus affecting insulin resistance after surgery. Our findings in this study suggest that concomitant use of 0.5 μg·kg-1·min-1 remifentanil as an intraoperative analgesic with the aim of improving insulin resistance may offer new possibilities for enhanced recovery after surgery.
Regarding the patient enrollment, this study included patients undergoing both open surgery and laparoscopic surgery, which differ in the degree of surgical invasiveness. In the study, stratified randomization was performed for each type of surgery, and there was no bias among patients registered in the 2 groups. We compared data for patients who underwent open surgery and laparoscopic surgery within each group and found no significant difference in variables.
This study had several limitations. There were a number of dropout cases (due to operative blood loss, type of surgical procedure applied, etc.) and a planned sample size (22 patients per group) was not investigated in this study. We did not investigate the endpoints, which were used to assess recovery after surgery in other studies, such as length of hospital stay and occurrence of postoperative complications [23], so that we were unable to conclude whether the use of high-dose remifentanil may enhance recovery after surgery. Several previous studies, however, have reported that reducing postoperative insulin resistance enhances recovery after surgery [1-3, 24]. We used HOMA-IR to assess insulin resistance. Because the results of this study showed a tendency to reduce insulin resistance in postoperative patients, it will be necessary to confirm these results with greater accuracy by limiting studies to small numbers of patients and using a method such as the glucose clamp test.
The results of this study support the hypothesis that the intraoperative use of high-dose remifentanil may reduce postoperative insulin resistance patients undergoing elective gastrectomy. However, the hypothesis that it may reduce postoperative protein catabolism was not established.
Acknowledgements
We would like to thank Professor Hisahiro Takahashi, Department of Nutrition, Kanagawa University of Human Services, Kanagawa, Japan, for assistance in patient allocation, randomization, and maintaining patient records.
Financial support and sponsorship
This word was supported by Kanagawa Cancer Center, Yokohama, Japan
Competing Interests
None declared.
References
1. Fearon KC, Ljungqvist O, Von Meyenfeldt M, Revhaug A, Dejong CH, Lassen K, Nygren J, Hausel J, Soop M, Andersen J, Kehlet H. Enhanced recovery after surgery: A consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24:466-77
2. Kehlet H, Wilmore DW. Evidence based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189-98
3. Oliveira KG, Balsan M, Oliveira Sde S, Aguilar-Nascimento JE. Does abbreviation of preoperative fasting to two hours with carbohydrates increase the anesthetic risk? Rev Bras Anestesiol. 2009;59:577-84
4. Ouattara A, Lecomte P, Le Manach Y, Landi M, Jacqueminet S, Platonov I, Bonnet N, Riou B, Coriat P. Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology. 2005;103:687-94
5. Ammori JB, Sigakis M, Englesbe MJ, O'Reilly M, Pelletier SJ. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007;140:227-33
6. Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2:69-78
7. Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95:4338-44
8. Winterhalter M, Brandl K, Rahe-Meyer N, Osthaus A, Hecker H, Hagl C, Adams HA, Piepenbrock S. Endocrine stress response and inflam-matory activation during CABG surgery. A randomized trial comparing remifentanil infusion to intermittent fentanyl. Eur J Anaesthesiol. 2008;25:326-35
9. Ihn CH, Joo JD, Choi JW, Kim DW, Jeon YS, Kim YS, Jung HS, Kwon SY. Comparison of stress hormone response, interleukin-6 and anaes-thetic characteristics of two anaesthetic techniques: volatile induction and maintenance of anaesthesia using sevoflurane versus total intravenous anaesthesia using propofol and remifentanil. J Int Med Res. 2009;37:1760-71
10. Weale NK, Rogers CA, Cooper R, Nolan J, Wolf AR. Effect of remifentanil infusion rate on stress response to the pre-bypass phase of paediatric cardiac surgery. Br J Anaesth. 2004;92:187-94
11. Yamada T, Hayashi T, Cho H, Yoshikawa T, Taniguchi H, Fukushima R, Tsuburaya A. Usefulness of enhanced recovery after surgery protocol as compared with conventional perioperative care in gastric surgery. Gastric Cancer. 2012;15:34-41
12. Mohri Y, Tanaka K, Ohi M, Yokoe T, Miki C, Kusunoki M. Prognostic Significance of Host- and Tumor-Related Factors in Patients with Gastric Cancer. World J Surg. 2010;34:285-90
13. Miyahara H, Tango T. Medical Statistics Handbook 11: Sample Size. 11.4.2 Testing for differences between population means: 274. 1995 [In Japanese]
14. Itou K, Fukuyama T, Sasabuchi Y, Yasuda H, Suzuki N, Hinenoya H, Kim C, Sanui M, Taniguchi H, Miyao H, Seo N, Takeuchi M, Iwao Y, Sakamoto A, Fujita Y, Suzuki T. Safety and efficacy of oral rehydration therapy until 2 h before surgery: a multicenter randomized controlled trial. J Anesth. 2012;26:20-7
15. Taniguchi H, Sasaki T, Fujita H, Takamori M, Kawasaki R, Momiyama Y, Takano O, Shibata T, Goto T. Preoperative fluid and electrolyte management with oral rehydration therapy. J Anesth. 2009;23:222-9
16. Katsuki A, Sumida Y, Gabazza EC, Murashima S, Furuta M, Ara-ki-Sasaki R, Hori Y, Yano Y, Adachi Y. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362-5
17. Hermans MP, Levy JC, Morris RJ, Turner RC. Comparison of tests of beta-cell function across a range of glucose tolerance from normal to diabetes. Diabetes. 1999;48:1779-86
18. Yamashita S. Blood 3-methyl-histidine as an index of protein catabo-lism; standard values and changes in values in severe cases. Yamaguchi Igaku. 2007;56:193-200 [In Japanese]
19. Schricker T, Wykes L, Eberhart L, Lattermann R, Mazza L, Carli F. The anabolic effect of epidural blockade requires energy and substrate supply. Anesthesiology. 2002;97:943-51
20. Yamasaki K, Inagaki Y, Mochida S, Funaki K, Takahashi S, Sakamoto S. Effect of intraoperative acetated Ringer's solution with 1% glucose on glucose and protein metabolism. J Anesth. 2010;24:426-31
21. Myles PS, Hunt JO, Fletcher H, Watts J, Bain D, Silvers A, Buckland MR. Remifentanil, fentanyl, and cardiac surgery: a double-blinded, randomized, controlled trial of costs and outcomes. Anesth Analg. 2002;95:805-12
22. Matsuhisa M, Yamasaki Y, Emoto M, Shimabukuro M, Ueda S, Fu-nahashi T, Matsuzawa Y. A novel index of insulin resistance determined from the homeostasis model assessment index and adiponectin levels in Japanese subjects. Diabetes Res Clin Pract. 2007;77:151-4
23. Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery (Review). Cochrane Database Syst Rev. 2011;16:CD007635
24. Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. ESPEN guidelines on parenteral nutrition: Surgery. Clin Nutr. 2009;28:378-86
Author contact
![]() Corresponding author: Hideki Taniguchi, School of Nutrition & Dietetics, Kanagawa University of Human Services, and Department of Anesthesiology, Kanagawa Cancer Center, 1-10-1 Heisei, Yokosuka, Kanagawa 238-8522, Japan. Telephone: +81-46-828-2500 Fax: +81-46-828-2501 Email: hstanicom.
Corresponding author: Hideki Taniguchi, School of Nutrition & Dietetics, Kanagawa University of Human Services, and Department of Anesthesiology, Kanagawa Cancer Center, 1-10-1 Heisei, Yokosuka, Kanagawa 238-8522, Japan. Telephone: +81-46-828-2500 Fax: +81-46-828-2501 Email: hstanicom.

 Global reach, higher impact
Global reach, higher impact