3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(6):730-737. doi:10.7150/ijms.6104 This issue Cite
Research Paper
Status of Essential Trace Minerals and Oxidative Stress in Viral Hepatitis C Patients with Nonalcoholic Fatty Liver Disease
1. Micro-Nutrition & Biomedical Nutrition Labs, Institute of Biomedical Nutrition, Hung Kuang University, Taichung 433, Taiwan, Republic of China
2. Department of Internal Medicine, Kuang Tien General Hospital, Taichung 433, Taiwan, Republic of China
Received 2013-2-20; Accepted 2013-4-10; Published 2013-4-17
Abstract
Background: Nonalcoholic fatty liver disease (NAFLD) may be an important factor leading to altered trace mineral homeostasis, thereby accelerating the progression of hepatitis C virus (HCV) infection. Our aim was to determine whether NAFLD influenced the status of certain essential trace minerals and oxidative stress in chronic HCV-infected patients.
Design and Methods: Blood biochemical parameters were determined in a group of 30 healthy, non-obese, non-diabetic participants (CNL group), and hepatitis C patients without NAFLD (HCV group, n = 30) and with NAFLD (HCV-NAFLD group, n = 32).
Results: Concentrations of thiobarbituric acid reactive substances (TBARS; a measure of oxidative stress), C-reactive protein (CRP), ferritin, aminotransferases, lipid profiles, and insulin metabolism were markedly abnormal in both patient groups than in CNL subjects. Compared to patients in the HCV group, those with HCV-NAFLD group had lower high-density lipoprotein concentrations, higher low-density lipoprotein and homeostasis model assessment-insulin resistance (HOMA-IR) values, disrupted antioxidant enzyme activities, and elevated TBARS concentrations, as well as decreased plasma concentrations of trace minerals zinc (Zn) and selenium (Se) and increased copper (Cu). The alterations in mineral homeostasis were also linked to TBARS, CRP, ferritin, lipoproteins, and HOMA-IR values in the HCV-NAFLD group.
Conclusions: There is a progressive deterioration in the homeostasis of minerals (Zn, Se, and Cu) in HCV-NAFLD patients, which may reflect greater oxidative stress and inflammation. These results suggest that the disturbance in mineral metabolism by NAFLD has an impact on the effectiveness of treatment for chronic HCV infection.
Keywords: chronic hepatitis C, nonalcoholic fatty liver disease, essential trace minerals, oxidative stress, inflammation
Introduction
Hepatitis C virus (HCV) is a major cause of chronic liver diseases. HCV infection frequently does not resolve, leading to chronic hepatitis with increasing risk of hepatic fibrosis, steatosis, cirrhosis, and hepatocellular carcinoma [1]. Although the combination of pegylated interferon-a and ribavirin is the treatment for chronic HCV infection with proven efficacy [2], this therapeutic strategy still has a low sustained virologic response (SVR) in patients who have HCV genotype 1 and a high viral load [3]. Potential risk factors associated with lower rates of SVR in patients include high baseline HCV-RNA and aminotransferase levels, older age, obesity, insulin resistance (IR), and hepatic fibrosis [4,5]. Recent studies have also shown that alterations in the plasma concentrations of several trace minerals is a major factor associated with the pathogenesis of such complications [6-9].
Essential trace minerals such as zinc (Zn), selenium (Se), and copper (Cu) not only act as antioxidants but also play vital roles in multiple metabolic processes in the liver [10]. Disturbances in the homeostasis of these minerals can cause oxidative stress and inflammatory changes, thus enhancing HCV replication, exacerbating hepatic fibrosis and IR, and reducing the effectiveness of anti-viral therapy in chronic HCV-infected patients [6,7,9]. Liver diseases may alter the regulation of trace mineral metabolism and homeostasis. However, Zn supplementation prevents increases in transaminases, reduces liver fibrosis and triglyceride levels, and improves the long-term outcome of these patients [7,11,12]. Blood Se concentrations decrease in proportion to the severity of HCV-induced hepatic injury. Se supplementation may be effective therapy for reducing IR and hepatic fibrosis in patients with chronic HCV infection [9,13]. Therefore, the maintenance of essential metal homeostasis is a crucial therapeutic target for hepatitis C [6]. Disturbances in plasma concentrations of these minerals can accelerate deterioration of hepatitis C and prevent the achievement of SVR.
Recently, nonalcoholic fatty liver disease (NAFLD) has also been shown to be associated with decreased SVR in HCV-infected patients [14]. NAFLD is characterized by the presence of excessive fat, predominantly triglyceride accumulation, in hepatocytes. NAFLD has been observed in chronic HCV-infected patients, including those who are not obese [15]. NAFLD is one of the most common causes of abnormal aminotransferase concentrations [16]. Additionally, the presence of NAFLD correlates directly with serum and intra-hepatic titers of HCV-RNA and with the severity of IR severity [17,18]. The mechanisms through which the HCV causes the liver damage associated with NAFLD are not fully understood. Altered trace mineral status may participate in NAFLD, thereby accelerating the progression of hepatitis C. However, the association of these trace minerals with NAFLD has not been well established in patients with chronic hepatitis C.
In the present preliminary study, the plasma concentrations of trace minerals (Zn, Cu, and Se), oxidative stress, inflammatory status, and related metabolic variables were compared between non-obese, non-diabetic patients with hepatitis C and non-obese, non-diabetic patients with hepatitis C and NAFLD. The relationship between trace mineral concentrations and several blood variables were also examined in hepatitis C patients with NAFLD.
Patients and Methods
Subjects
Between January 2010 and May 2011, a total of sixty-seven patients at the hepatology unit of Kuang Tien General Hospital (Taichung, Taiwan) were enrolled. Participants were excluded if they had a history of alcohol consumption, cigarette smoking, known coronary heart disease, diabetes, cancer, or if they tested positive for hepatitis B virus, had a body mass index (BMI) higher than 27, and received supplementation with antioxidants, and vitamins/minerals.
The HCV-infected patients included in this study demonstrated high viral loads of serum HCV-RNA of genotype 1b. They had been diagnosed with chronic hepatitis C on the basis of abnormal serum alanine aminotransferase (ALT) for at least six months and tested with positive for HCV-RNA as assessed by polymerase chain reaction analysis. Furthermore, the study population included hepatitis C patients with and without non-alcoholic fatty liver disease (NAFLD; n =34 and n=35, respectively). Healthy subjects of similar age and gender from the health evaluation center volunteered as controls (CNL; n = 30). All subjects underwent a routine clinical examination, including physical examination, biochemical tests, and liver ultrasonography. The study protocol was approved by the Ethics in Human Research Committee of Kuang Tien General Hospital.
Ultrasonographic examination
Though fatty liver can be assessed by invasive liver biopsy, this method remains inconveniences and some complications [19]. In the present study, liver ultrasonography was examined in all participants by the same ultrasound operator. Fatty liver is defined as liver parenchyma with echogenicity higher than the right kidney cortex on two different probe positions. Further, the degree of fatty infiltration is graded from 0 to 3. Grade 1 (mild) indicates minimal diffuse increase in hepatic echogenity with normal visualization of the intrahepatic vessels and diaphragm. Grade 2 (medium) indicates medium grade diffuse increase in hepatic echogenity and vascular blurring. Grade 3 (severe) produces apparent increase in echogenity [20].
Biochemical analyses
In all subjects, between 08:00 and 09:30, peripheral venous blood was collected in BD- Vacutainer tubes (sodium heparin) after an overnight fast of 12 h. The plasma lipid profile (i.e., cholesterol, total triglyceride, high density lipoprotein [HDL], and low density lipoprotein [LDL]), and markers of hepatic function including ALT, aspartate amino- transferase (AST), and g-glutamyl transpeptidase (GGT) were determined with a Hitachi 7050 automatic analyzer (Hitachi Corp., Tokyo, Japan) using commercially available assay kits. Body mass index (BMI), waist-to-hip ratio (WHR), insulin resistance expressed as homeostasis model assessment-insulin resistance (HOMA-IR) index, and several blood tests were assessed.
In addition, quantitative detection of serum HCV-RNA was performed by Amplicor-HCV monitor assay (Roche Molecular Diagnostics) and expressed as the log of copies of RNA per milliliter. RNA was extracted from serum samples following the manufacturer's instructions (QIAamp viral RNA kit from Qiagen Inc.). This assay had a lower limit of 100 copies/mL.
Determination of essential minerals
The concentrations of plasma Zn and Cu were measured with a flame atomic absorption spectrophotometer (932 plus, GBC, Australia) using an air-acetylene flame without background correction at 213.9 and 324.71 nm, respectively. Triplicate absorbance readings were taken for each sample in the peak-height mode. Samples were digested in a mixture of H2O2 and HNO3 in a start D microwave-assisted digestion system (Milestone Microwave Labstation, ETHOSD, USA) and subsequently brought up the volume with double-deionized water.
In addition, the accessory hydride generation system (HG 3000), from GBC, was used for determining erythrocyte Se concentrations. Hollow cathode lamps were employed at the 196.0 nm wavelength and 1.0 nm band pass. The temperature programmed for the atomization was based on recommendation of the manufacturer. Samples were digested for a total of 10.5 h with initial temperature of 60°C for 1-1.5 h, followed by increasing temperatures on 20°C increments and finally heated up to 225°C for 2 h in a mixture of 3.2 mL nitric acid (16N), and 0.8 mL concentrated perchloric acid to convert all Se species to selenate. The reduction of selenate was completed within 30 min at a block temperature of 120°C. Accuracy of the method was confirmed by comparing to serum reference materials (level 2, NO0371, Seronorm, Nycomed, Oslo, Norway).
Measurement of oxidative stress
Lipid peroxidation was determined by assaying the formation of thiobarbituric acid reactive substances (TBARS). Plasma samples were mixed with 3% sodium dodecyl sulfate, 0.1 N HCl, 10% phosphortungstic acid, and 0.7% thiobarbituric acid and then incubated at 95°C for 60 min. The TBARS was extracted into n-butanol and the fluorescence of the n-butanol layer was measured using a spectrofluorometer (Perkin-Elmer, Turku, Finland) with excitation at 485 nm and emission at 530 nm [21].
Determination of antioxidant enzymes
The erythrocyte pellet was washed with cold isotonic saline and then diluted with saline. The activity of superoxide dismutase (SOD) was determined using a commercial RANSOD kit (Randox, San Diego); one unit was defined as the amount of enzymes necessary to produce 50% inhibition in the rate of p-iodonitrotetrazolium reduction. The activity of SOD was expressed in unit per milligram of protein (U/mg prot). In addition, erythrocyte glutathione peroxidase (GPx) activity was measured with GPx assay kits (Cayman Chemical, Ann Arbor, Michigan, USA). Oxidized glutathione, produced upon reduction of an organic hydroperoxide by GPx, was recycled to its reduced state by glutathione reductase and NADPH. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm; it was directly proportional to the GPx activity. Protein content was determined using the Coomassie protein assay (Pierce, Rockford, IL, USA) with bovine serum albumin as the standard.
Measurement of inflammatory parameters
Plasma concentrations of C-reactive protein (CRP) were measured using the human CRP ELISA kit (E-80CRP, Immunology Consultants Laboratory, Inc, Newberg, OR, USA). The intra- and inter-assay CVs (coefficient of variation) were < 3% and < 5%, respectively. The absorbance at 450 nm was measured, and readings were interpolated into the standard curve. Ferritin concentrations were also measured using human ferritin enzyme immunoassay test kit (IBL Immunobiological Laboratories, Hamburg, Germany) according to the manufacturer's instructions.
Statistical analysis
Each value was expressed as the mean ± SD or medians (inter-quartile range, IQR), depending on the normality of data distribution (Shapiro-Wilk test). The differences among three groups were then analyzed by one-way analysis of variance (ANOVA) or Kruskal- Wallis ANOVA, when results were non-parametrically distribution. The significance of the selected variables was also determined using χ2 analysis and Student's t-test. A two-tailed p value less than 0.05 was considered statistically significant. Pearson's correlation or Spearman's correlation coefficients depend on the normality of residuals were performed to identify relationships of the blood variables.
Results
Clinical characteristics
A total of 35 HCV group patients were initially included. However, 5 patients were excluded due to incomplete baseline information (n = 3) and insufficient sample (n = 2). All data were analysis for the remaining 30 patients. Two participants in the HCV-NAFLD group were also excluded from the analysis due to severe cold symptoms and diarrhea.
There were no significant differences in age, sex, and BMI among the three groups (p > 0.05) (Table 1). Compared with control subjects (CNL), participants in the HCV and HCV- NAFLD groups had abnormally high concentrations of AST and ALT, both blood markers of hepatic function. The patients in the HCV-NAFLD group have showed significantly higher plasma GGT activities, compared to the CNL or HCV group.
Patients in the HCV-NAFLD group had higher plasma concentrations of total cholesterol and LDL compared to the CNL and HCV groups. Plasma concentrations of HDL were lowest in the HCV-NAFLD group. HCV-NAFLD patients showed higher plasma triglyceride concentration than the HCV group. In addition, patients in HCV-NAFLD group have higher systolic blood pressure, compared to the CNL and HCV groups, such that the levels of systolic pressure were as followed: HCV-NAFLD > HCV > CNL.
Fasting plasma concentrations of glucose and insulin, and HOMA-IR values were also all markedly higher in the HCV and HCV-NAFLD groups than in the CNL group. High HOMA-IR values indicate low insulin sensitivity; HOMA-IR was highest for those in the HCV-NAFLD group.
Clinical and biochemical characteristics of the study population 1-3
| HCV (n = 30) | HCV-NAFLD (n = 32) | CNL (n = 30) | |
|---|---|---|---|
| Gender (M : F) | 17/13 | 17/15 | 16/14 |
| Age (year) | 47 ± 12 | 49 ± 13 | 46 ± 11 |
| Height (cm) | 165 ± 8 | 163 ± 7 | 162 ± 9 |
| Weight (kg) | 65 ± 10 | 63 ± 8 | 61 ± 9 |
| BMI (kg/m2) | 24 ± 2 | 24 ± 2 | 23 ± 2 |
| WHR | 0.81 (0.79-0.88) | 0.85 (0.80-0.86) | 0.82 (0.77-0.85) |
| Systolic P. (mmHg) | 120 (116-130) b | 134 (120-147) c | 110 (103-112) a |
| Diastolic P. (mmHg) | 81 ± 7 b | 85 ± 10 b | 70 ± 7 a |
| AST (U/L) | 38 (28-67) b | 57 (38-93) b | 22 (13-30) a |
| ALT (U/L) | 47 (29-89) b | 83 (63-116) b | 16 (9-22) a |
| GGT (U/L) | 37 (22-69) b | 73 (57-117) c | 9 (8-19) a |
| Sugar AC (mmol/L) | 5.2 ± 0.6 b | 5.9 ± 0.6 c | 4.7 ± 0.4 a |
| Insulin (mU/L) | 22.8 ± 4.8 b | 29.0 ± 4.7 c | 11.3 ± 3.0 a |
| HOMA-IR index | 5.0 (4.4-6.1) b | 7.8 (6.6-8.4) c | 2.4 (1.7-2.9) a |
| Total cholesterol (mg/dL) | 177 (164-199) b | 198 (186-229) c | 136 (105-182) a |
| Triglycerides (mg/dL) | 75 (67-91) a | 114 (84-131) b | 90 (67-122) ab |
| LDL (mg/dL) | 106 (94-124) b | 133 (116-154) c | 72 (50-92) a |
| HDL (mg/dL) | 41 (37-44) b | 36 (33-40) a | 54 (44-59) c |
| CRP (mg/dL) | 1.5 (0.9-2.1) b | 2.8 (2.1-3.9) c | 0.3 (0.1-0.7) a |
| Ferritin (ng/mL) | 162 (134-208) b | 360 (293-491) c | 39 (28-55) a |
| HCV-RNA (log) | 5.86 ± 0.83 | 6.00 ± 1.36 | - |
| Fatty liver | |||
| Grade 0 | 30 | - | 30 |
| Grade 1 | - | 15 | - |
| Grade 2-3 | - | 17 | - |
1 Values are mean ± SD or medians (IQR). 2 HCV = hepatitis C patients; HCV-NAFLD = hepatitis C patients with non-alcoholic fatty liver disease; CNL = healthy controls. 3 Values in the same row with different superscripts are significantly different (p < 0.05). 4 BMI = body mass index; WHR = waist-to-hip ratio; AST = aspartate transaminase; ALT = alanine transaminase; GGT = γ-Glutamyl transferase; Sugar AC = fasting glucose; HOMA-IR = homeostasis model assessment-insulin resistance; LDL = low-density lipoprotein; HDL = high-density lipoprotein; CRP = C-reactive protein. 5 Ultrasonographic fatty liver (Grade 1 = mild fatty liver; Grade 2 = moderate fatty liver; Grade 3 = severe fatty liver).
Plasma CRP and ferritin were significantly higher in both HCV and HCV-NAFLD groups than in the CNL group. Furthermore, the concentrations of CRP and ferritin were highest in the HCV-NAFLD group.
Concentrations of essential trace minerals
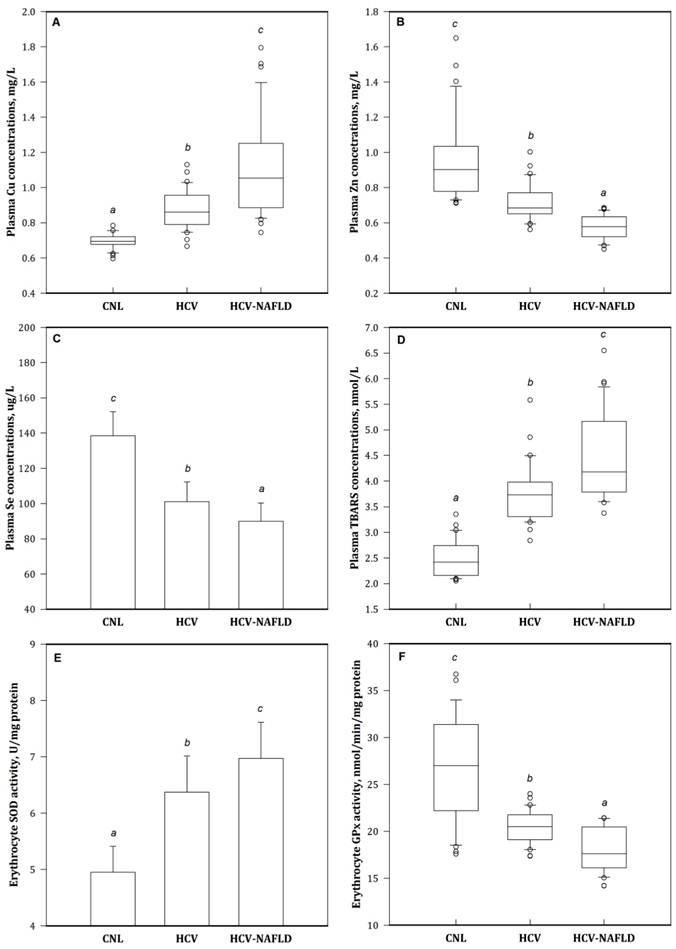
Compared to the CNL group, the concentrations of Cu, Zn, and Se were abnormal in the HCV and HCV-NAFLD patient groups. Plasma concentrations of Zn and Se were the lowest and plasma concentrations of Cu were the highest in the HCV-NAFLD group (Fig. 1).
Oxidative-antioxidant status
Patient in the HCV and HCV-NAFLD groups had considerably higher plasma concentrations of TBARS (an index of oxidative stress), lower antioxidant enzyme GPx activity, and higher SOD activity in erythrocytes than the CNL group (Fig. 1). The highest concentration of TBARS was observed in the HCV-NAFLD group.
Correlations among measured blood variables in HCV-NAFLD patients
Patients in the HCV-NAFLD group plasma concentrations of CRP, ferritin, and TBARS were correlated with abnormal concentrations of trace minerals (Table 2). In addition, there were associations between plasma trace minerals with HDL, LDL concentrations, and HOMA-IR values in these individuals.
Correlations between plasma trace mineral status and biochemical variables in the HCV-NAFLD group1, 2
| Cu | Zn | Se | |||
|---|---|---|---|---|---|
| r | |||||
| TBARS | 0.41 | - | -0.40 | ||
| CRP | 0.42 | -0.42 | -0.46 | ||
| Ferritin | - | -0.60 | -0.43 | ||
| HOMA-IR | - | -0.41 | -0.48 | ||
| HDL | -0.45 | 0.44 | - | ||
| LDL | 0.45 | - | -0.41 | ||
1 HCV-NAFLD = hepatitis C patients with non-alcoholic fatty liver disease. 2 r = Pearson's or Spearman's correlation coefficients; p < 0.05. 3 Cu = copper; Zn = zinc; Se = selenium; TBARS = thiobarbituric acid reactive substances; CRP = C-reactive protein; HOMA-IR = homeostasis model assessment-insulin resistance; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Discussion
The present preliminary investigation demonstrated that patients in the HCV and HCV-NAFLD groups had altered blood lipid profiles, higher IR, elevated liver enzyme activities, imbalances in antioxidant defense, and increased inflammatory responses compared to controls. Disturbances in the concentrations of essential trace minerals (Zn, Cu, and Se) compared to control subjects were also found in these patients, particularly in those with non-alcoholic fatty liver disease.
The HCV patients had low circulating Zn and Se concentrations and high Cu concentrations compared to controls. These results are consistent with those of previous studies [6,9,13,22,23]. Patients in the HCV-NAFLD group had lower plasma concentrations of both Zn and Se and higher plasma concentrations of Cu than did patients in the HCV group. The development of NAFLD may thus accelerate the disrupted homeostasis of these trace minerals in patients with chronic HCV infection.
Zn administration induces antioxidant and anti-inflammatory effects that can result in reduced hepatocyte injury [24]. Zn is a structural component of peroxisome proliferator- activated receptors, and Zn deficiency can thus interfere with lipid metabolism [25]. NAFLD patients have been shown to have a lower dietary intake of Zn, suggesting that inadequate Zn intake is associated with the development and progression of NAFLD [26]. Excessive lipid accumulation in the liver is associated with the formation of ROS and consequently with increased expression of Zn-finger protein ZNF267 mRNA [27]. Up-regulation of Zn- importing proteins by pro-inflammatory cytokines and oxidative stress reduces plasma Zn concentrations [28]. Dietary intake data were not available in this preliminary study, which we consider to be a limitation.
Se is another potent antioxidant that acts as an anti-inflammatory agent. Increased inflammation decreases the absorption of Se, resulting in low plasma Se concentrations [29]. GPx activity, which is regulated by Se, has been reported to be low in NAFLD patients [30]. Suboptimal Se intake is identified as a risk factor in the pathogenesis of NAFLD [31]. Se supplementation has been observed to significantly inhibit adipocyte hypertrophy and abdominal fat accumulation and to decrease fatty liver formation [32]. Furthermore, Cu exposure can enhance lipid synthesis, increasing hepatic lipid deposition [33]. In contrast, reduced hepatic Cu concentrations are found in NAFLD patients and are associated with hepatic steatosis [34]. The release of Cu during tissue damage mediated by inflammatory responses may account for increased circulating Cu concentrations [35]. In HCV-NAFLD patients, we observed that imbalances in essential trace minerals (Zn, Se, and Cu) were associated with increased oxidative stress and inflammatory markers. Interference with the homeostasis of these minerals homeostasis by NAFLD may thus contribute to the progression of hepatitis C.
Plasma concentrations of essential metals (A)Cu, (B)Zn, (C)Se, lipid peroxidation byproduct (D)TBARS, and erythrocyte antioxidant enzymes (E)SOD and (F)GPx activities in HCV-NAFLD patients. Bars are mean (SD) or median (IQR). Values above the box plots are outliers. Bars with different superscripts are significantly different (p < 0.05). HCV-NAFLD = hepatitis C patients with non-alcoholic fatty liver disease. Cu= copper; Zn = zinc; Se = selenium; TBARS = thiobarbituric acid reactive substances; SOD = superoxide dismutase; GPx = glutathione peroxide.
We observed that HCV-infected patients without NAFLD had higher plasma cholesterol and LDL concentrations than did healthy control patients. Furthermore, patients with both NAFLD and HCV had higher concentrations of plasma cholesterol and LDL as well as lower HDL than did patients with HCV alone. This data supports the hypothesis that the combined effects of NAFLD and HCV infection interfere with lipid metabolism. Several studies have found that blood lipid profiles may reflect the HCV-RNA load and predict the efficacy of hepatitis C antiviral therapy [36]. Low HDL levels have been shown to be associated with reduced plasma Zn and increased Cu levels in hypercholesterolemic patients [37]. Additionally, Se has been demonstrated to affect the maintenance of lipid and glucose metabolism and to decrease plasma LDL and cholesterol concentrations [38,39].
Altered HDL and HOMA-IR status are implicated in oxidative stress and were shown to be predictors of a poor response to pegylated interferon-a/ribavirin in chronic HCV-infected patients [40]. HCV infection may induce IR through a pro-inflammatory cytokine-mediated pathway and oxidative stress [41]. Relative to the HCV group, patients in the HCV-NAFLD group had markedly elevated HOMA-IR values. IR is frequently accompanied by lipid accumulation; individuals with NAFLD have an increased risk for the development of IR [42]. The presence of NAFLD in the subjects with IR implies further oxidative stress and inflammation [43]. The plasma concentrations of Zn (r = -0.38, p = 0.04) and Se (r = -0.54, p = 0.00) inversely correlated with HOMA-IR values in HCV patients, which agrees with the results of other studies [8,9]. In the present study, plasma mineral concentrations also correlated with HOMA-IR and HDL values in the HCV-NAFLD patient group.
Recent evidence shows that patients with chronic hepatitis C have significantly higher oxidative stress, which can lead to chronic inflammation [44]. HCV-NAFLD patients have been observed to have markedly elevated concentrations of TBARS and inflammatory markers such as CRP and ferritin compared to HCV patients. Hepatic accumulation of fat may involve the development of redox imbalance and lipid peroxidation, enhancing the release of pro-inflammatory cytokines and the production of ROS [45]. Increased CRP has been associated with metabolic syndrome, NAFLD, cardiovascular diseases, and SVR [46,47]. Elevated ferritin concentrations have a significant positive correlation with tumor necrosis factor-a and TBARS concentrations in NAFLD patients [48]. In the present study, the TBARS values correlated negatively with plasma concentrations of Zn (r = -0.50, p = 0.01) and Se (r = -0.60, p = 0.00) in HCV patients. In these patients, plasma mineral concentrations were also negatively associated with ferritin concentrations (data not shown). Moreover, concentrations of essential metals correlated with oxidative stress and inflammation in HCV-NAFLD patients. NAFLD in HCV patients is associated with oxidative damage, likely due to imbalances in trace metals.
In conclusion, the concomitant presence of HCV infection and NAFLD results in more severe perturbations in the homeostasis of essential minerals (Zn, Se, and Cu) status, amplifying oxidative stress and inflammation. Such conditions may cause a low SVR to anti-HCV therapy. While the mechanism underlying the association between plasma minerals and NAFLD in patients with chronic hepatitis C is unknown, the development of NAFLD is a potential contributor to the disruption of homeostasis of these minerals. Improving the status of these minerals may thus alleviate the progression of NAFLD and increase SVR rates in patients with chronic hepatitis C.
Acknowledgements
Research supported in part by a grant from Kuang-Tien General Hospital, Taichung, Taiwan.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889-900
2. Corouge M, Pol S. New treatments for chronic hepatitis C virus infection. Med Mal Infect. 2011;41:579-87
3. Ishikawa T. Can zinc enhance response interferon therapy for patients with HCV-related liver disease? World J Gastroenterol. 2012;18:3196-200
4. Huang CF, Yang JF, Dai CY, Huang JF, Hou NJ, Hsieh MY. et al. Efficacy and safety of pegylated interferon combined with ribavirin for the treatment of older patients with chronic hepatitis C. J Infect Dis. 2010;201:751-9
5. Petta S, Amato M, Cabibi D, Cammà C, Di Marco V, Giordano C. et al. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology. 2010;52:1543-52
6. Ko WS, Guo CH, Yeh MS, Lin LY, Hsu GS, Chen PC. et al. Blood micronutrient, oxidative stress, and viral load in patients with chronic hepatitis C. World J Gastroenterol. 2005;11:4697-702
7. Murakami Y, Koyabu T, Kawashima A, Kakibuchi N, Kawakami T, Takaguchi K, Kita K, Okita M. Zinc supplementation prevents the increase of transaminase in chronic hepatitis C patients during combination therapy with pegylated interferon alpha-2b and ribavirin. J Nutr Sci Vitaminol (Tokyo). 2007;53:213-8
8. Himoto T, Yoneyama H, Deguch A, Kurokochi K, Inukai M, Masugata H. et al. Insulin resistance derived from zinc deficiency in non-diabetic patients with chronic hepatitis C. Exp Ther Med. 2010;1:707-11
9. Himoto T, Yoneyama H, Kurokohchi K, Inukai M, Masugata H, Goda F. et al. Selenium deficiency is associated with insulin resistance in patients with hepatitis C virus-related chronic liver disease. Nutr Res. 2011;31:829-35
10. Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4alpha and peroxisome proliferator-activated receptor-alpha. Hepatology. 2009;50:1241-50
11. Takahashi M, Saito H, Higashimoto M, Hibi T. Possible inhibitory effect of oral zinc supplementation on hepatic fibrosis through downregulation of TIMP-1: A pilot study. Hepatol Res. 2007;37:405-9
12. Matsuoka S, Matsumura H, Nakamura H, Oshiro S, Arakawa Y, Hayashi J. et al. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J Clin Biochem Nutr. 2009;45:292-303
13. Khan MS, Dilawar S, Ali I, Rauf N. The possible role of selenium concentration in hepatitis B and C patients. Saudi J Gastroenterol. 2012;18:106-10
14. Soresi M, Tripi S, Franco V, Giannitrapani L, Alessandri A, Rappa F, Vuturo O, Montalto G. Impact of liver steatosis on the antiviral response in the hepatitis C virus-associated chronic hepatitis. Liver Int. 2006;26:1119-25
15. Kim HJ, Kim HJ, Lee KE, Kim DJ, Kim SK, Ahn CW, Lim SK, Kim KR, Lee HC, Huh KB, Cha BS. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169-75
16. Ji BL, Li R, Zhang SH, Gong LL, Wang ZH, Ren W, Li QF. The lipid accumulation product is highly related to serum alanine aminotransferase level in male adults. Nutr Res. 2012;32:581-7
17. Ong JP, Younossi ZM, Speer C, Olano A, Gramlich T, Boparai N. Chronic hepatitis C and superimposed nonalcoholic fatty liver disease. Liver. 2001;21:266-71
18. Younossi ZM, McCullough AJ, Ong JP, Barnes DS, Post A, Tavill A. et al. Obesity and non-alcoholic fatty liver disease in chronic hepatitis C. J Clin Gastroenterol. 2004;38:705-9
19. Estep JM, Birerdinc A, Younossi Z. Non-invasive diagnostic tests for non-alcoholic fatty liver disease. Curr Mol Med. 2010;10:166-72
20. Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49:450-65
21. Guo CH, Chen PC, Lin KP, Shih MY, Ko WS. Trace metal imbalance associated with oxidative stress and inflammatory status in anti-hepatitis C virus antibody positive subjects. Environ Toxicol Pharmacol. 2012;33:288-96
22. Matsumura H, Nirei K, Nakamura H, Arakawa Y, Higuchi T, Hayashi J. et al. Zinc supplementation therapy improves the outcome of patients with chronic hepatitis C. J Clin Biochem Nutr. 2012;51:178-84
23. Kolachi NF, Kazi TG, Afridi HI, Kazi N, Kandhro GA, Shah AQ. et al. Distribution of copper, iron, and zinc in biological samples (scalp hair, serum, blood, and urine) of Pakistani viral hepatitis (A-E) patients and controls. Biol Trace Elem Res. 2011;143:116-30
24. Himoto T, Hosomi N, Nakai S, Deguchi A, Kinekawa F, Matsuki M. et al. Efficacy of zinc administration in patients with hepatitis C virus-related chronic liver disease. Scand J Gastroenterol. 2007;42:1078-87
25. Shen H, MacDonald R, Bruemmer D, Stromberg A, Daugherty A, Li XA, Toborek M, Hennig B. Zinc deficiency alters lipid metabolism in LDL receptor deficient mice treated with rosiglitazone. J Nutr. 2007;137:2339-45
26. Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y. et al. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46-52
27. Schnabl B, Czech B, Valletta D, Weiss TS, Kirovski G, Hellerbrand C. Increased expression of Zinc finger protein 267 in non-alcoholic fatty liver disease. Int J Clin Exp Pathol. 2011;4:661-6
28. Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA. 2005;102:6843-8
29. Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, Guralnik J, Fried LP. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18-26
30. Das SK, Balakrishnan V, Mukherjee S, Vasudevan DM. Evaluation of blood oxidative stress-related parameters in alcoholic liver disease and nonalcoholic fatty liver disease. Scand J Clin Lab Invest. 2008;68:323-34
31. Clarke C, Baghdadi H, Howie AF, Mason JI, Walker SW, Beckett GJ. Selenium supplementation attenuates procollagen-1 and interleukin-8 production in fat-loaded human C3A hepatoblastoma cells treated with TGFbeta1. Biochim Biophys Acta. 2010;1800:611-8
32. Kim JE, Choi SI, Lee HR, Hwang IS, Lee YJ, An BS. et al. Selenium significantly inhibits adipocyte hypertrophy and abdominal fat accumulation in OLETF rats via induction of fatty acid β-oxidation. Biol Trace Elem Res. 2012;150:360-70
33. Chen QL, Luo Z, Liu X, Song YF, Liu CX, Zheng JL, Zhao YH. Effects of waterborne chronic copper exposure on hepatic lipid metabolism and metal-element composition in Synechogobius hasta. Arch Environ Contam Toxicol. 2012 [Epub ahead of print]
34. Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A. et al. A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010;105:1978-85
35. Guo CH, Wang CL, Chen PC, Yang TC. Linkage of some trace elements, peripheral blood lymphocytes, inflammation, and oxidative stress in ESRD patients undergoing either hemodialysis or peritoneal dialysis. Periton Dialysis Int. 2011;31:583-91
36. Liu S, Shi W, Li G, Jin B, Chen Y, Hu H, Liu L, Xie F, Chen K, Yin D. Plasma reactive carbonyl species levels and risk of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26:1010-5
37. Thuillier-Juteau Y, Jaudon MC, Clavel JP, Delattre J, Galli A. Serum zinc and copper in hypercholesterolemia. Pathol Biol (Paris). 1987;35:387-90
38. Dhingra S, Bansal MP. Hypercholesterolemia and LDL receptor mRNA expression: modulation by selenium supplementation. Biometals. 2006;19:493-501
39. Seale LA, Hashimoto AC, Kurokawa S, Gilman CL, Seyedali A, Bellinger FP. et al. Disruption of the selenocysteine lyase-mediated selenium recycling pathway leads to metabolic syndrome in mice. Mol Cell Biol. 2012;32:4141-54
40. Harrison SA, Rossaro L, Hu KQ, Patel K, Tillmann H, Dhaliwal S. et al. Serum cholesterol and statin use predict virological response to peginterferon and ribavirin therapy. Hepatology. 2010;52:864-74
41. Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: Direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-8
42. Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009;50(Suppl):S74-9
43. Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog Lipid Res. 2009;48:1-26
44. El-Kannishy G, Arafa M, Abdelaal I, Elarman M, El-Mahdy R. Persistent oxidative stress in patients with chronic active hepatitis-C infection after antiviral therapy failure. Saudi J Gastroenterol. 2012;18:375-9
45. Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of non- alcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59-69
46. Riquelme A, Arrese M, Soza A, Morales A, Baudrand R, Pérez-Ayuso RM. et al. Non- alcoholic fatty liver disease and its association with obesity, insulin resistance and increased serum levels of C-reactive protein in Hispanics. Liver Int. 2009;29:82-8
47. Huang CF, Hsieh MY, Yang JF, Chen WC, Yeh ML, Huang CI. et al. Serum hs-CRP was correlated with treatment response to pegylated interferon and ribavirin combination therapy in chronic hepatitis C patients. Hepatol Int. 2010;4:621-7
48. Helaly MAH, Hatata ESZ, Abdel-Khalek EES, Ala IAA. Elevated levels of serum ferritin may be related to non-alcoholic steatohepatitis. Eur J Basic Med Sci. 2011;1:13-20
Author contact
![]() Corresponding author: E-mail: ker200448com.tw (W-S. Ko). Tel: 886-4-26625111 ex 2829, 2191. Fax: 886-4-2633-8212
Corresponding author: E-mail: ker200448com.tw (W-S. Ko). Tel: 886-4-26625111 ex 2829, 2191. Fax: 886-4-2633-8212

 Global reach, higher impact
Global reach, higher impact