3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(8):995-1002. doi:10.7150/ijms.5963 This issue Cite
Research Paper
Overexpression of Integrin-linked Kinase Promotes Lung Cancer Cell Migration and Invasion via NF-κB-mediated Upregulation of Matrix Metalloproteinase-9
1. Department of Respiratory Medicine,
2. Department of Pathology, The Fourth Affiliated Hospital of China Medical University, Shenyang 110032, People's Republic of China
# Mingjing Zhao and Ying Gao contributed equally to this work.
Received 2013-1-26; Accepted 2013-5-14; Published 2013-6-14
Abstract
Integrin-linked kinase (ILK) is a highly conserved serine-threonine protein kinase which has been implicated in the regulation of various cellular processes. Previously, we have demonstrated that overexpression of ILK correlates with malignant phenotype in non-small cell lung cancer. Furthermore, forced overexpression of ILK promotes lung cancer cell invasion and migration. However, the molecular mechanisms by which ILK enhances the invasive phenotype of lung cancer cells are still not fully understood. In the present study, we found that overexpression of ILK stimulated matrix metalloproteinase-9 (MMP-9) expression and activity in lung cancer cells. ILK-induced cell migration and invasion were significantly inhibited by MMP inhibitor doxycycline as well as by anti-MMP-9 neutralizing antibody. In addition, overexpression of ILK induced phosphorylation and nuclear translocation of nuclear factor-κB (NF-κB) subunit p65. Finally, upregulation of MMP-9 was severely abolished by either BAY 11-7028, a specific NF-κB inhibitor, or small interfering RNA targeted to NF-κB p65 in ILK overexpression cells. Taken together, these findings suggest that ILK promotes lung cancer cell migration and invasion via NF-κB-mediated upregulation of MMP-9.
Keywords: integrin-linked kinase, matrix metalloproteinase-9, migration, invasion, lung cancer cell, nuclear factor-κB
Introduction
Integrin-linked kinase (ILK) is a highly conserved serine-threonine protein kinase which interacts with the cytoplasmic domains of integrin subunits [1-2]. As a component of the phosphatidylinositol 3-kinase (PI-3K) pathway located upstream of protein kinase B (PKB/AKT), ILK is implicated in the regulation of various cellular processes, including cell migration and invasion [3-5]. Activated ILK can directly phosphorylate PKB/AKT and glycogen synthase kinase-3β (GSK-3β), resulting in activation of PKB/AKT and inhibition of GSK-3β, respectively [6-7]. Lately several groups have reported the abnormal overexpression of ILK in a diverse set of cancers, such as colon [8-9], gastric [10], prostate [11] and ovarian cancers [12], malignant melanomas [13], malignant pleural mesothelioma [14] and non-small cell lung cancer (NSCLC) [15-17]. In addition, overexpression of ILK has been shown to promote cell migration and invasion [18-20]. However, the underlying molecular mechanisms still remain to be fully elucidated.
Matrix metalloproteinases (MMPs) belong to a large family of zinc-dependent endopeptidases which are responsible for degrading a wide range of extracellular matrix (ECM) components [21]. Degradation of ECM surrounding tumor cells is a necessary requisite for invasion and metastasis of tumors [22]. Consequently, MMPs play a crucial role in tumor cell migration and invasion. Overexpression of MMPs has been found in a variety of human cancers including NSCLC [23-25]. Moreover, it has been shown that the expression of MMPs is regulated by ILK. Studies have demonstrated that downregulation of ILK reduces the expression of MMP-9 in bladder cancer cells, resulting in the inhibition of cell migration and invasion [26-27]. Ectopic expression of ILK induces MMP-9 expression and promotes cell migration in podocytes [28].
Nuclear factor-κB (NF-κB) is a transcription factor and has been implicated in the regulation of MMPs expression. Inhibition of NF-κB activity reduces the expression of MMP-9 in human metastatic lung and cervical cancer cells [29-30], as well as in breast cancer cells [31]. Additionally, Wani et al. (2011) have documented that overexpression of ILK results in phosphorylation and nuclear translocation of NF-κB subunit p65, indicating activation of the NF-κB signaling pathway [32]. These findings suggest potential roles of MMP-9 and NF-κB in ILK-induced migration and invasion of lung cancer cells.
Previously, we reported that ILK upregulation correlates with malignant phenotype in NSCLC and promotes lung cancer cell invasion and migration via regulating epithelial-mesenchymal transition (EMT)-related genes [33]. In this study, we investigated the role of MMP-9 in cell migration and invasion induced by ILK overexpression in a human lung cancer cell line (A549). We demonstrated that MMP-9 expression is regulated by ILK in lung cancer cells. Moreover, inhibition of MMP-9 results in prevention of ILK-induced cell migration and invasion. Finally, NF-κB is involved in ILK-induced upregulation of MMP-9 in lung cancer cells.
Materials and Methods
Cell culture
The human lung cancer cell line A549 was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were maintained in RPMI 1640 (Gibco, Grand Island, NY, USA) containing 10 % fetal bovine serum (FBS, Hyclone, Logan, UT, USA), penicillin (100 U/mL), streptomycin (100 μg/mL) and 25 mM HEPES buffer. All cells were kept in a humidified (37 °C, 5 % CO2) incubator and passaged routinely. Cells at logarithmic growth phase were used in this study. The NF-κB inhibitor BAY 11-7028 (10 μM) (Beyotime Institute of Biotechnology, Haimen, China) was added to cell cultures for 48 h before analysis.
Stable transfection
ILK overexpression cell line was established as described before [33]. In brief, we transfected pcDNA3.1-ILK or pcDNA3.1-vector plasmids into A549 cells to overexpress ILK. Stably transfected cell lines were obtained by G418 (Invitrogen, Carlsbad, CA, USA) selection. The pcDNA3.1-ILK cell line contained a higher level of ILK protein was used in this study.
Quantitative real-time PCR
Total RNA was extracted from cells using Trizol reagent (Life Technologies, Rockville, MD, USA) according to the manufacturer's instructions. First-strand complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). Quantitative real-time PCR reactions were carried out with cDNA (1 μL) and the SYBR Green Master Mix (Takara) on a Real-Time Quantitative Thermal Block (Biometra, Göttingen, Germany). The sequences of primers are as follows: ILK, forward: 5'-ATGGAACCCTGAACAAACACT-3', reverse: 5'-AGCACATTTGGATGCGAGAAA-3'; MMP-9, forward: 5'-GCTACGTGACCTATGACATCCT-3', reverse: 5'-TCCTCCAGAACAGAATACCAGT-3'; NF-κB p65, forward: 5'-CCCATCTTTGACAATCGTGC-3', reverse: 5'-CTGGTCCCGTGAAATACACC -3'; β-actin, forward 5'-CTTAGTTGCGTTACACCCTTTCTTG-3', reverse 5'-CTGTCACCTTCACCGTTCCAGTTT-3'. β-Actin was served as an internal control. The specificity of the PCR was confirmed by melting curve analysis. Data were treated using the comparative threshold cycle (CT) method [34].
Western blot analysis
Protein extracts were prepared using radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology). The concentrations of extract proteins were measured by a bovine serum albumin standard line. Western blot was performed as previously described [33]. The following antibodies were used in this study: anti-MMP-9, anti-lamp2, anti-histone H4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ILK, anti-p-S473-AKT, anti-p-S9-GSK-3β, anti-p-S536-NF-κB p65, anti-p-S32-IκB, anti-NF-κB p65 (Cell Signaling Technology, Boston, MA, USA) and anti-β-actin antibodies (Sigma-Aldrich, St Louis, MO, USA). Signals were detected using an ECL plus chemiluminescence kit (Millipore, Bedford, MA, USA).
Gelatin zymography
The proteolytic activity of MMP-9 was measured by gelatin zymography protease assays as described by Kao et al. (2012) [30]. Briefly, the collected media of an appropriate volume were prepared with sodium dodecyl sulphate (SDS) sample buffer without boiling or reduction and then subjected to 0.1 % gelatin-8 % SDS polyacrylamide gel electrophoresis (SDS-PAGE) at 4 °C. After electrophoresis, gels were washed with 2.5 % Triton X-100 and then incubated in reaction buffer (40 mM Tris-HCl, pH 8.0, 10 mM CaCl2, 0.01 % NaN3) for 12 h at 37 °C. Finally, the gel was stained with Coomassie brilliant blue R-250.
Wound healing assay
Cells were plated in six-well plates at a density of 1 × 105 cells per well and grown to approximately 80 % confluency. The monolayer was scraped with a sterile 200 μL pipette tip after removal of the culture medium. Subsequently, the culture was washed twice with serum-free medium. After that, cells were maintained in RPMI 1640 containing 10 % FBS for an additional 12 or 24 h. The migration ability of the cells was assessed by measuring the width of the monolayer wound at 12 and 24 h after scraping. The contribution of MMP-9 to the migration of pcDNA3.1-ILK cells was determined by performing wound healing assay in the presence of doxycycline (20 μg/mL) or anti-MMP-9 antibody (10 μg/mL) (Santa Cruz). The addition of an irrelevant IgG was used as control.
In vitro Matrigel invasion assay
In vitro migration assay was conducted as described before [33]. Briefly, 5 × 104 cells in 500 μL of serum-free medium, with or without doxycycline (20 μg/mL) and anti-MMP-9 antibody (10 μg/mL) (Santa Cruz), were loaded into the upper chamber. The RPMI 1640 medium containing 10 % FBS was used as chemoattractant and loaded into the lower chamber. After 24 h incubation, the non-invasive cells were removed by wiping with a cotton swab, and the migrated cells were fixed and stained with hematoxylin. Six random fields at a magnification of 200× were counted for quantification of cell migration. Migration assay performed with 5 × 104 cells in serum-free medium (500 μL) containing an irrelevant IgG was used as control.
Transfection with small interfering RNA (siRNA) targeted to NF-κB subunit p65
The siRNA sequence used for knockdown of NF-κB p65 expression was 5'-GCCCUAUCCCUUUACGUCA-3' [35]. A scrambled sequence which does not affect any known cellular mRNA was served as a negative control. Transfection was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Statistical analysis
The statistical analyses were performed with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). The values were expressed as means ± SD. Differences between groups were analyzed by one-way ANOVA. A P-value < 0.05 was considered statistically significant.
Results
Overexpression of ILK stimulates MMP-9 expression and activity in lung cancer cells
Previously, we have established an ILK overexpression cell line in which the expression of ILK was increased at both mRNA and protein levels (Supplementary Material: Fig. S1) [33]. To investigate whether the expression of MMP-9 is regulated by ILK, we examined the mRNA and protein levels of MMP-9 in these cells. As shown in Fig. 1A, the mRNA level of MMP-9 was significantly increased in pcDNA3.1-ILK cells compared with pcDNA3.1-vector cells and mock control cells (control cells without transfection). In addition, overexpression of ILK also induced MMP-9 protein expression (Fig. 1B and 1C, P < 0.01). The elevated phosphorylation of PKB/AKT and GSK-3β proteins further confirmed the kinase activity of ILK in the transfected cells (Fig. 1B and 1C, P < 0.05). Moreover, MMP-9 activity was appreciably increased in pcDNA3.1-ILK cells as evidenced by zymographic analysis (Fig. 1D, P < 0.01). These data demonstrated that overexpression of ILK stimulated MMP-9 expression and activity.
MMP-9 is required for ILK-induced migration and invasion of lung cancer cells
The observation that MMP-9 is upregulated by ILK overexpression suggests that MMP-9 may play an important role in ILK-induced cell migration and invasion. Therefore, we analyzed the effects of MMP-9 inhibitor doxycycline and anti-MMP-9 antibody on the migration and invasion of ILK overexpression cells. As shown in Fig. 2A and 2B, the addition of doxycycline significantly impaired the wound healing capacity in pcDNA3.1-ILK cells. Similarly, cell migration was severely retarded in the presence of anti-MMP-9 neutralizing antibody (Fig. 2A and 2B). However, the control (irrelevant) IgG did not show any retarding effect on the migration of ILK overexpression cells (Fig. 2A and 2B).
Next, the Transwell invasion assay was carried out to further explore whether MMP-9 is required for ILK-induced migration and invasion of lung cancer cells. We found that inhibition of MMP-9, by either doxycycline or anti-MMP-9 neutralizing antibody, dramatically suppress migration and invasion of ILK overexpression cells, as demonstrated by a remarkable decrease in the number of migrated and invaded cells (Fig. 2C and 2D, P < 0.01). The addition of the control (irrelevant) IgG had no effect (Fig. 2C and 2D). Collectively, these findings revealed that MMP-9 is required for ILK-induced migration and invasion of lung cancer cells.
ILK stimulates MMP-9 expression and activity in human lung cancer A549 cells. (A) MMP-9 mRNA level in pcDNA3.1-ILK cells compared with pcDNA3.1-vector cells and mock control cells as determined by quantitative real-time PCR. (B) Western blot analysis of MMP-9, p-AKT and p-GSK-3β protein expression in transfected cells. (C) Quantification of MMP-9, p-AKT and p-GSK-3β protein from three separate experiments, normalized to β-actin. (D) Gelatin zymography assay for the determination of MMP-9 activity. The intensities of gelatin-digested bands by MMP-9 were measured by densitometry and shown by the bar diagram. *P < 0.05, **P < 0.01 vs mock control cells.
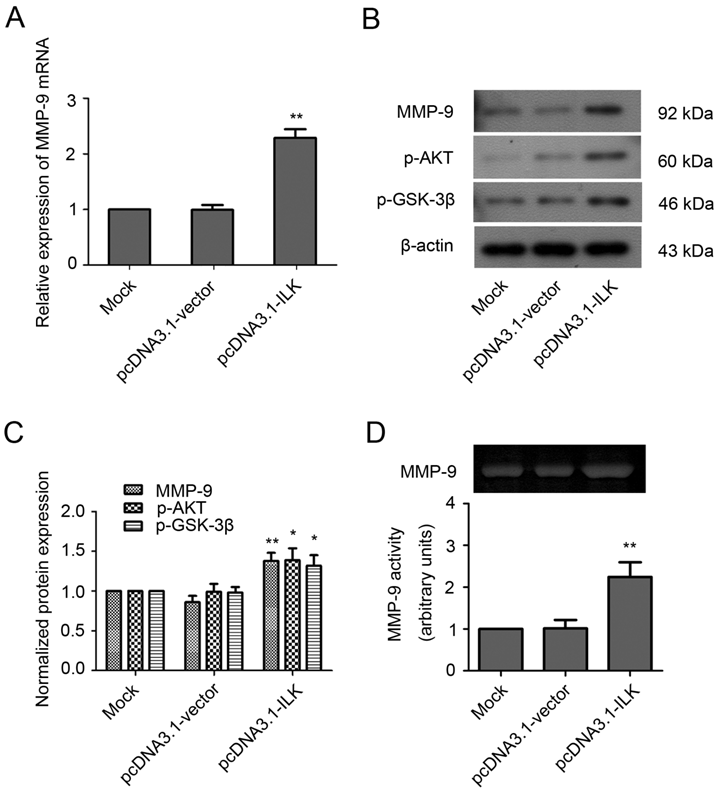
MMP-9 is required for ILK-induced lung cancer A549 cell migration and invasion in vitro. (A) Wound healing assay of the pcDNA3.1-ILK cells in the absence and presence of doxycycline, anti-MMP-9 neutralizing antibody and control (irrelevant) IgG. (B) Bars represent the migration index of each cell, expressed as a value relative to the distance moved by the cell monolayer. (C) Transwell migration assay was performed in the absence and presence of doxycycline, anti-MMP-9 neutralizing antibody and control (irrelevant) IgG. (D) Values are expressed as the mean ± SD of three independent experiments. Mock control and pcDNA3.1-vector cells served as control. *P < 0.05, **P < 0.01 vs pcDNA3.1-ILK cells.
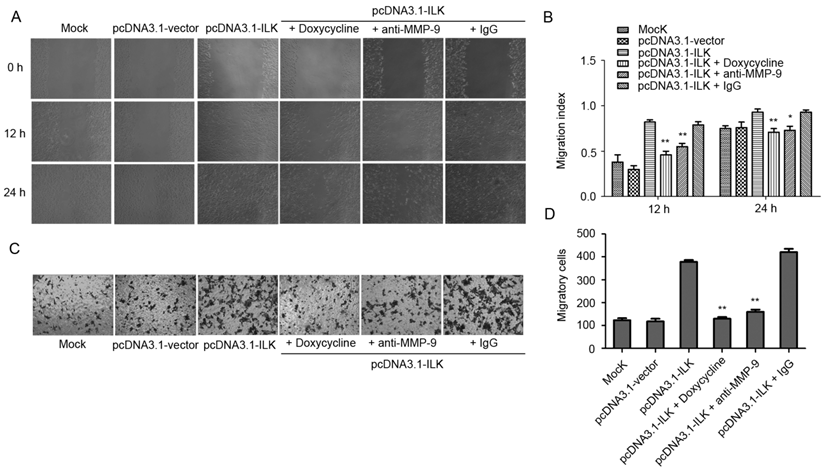
Activation of NF-κB in ILK overexpression cells. (A) Phosphorylation levels of NF-κB p65 and IκB protein in pcDNA3.1-ILK cells compared with pcDNA3.1-vector cells and mock control cells as determined by western blot analysis. (B) Densitometric quantification data are expressed as the intensity ratio of the target proteins to β-actin (mean ± SD). (C) ILK affects the subcellular localization of NF-κB p65. Nuclear and cytoplasmic extracts from mock control, pcDNA3.1-vector and pcDNA3.1-ILK cells were subjected to western blot analysis using anti-NF-κB p65 antibody. Histone H4 and lamp 2 were used as input for nuclear and cytoplasmic extracts, respectively. (D) Quantification of NF-κB p65 protein from three separate experiments, normalized to β-actin. *P < 0.05, **P < 0.01 vs mock control cells.
ILK induces activation of NF-κB signaling in lung cancer cells
ILK has been shown to regulate downstream genes through NF-κB signaling pathway in melanoma cells [32]. To test whether NF-κB signaling is activated by ILK in lung cancer cells, we examined the status of NF-κB in transfected A549 cells. As indicated in Fig. 3A and 3B, ILK overexpression resulted in enhanced phosphorylation of NF-κB p65 in pcDNA3.1-ILK cells compared with pcDNA3.1-vector cells and mock control cells. Moreover, a significant increase of IκB phosphorylation was also observed in pcDNA3.1-ILK cells, indicating the dissociation of IκB from NF-κB. To further explore whether NF-κB is activated, we determined the protein levels of NF-κB in the nuclear and cytoplasmic extracts prepared from pcDNA3.1-ILK, pcDNA3.1-vector and mock control cells, respectively. We found that the protein level of nuclear NF-κB was significantly increased in pcDNA3.1-ILK cells as compared with pcDNA3.1-vector cells and control cells (Fig. 3C and 3D, P < 0.05). Correspondingly, the cytoplasmic level of NF-κB p65 was decreased in pcDNA3.1-ILK cells (Fig. 3C and 3D, P < 0.05). These data demonstrated that overexpression of ILK induced phosphorylation and nuclear translocation of NF-κB p65, suggesting a potential role of NF-κB signaling in ILK overexpression cells.
ILK stimulates MMP-9 expression via NF-κB signaling pathway
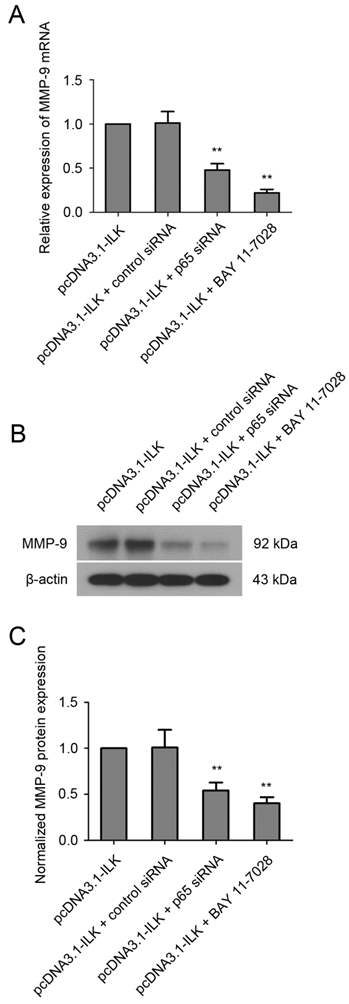
To find out whether ILK stimulates the expression of MMP-9 via NF-κB signaling pathway, cells were treated with NF-κB inhibitor BAY 11-7028 (10 μM) for 48 h before analysis. As shown in Fig.4A, the mRNA level of MMP-9 was significantly reduced in pcDNA3.1-ILK cells treated with BAY 11-7028. Additionally, such treatment resulted in remarkable reduction of MMP-9 protein expression in pcDNA3.1-ILK cells (Fig. 4B and 4C). To further investigate the role of NF-κB in ILK-induced upregulation of MMP-9, we decided to knock down NF-κB expression in pcDNA3.1-ILK cells using siRNA transfection. The expression of NF-κB protein was appreciably inhibited in pcDNA3.1-ILK cells transfected with siRNA targeted to NF-κB p65 (Supplementary Material: Fig. S2). We found that ILK-induced upregulation of MMP-9 was significantly attenuated by p65 siRNA at both mRNA and protein levels in pcDNA3.1-ILK cells (Fig 4). Taken together, these results suggested that ILK stimulated MMP-9 expression may through activation of the NF-κB signaling pathway.
Involvement of NF-κB in ILK-induced upregulation of MMP-9. (A) Quantitative real-time PCR analysis showing the mRNA level of MMP-9 in pcDNA3.1-ILK cells treated with or without NF-kB p65 siRNA and BAY 11-7028. (B) NF-κB is required for ILK-induced upregulation of MMP-9 protein as determined by western blot analysis. (C) Densitometric quantification data are expressed as the intensity ratio of the target proteins to β-actin (mean ± SD). **P < 0.01 vs pcDNA3.1-ILK cells.
Discussion
Overexpression of ILK has been found in several types of human cancers including NSCLC [15-17]. In our previously published paper, we found that increased ILK expression correlates with the Tumor-Node-Metastasis stage and lymph node metastasis of NSCLC. In addition, forced overexpression of ILK in A549 human lung cancer cells promotes cell migration and invasion in vitro which is mediated through the induction of EMT process [33]. However, the molecular mechanisms by which ILK employed to induce migration and invasion are more complex than anticipated and still remain to be fully elucidated [18-19].
Recently, it has been reported that knockdown of ILK results in reduction of MMP-9 expression and inhibits migration and invasion in bladder cancer cells [26-27]. A study by Kang et al. demonstrated that ectopic expression of ILK induces MMP-9 expression and promotes cell migration in podocytes [28]. Hence, we first examined whether MMP-9 expression is altered when ILK is overexpressed in lung cancer cells. Our results showed that overexpression of ILK induced MMP-9 expression at both mRNA and protein levels, indicating that MMP-9 is also regulated by ILK in lung cancer cells.
In addition to EMT, degradation of the ECM by MMPs is another critical step in cancer cell invasion [21-22]. The observation that MMP-9 is upregulated by ILK suggests that MMP-9 may function in ILK-induced migration and invasion of lung cancer cells. Consequently, we investigated the role of MMP-9 in cell migration and invasion induced by ILK in A549 lung cancer cells in the current study, which has never been reported before. The effect of MMP-9 inhibition caused by either doxycycline or anti-MMP-9 neutralizing antibody on the migration and invasion of ILK overexpression cells was assessed. The results showed that the wound healing capacity was significantly impaired in pcDNA3.1-ILK cells treated with either doxycycline or anti-MMP-9 antibody. Moreover, similar results were also obtained from Transwell invasion assay. The data presented here provide direct evidence for a critical role of MMP-9 in ILK-induced migration and invasion of lung cancer cells. However, it should be noted that doxycycline is a broad-spectrum MMP inhibitor which has been reported to inhibit MMP-2 and MMP-9 predominantly [36-37]. Previous study showed that downregulation of ILK also reduces MMP-2 expression in bladder cancer cells [26]. Another report demonstrated that ILK induces MMP-2 expression and promotes migration and invasion of human kidney proximal tubular epithelial cells [38]. Consequently, we speculate that MMP-2 expression may be regulated by ILK in lung cancer cells. Further investigation is required to confirm whether MMP-2 contributes to ILK-induced migration and invasion of lung cancer cells.
The transcription factor NF-κB plays a vital role in promoting cancer cell migration and invasion [39]. Inhibition of NF-κB activity has been shown to reduce MMP-9 expression in multiple types of human cancer cells, including cervical [29], breast [31, 40] and lung cancer cells [30]. More importantly, ectopic expression of ILK results in activation of NF-κB signaling pathway in melanoma cells. Hence, we examined whether NF-κB is involved in ILK-induced upregulation of MMP-9 in lung cancer cells. Increased levels of phosphorylation and nuclear translocation of NF-κB p65 were found in ILK overexpression cells. Furthermore, inhibition of NF-κB, by either siRNA or a specific inhibitor, led to significant reduction of MMP-9 expression in ILK overexpression cells. These results suggest that ILK stimulates MMP-9 expression at least partly through activation of the NF-κB signaling pathway in lung cancer cells. Interestingly, we have demonstrated that NF-κB pathway is also involved in ILK-induced EMT in our previous study [33], suggesting multiple functions for this transcription factor [41]. Future research should address whether NF-κB directly binds to MMP-9 and controls the transcription of MMP-9 in lung cancer cells.
In summary, the results of the present study indicate that ILK promotes lung cancer cell migration and invasion at least partly through NF-κB-mediated upregulation of MMP-9. Our findings shed new light on how ILK enhances the invasive phenotype of lung cancer cells. On these grounds, we propose that targeting the ILK/NF-κB/MMP-9 pathway may be a potential therapeutic strategy for preventing lung cancer invasion and metastasis.
Supplementary Material
Fig.S1 - S2.
Acknowledgements
This study was supported by a grant from the Department of Science and Technology of Liaoning Province (Grant No.: 2012225016).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J. et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91-6 doi:10.1038/379091a0
2. Wu C, Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complexes. J Cell Biol. 2001;155:505-10 doi:10.1083/jcb.200108077. jcb.200108077 [pii]
3. Legate KR, Montanez E, Kudlacek O, Fassler R. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20-31 doi:nrm1789 [pii] 10.1038/nrm1789
4. Troussard AA, Mawji NM, Ong C, Mui A, St -Arnaud R, Dedhar S. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem. 2003;278:22374-8 doi:10.1074/jbc.M303083200 M303083200 [pii]
5. Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51-63 doi:nrc1524 [pii] 10.1038/nrc1524
6. Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211-6
7. Persad S, Attwell S, Gray V, Delcommenne M, Troussard A, Sanghera J. et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci U S A. 2000;97:3207-12 doi:10.1073/pnas.060579697 060579697 [pii]
8. Bravou V, Klironomos G, Papadaki E, Stefanou D, Varakis J. Integrin-linked kinase (ILK) expression in human colon cancer. Br J Cancer. 2003;89:2340-1 doi:10.1038/sj.bjc.6601482 6601482 [pii]
9. Li R, Liu B, Yin H, Sun W, Yin J, Su Q. Overexpression of integrin-linked kinase (ILK) is associated with tumor progression and an unfavorable prognosis in patients with colorectal cancer. J Mol Histol. 2012 doi:10.1007/s10735-012-9463-6
10. Ito R, Oue N, Zhu X, Yoshida K, Nakayama H, Yokozaki H. et al. Expression of integrin-linked kinase is closely correlated with invasion and metastasis of gastric carcinoma. Virchows Arch. 2003;442:118-23 doi:10.1007/s00428-002-0718-6
11. Graff JR, Deddens JA, Konicek BW, Colligan BM, Hurst BM, Carter HW. et al. Integrin-linked kinase expression increases with prostate tumor grade. Clin Cancer Res. 2001;7:1987-91
12. Ahmed N, Riley C, Oliva K, Stutt E, Rice GE, Quinn MA. Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol. 2003;201:229-37 doi:10.1002/path.1441
13. Dai DL, Makretsov N, Campos EI, Huang C, Zhou Y, Huntsman D. et al. Increased expression of integrin-linked kinase is correlated with melanoma progression and poor patient survival. Clin Cancer Res. 2003;9:4409-14
14. Watzka SB, Setinek U, Stubenberger EB, Totsch M, Dekan G, Marcher M. et al. Integrin-linked kinase, phosphorylated AKT and the prognosis of malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2011;39:180-4 doi:S1010-7940(10)00444-6 [pii] 10.1016/j.ejcts.2010.05.007
15. Takanami I. Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer. 2005;5:1. doi:1471-2407-5-1 [pii] 10.1186/1471-2407-5-1
16. Watzka SB, Rauscher-Potsch I, Stubenberger E, Getman V, Setinek U, Totsch M. et al. Immunoreactivity of integrin-linked kinase in primary non-small-cell lung cancer and survival after curative resection. Eur J Cardiothorac Surg. 2010;38:254-9 doi:S1010-7940(10)00136-3 [pii] 10.1016/j.ejcts.2010.02.006
17. Yu J, Shi R, Zhang D, Wang E, Qiu X. Expression of integrin-linked kinase in lung squamous cell carcinoma and adenocarcinoma: correlation with E-cadherin expression, tumor microvessel density and clinical outcome. Virchows Arch. 2011;458:99-107 doi:10.1007/s00428-010-1016-3
18. Oneyama C, Morii E, Okuzaki D, Takahashi Y, Ikeda J, Wakabayashi N. et al. MicroRNA-mediated upregulation of integrin-linked kinase promotes Src-induced tumor progression. Oncogene. 2012;31:1623-35 doi:onc2011367 [pii] 10.1038/onc.2011.367
19. Qian Y, Zhong X, Flynn DC, Zheng JZ, Qiao M, Wu C. et al. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene. 2005;24:3154-65 doi:1208525 [pii] 10.1038/sj.onc.1208525
20. Mi Z, Guo H, Wai PY, Gao C, Kuo PC. Integrin-linked kinase regulates osteopontin-dependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells. Carcinogenesis. 2006;27:1134-45 doi:bgi352 [pii] 10.1093/carcin/bgi352
21. Woessner JF Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145-54
22. Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781-92
23. Cakarovski K, Leung JY, Restall C, Carin-Carlson A, Yang E, Perlmutter P. et al. Novel inhibitors of urokinase-type plasminogen activator and matrix metalloproteinase expression in metastatic cancer cell lines. Int J Cancer. 2004;110:610-6 doi:10.1002/ijc.20135
24. Patel BP, Shah SV, Shukla SN, Shah PM, Patel PS. Clinical significance of MMP-2 and MMP-9 in patients with oral cancer. Head Neck. 2007;29:564-72 doi:10.1002/hed.20561
25. Passlick B, Sienel W, Seen-Hibler R, Wockel W, Thetter O, Mutschler W. et al. Overexpression of matrix metalloproteinase 2 predicts unfavorable outcome in early-stage non-small cell lung cancer. Clin Cancer Res. 2000;6:3944-8
26. Zhu J, Pan X, Zhang Z, Gao J, Zhang L, Chen J. Downregulation of integrin-linked kinase inhibits epithelial-to-mesenchymal transition and metastasis in bladder cancer cells. Cell Signal. 2012;24:1323-32
27. Matsui Y, Assi K, Ogawa O, Raven PA, Dedhar S, Gleave ME. et al. The importance of integrin-linked kinase in the regulation of bladder cancer invasion. Int J Cancer. 2012;130:521-31 doi:10.1002/ijc.26008
28. Kang YS, Li Y, Dai C, Kiss LP, Wu C, Liu Y. Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int. 2010;78:363-73 doi:ki2010137 [pii] 10.1038/ki.2010.137
29. Kim YS, Sull JW, Sung HJ. Suppressing effect of resveratrol on the migration and invasion of human metastatic lung and cervical cancer cells. Mol Biol Rep. 2012;39:8709-16 doi:10.1007/s11033-012-1728-3
30. Kao SJ, Su JL, Chen CK, Yu MC, Bai KJ, Chang JH. et al. Osthole inhibits the invasive ability of human lung adenocarcinoma cells via suppression of NF-kappaB-mediated matrix metalloproteinase-9 expression. Toxicol Appl Pharmacol. 2012;261:105-15 doi:S0041-008X(12)00123-8 [pii] 10.1016/j.taap.2012.03.020
31. Li Q, Zhang L, Han Y, Jiang Z, Wang Q. Propofol reduces MMPs expression by inhibiting NF-kappaB activity in human MDA-MB-231 cells. Biomed Pharmacother. 2012;66:52-6 doi:S0753-3322(11)00178-8 [pii] 10.1016/j.biopha.2011.10.006
32. Wani AA, Jafarnejad SM, Zhou J, Li G. Integrin-linked kinase regulates melanoma angiogenesis by activating NF-kappaB/interleukin-6 signaling pathway. Oncogene. 2011;30:2778-88 doi:onc2010644 [pii] 10.1038/onc.2010.644
33. Chen D, Zhang Y, Zhang X, Li J, Han B, Liu S. et al. Overexpression of integrin-linked kinase correlates with malignant phenotype in non-small cell lung cancer and promotes lung cancer cell invasion and migration via regulating epithelial-mesenchymal transition (EMT)-related genes. Acta Histochem. 2012. doi: S0065-1281(12)00076-1[pii]10.1016/j.acthis. 2012 05.004
34. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8 doi:10.1006/meth.2001.1262 S1046-2023(01)91262-9 [pii]
35. Guo J, Verma UN, Gaynor RB, Frenkel EP, Becerra CR. Enhanced chemosensitivity to irinotecan by RNA interference-mediated down-regulation of the nuclear factor-kappaB p65 subunit. Clin Cancer Res. 2004;10:3333-41 doi:10.1158/1078-0432.CCR-03-0366 10/10/3333 [pii]
36. Lokeshwar BL, Selzer MG, Zhu BQ, Block NL, Golub LM. Inhibition of cell proliferation, invasion, tumor growth and metastasis by an oral non-antimicrobial tetracycline analog (COL-3) in a metastatic prostate cancer model. Int J Cancer. 2002;98:297-309 doi:10.1002/ijc.10168 [pii]
37. Seftor RE, Seftor EA, De Larco JE, Kleiner DE, Leferson J, Stetler-Stevenson WG. et al. Chemically modified tetracyclines inhibit human melanoma cell invasion and metastasis. Clin Exp Metastasis. 1998;16:217-25
38. Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503-16 doi:10.1172/JCI17913 112/4/503 [pii]
39. Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241-6 doi:10.1172/JCI11991
40. Maity G, Choudhury PR, Sen T, Ganguly KK, Sil H, Chatterjee A. Culture of human breast cancer cell line (MDA-MB-231) on fibronectin-coated surface induces pro-matrix metalloproteinase-9 expression and activity. Tumour Biol. 2011;32:129-38 doi:10.1007/s13277-010-0106-9
41. Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11-22 doi:S1550-4131(10)00450-X [pii] 10.1016/j.cmet.2010.12.008
Author contact
![]() Corresponding author: Dr. Xiaoge Wang, Department of Respiratory Medicine, The Fourth Affiliated Hospital of China Medical University, 4 East Chongshan Road, Shenyang 110032, People's Republic of China. Tel: 86-24-62255001; Fax: 86-24-62571119; E-mail: cm4hwxgn2005com
Corresponding author: Dr. Xiaoge Wang, Department of Respiratory Medicine, The Fourth Affiliated Hospital of China Medical University, 4 East Chongshan Road, Shenyang 110032, People's Republic of China. Tel: 86-24-62255001; Fax: 86-24-62571119; E-mail: cm4hwxgn2005com

 Global reach, higher impact
Global reach, higher impact