3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(6):683-690. doi:10.7150/ijms.5947 This issue Cite
Research Paper
Glutathione S-Transferase P1 Correlated with Oxidative Stress in Hepatocellular Carcinoma
1. Department of Hepatology, Qilu Hospital, Shandong University, Jinan 250012, China;
2. Department of infectious diseases, The third hospital of Zaozhuang city, Zaozhuang 277100, China;
3. Institute of Hepatology, Shandong University, Jinan 250012, China.
Received 2013-1-24; Accepted 2013-3-17; Published 2013-4-3
Abstract
Background: Glutathione-S-transferase P1 (GSTP1) is an important phase II enzyme that can protect cells from oxidative stress in various human cancers. However, few clinical studies were undertaken on the relationship between GSTP1 and oxidative stress in hepatocellular carcinoma (HCC). The present study was therefore aimed to evaluate the potential associations between GSTP1 and oxidative stress in HCC patients.
Methods: The GSTP1 expression in peripheral blood mononuclear cells (PBMCs) was determined by flow cytometry from 38 HCC patients and 38 chronic hepatitis B (CHB) patients. The GSTP1 mRNA level in PBMCs was determined by real-time quantitative polymerase chain reaction. Enzyme-linked-immunosorbent-assay (ELISA) was performed to measure the oxidative stress status, including plasma levels of malondialdehyde (MDA), xanthine oxidase (XOD), reduced glutathione hormone (GSH) and glutathione-S-transferases (GST).
Results: Significantly decreased GSTP1 protein expression was found in HCC patients than in CHB patients (P<0.05). The GSTP1 mRNA expression of HCC patients was also decreased compared with CHB patients (P<0.05). MDA and XOD levels were significantly higher in HCC patients than in CHB patients, while plasma GSH and GST levels were statistically lower in HCC patients than in CHB patients. GSTP1 expression level was correlated with plasma levels of MDA (P<0.01), XOD (P = 0.01) and GSH (P< 0.01), GST (P< 0.01).
Conclusion: We demonstrated that the reduced GSTP1 expression might contribute to oxidative stress in the development of HCC from CHB.
Keywords: Glutathione S transferases, flow cytometry, oxidative stress, hepatocellular carcinoma, chronic hepatitis B.
Introduction
As a global epidemic disease, about 400 million people are chronically infected with hepatitis B virus (HBV) [1]. This chronic infection could result in hepatocellular carcinoma (HCC), which accounts for 320 000 deaths each year worldwide [2]. HCC ranks the third in the leading causes of cancer-related death around the world, due to the poor prognosis [3]. Therefore, HCC becomes one of the major health threats. However, the mechanism of HCC development is not well understood. To our knowledge, both the immune response to HBV and oxidative stress are involved in the pathogenesis of HCC [4, 5].
Previous studies have reported that oxidative stress takes part in pathogenesis of both HCC and CHB [5, 6]. Bolukbas et al reported that increased oxidative stress was involved in various forms of HBV infection [7]. Another recent study demonstrated that oxidative stress could enhance the malignant potential of HCC through the telomerase activation [8]. However, few investigations have focused on the difference of oxidative stress status between HCC and CHB patients, while the difference of antioxidants such as reduced glutathione hormone (GSH) and glutathione-S-transferases (GST), is also obscure between the two groups.
GST is a family of phase II enzymes that can conjugate GSH with various environmental etiological factors, and it is ubiquitously expressed in normal and malignant tissues [9]. The family is composed of eight isoforms: alpha (GSTA), mu (GSTM), pi (GSTP), theta (GSTT), tau (GSTZ), sigma (GSTS), omicron (GSTO) and kappa (GSTK) [10]. With respect to cancers, the most studied member among them is GSTP1, who can protect cells from cytotoxic and carcinogenic agents. It has been reported that GSTP1 Ile105Val polymorphism of GSTP1 was associated with risk of various cancers, such as colorectal cancer and esophageal cancer [11, 12]. In another clinical research, Chen, et al, demonstrated that GSTP1 gene polymorphisms could increase age-related susceptibility to HCC [13]. Moreover, other researchers found that GSTP1 genetic polymorphisms could affect GSTP1 mRNA expression, but the protein expression level of GSTP1 was not studied [14].
GSTP1 promoter methylation was also thoroughly investigated in HCC. For instance, a study on primary HCC patients reported that GSTP1 promoter methylation was more frequent in HCC patients than in cirrhosis patients [15]. An in vitro study revealed that GSTP1 expression was significantly reduced in HepG2.2.15 cells due to the promoter methylation, in which western blot was used to detect GSTP1 expression [16]. To our knowledge, there are few studies aimed to assay GSTP1 protein expression with flow cytometry in HCC patients. Along with technical advance in intracellular staining and antibody production, flow cytometry becomes a useful measurement of intracellular protein, which is a quick and efficient way [17]. Therefore, we employed flow cytometry for the first time to detect GSTP1 protein in patients with HCC and CHB in the current study.
Based on this background, we aimed to test the feasibility of detecting GSTP1 expression with flow cytometry and investigate oxidative stress status of HCC patients compared with CHB patients in the present research.
Materials and Methods
General characteristics
A total of consecutive 38 HCC patients, 38 CHB patients and 10 normal controls were enrolled in the present study from December 2009 to February 2012. The subjects were from the Department of Hepatology, Qilu Hospital of Shandong University. CHB diagnosis was based on HBsAg and HBV-DNA positive for at least 6 months. No sign of cirrhosis was found among CHB patients according to B ultrasound, computer tomography or liver biopsy. HCC was diagnosed with histopathology or typical HCC imaging patterns (angiography, computed tomography and/or magnetic resonance imaging), combined with serum alpha fetoprotein (AFP) level, who had CHB history for more than one year [18]. All of the above patients and their samples were collected before any therapy including medicine and operation. History of coronary artery disease, cancer, systemic or local infection, pregnancy, and concomitant chronic hepatitis C or hepatitis D, as well as other known liver diseases such as alcoholic hepatitis, metabolic or autoimmune disorders were considered as exclusion criteria. General characteristics and clinical parameters such as ALT, AST, TBIL and PTA were obtained from hospital records on the day after admission. Ten ml peripheral blood was collected from each patient. The study was performed with approval of the Qilu Hospital ethical committee of Shandong University, and written consent was signed by each subject.
Intracellular GSTP1 protein expression analysis by flow cytometry
Protein expression level of intracellular GSTP1 in peripheral mononuclear cells (PBMCs) was analyzed by flow cytometry. In detail, 100μl heparinized peripheral whole blood was fixed with 100μl IC Fixation Buffer (eBioscience, Inc. San Diego, CA, USA). Then, 2 ml H-Lyse Buffer (R&D Systems Inc. Minneapolis, Minnesota, USA) was added to each tube to lyse erythrocytes. After 10 minutes, the mixture was centrifuged and resuspended in Permeabilization Buffer (eBioscience, Inc. San Diego, CA, USA). After centrifugalization, samples were incubated with GSTP1 antibody (Epitomics, Inc. Burlingame, CA, USA) for 20 minutes, while nothing was added to tubes of negative control. Then they were washed once with Permeabilization Buffer and incubated with FITC-labeled goat anti-rabbit IgG antibody (KPL, Gaithersburg, MD, USA) for 20 minutes. After centrifugalization, they were resuspended in 200 μl phosphate buffer saline (PBS). Stained cells were analyzed on a FACSCalibur cytometer (BD Biosciences, San Jose, CA). WinMDI 2.9 was employed to analyze positive percentage and mean fluorescence intensity (MFI), while regions were gated with lymphocytes.
RNA extraction and cDNA Synthesis
RNA was isolated using Trizol (Invitrogen Life Science, Carlsbad, California) from PBMCs of HCC patients and CHB patients. Then, RNA was converted into cDNA by PrimerScript TM RT Reagent Kit (Perfect Real Time; Takara, Japan) according to the manufacturer's instruction.
Detection of GSTP1 mRNA by quantitative reverse transcription polymerase chain reaction (QRT-PCR)
GSTP1 cDNA sequences were amplified by QRT-PCR, following a recent study [16]. In detail, it was performed in a total volume of 20 μl containing 0.5 mmol/L (mM) of each primer (Forward primer, ATGACTATGTGAAGGCACTG; Reverse primer, AGGTTCACGTACTCAGGGGA), 10 × SYBR Green (Toyobo Co., Ltd, Tokyo, Japan), 0.5 mM of cDNA, following 40 cycles composed of denaturation at 94℃ for 30 seconds, annealing at 60℃ for 45 seconds, and extension at 72℃ for minutes, and a final 7 minutes extension at 72℃. β-actin was used as an internal reference gene and GSTP1 mRNA expression level was calculated as GSTP1/β-actin in subjects.
Detection of plasma MDA, XOD, GSH and GST levels with Enzyme-linked-immunosorbent-assay (ELISA)
Oxidant and antioxidant levels in HCC patients and CHB patients were evaluated by plasma levels of MDA, XOD, GSH and GST. Human OXISelectTM MDA Adduct ELISA kit (Cell Biolabs, INC, USA), XOD ELISA kit (Uscn Life Science Inc., Wuhan, China), GSH ELISA kit (Uscn Life Science Inc., Wuhan, China), and GST ELISA kit (Uscn Life Science Inc., Wuhan, China) were employed to detect their plasma levels, respectively. Standard protocols provided by the manufacturers were exactly followed.
Statistical analysis
SPSS 13.0 statistical software (SPSS Inc., Chicago, IL) was used to analyze data. GSTP1 positive percentage and MFI differences between HCC patients and CHB patients were assayed by Independent-Samples t Test. Mann-Whitney U-test was employed to compare GSTP1 mRNA expression levels between the two groups, while laboratory parameters difference were analyzed with Independent-Samples t Test. Bivariate correlation was used to analyze the relation between GSTP1 level and laboratory parameters of HCC and CHB patients. All statistical analyses were two-sided, and P value <0.05 was considered to be statistically significant.
Results
Analysis of general characteristics
There was no statistical difference in gender between HCC patients and CHB patients (χ2=1.49, P=0.33), while HCC patients were significantly older than CHB patients evaluated by Mann-Whitney U test (P<0.01). No statistical difference in ALT, AST, TBIL and PTA was found between the two groups (Table 1).
Characteristics of HCC patients and CHB patients.
| Characteristics | CHB patients | HCC patients | P value |
|---|---|---|---|
| Age(years) | < 0.05 | ||
| <40 | 20 | 1 | |
| 40-60 | 15 | 13 | |
| >60 | 3 | 24 | |
| Gender | 0.33 | ||
| Female | 15 | 10 | |
| Male | 23 | 28 | |
| HBeAg(+) | 24 | 20 | 0.49 |
| HBeAg(-) | 14 | 18 | |
| HBV-DNA(copies/ml) | 0.36 | ||
| <1×105 copies/ml | 17 | 22 | |
| >1×105 copies/ml | 21 | 16 | |
| ALT(IU/dL) | 187.6(243.19) | 116.18(120.03) | 0.10 |
| AST(IU/dL) | 122.66(166.03) | 120.39(146.88) | 0.59 |
| TBIL(μmol/L) | 68.81(110.36) | 70.73(138.17) | 0.98 |
| PTA(%) | 99.13(8.87) | 75.42(19.55) | 0.47 |
Values shown as mean(SD).
Difference of GSTP1 protein and mRNA expression levels between HCC patients and CHB patients
No significant difference of GSTP1 protein expression was found between normal controls and CHB patients (3.04±0.65% vs 2.72±1.06%, P>0.05). As shown in Fig. 1, protein expression level of GSTP1 in HCC patients was significantly decreased in comparison with CHB patients. The positive percentage and MFI of GSTP1 in HCC patients were statistically lower than in CHB patients (1.13±0.55% vs 2.72±1.06%, P=0.005; 2.63±1.02 vs 5.63±2.33, P=0.002). The results were illustrated in Fig. 1.
GSTP1 mRNA expression in HCC patients was significantly lower than in CHB patients (median, 0.04; range, 0.005-0.33 vs. median, 0.06; range, 0.02-1.05; P=0.01) analyzed by the Mann-Whitney U-test (Fig. 1).
Oxidant and antioxidant levels in HCC patients and CHB patients
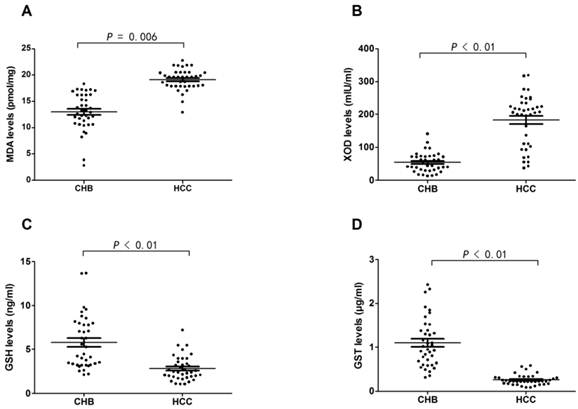
MDA and XOD levels were significantly higher in HCC patients than CHB patients (19.13±2.01 pmol/mg vs 12.99±3.55 pmol/mg, P=0.006; 183.23±78.09 mIU/ml vs 53.96±29.28 mIU/ml, P<0.01). In contrast, GSH and GST levels were significantly lower in HCC patients than CHB patients (2.81±1.40 ng/ml vs 5.79±3.03 ng/ml, P<0.01; 0.26±0.12 μg/ml vs 1.10±0.56 μg/ml, P<0.01), which meant that HCC patients were under much more severer imbalance of oxidative stress and antioxidant defense in contrast to CHB patients (Fig. 2).
(A) Typical results of intracellular flow cytometry for GSTP1 in HCC and CHB patients. (a) Negative control with only FITC-conjugated affinity purified antibody fluorescein labeled goat anti-rabbit IgG (H+L); (b) a representative result of intracellular flow cytometry for HCC patients. (c) a representative result of intracellular flow cytometry for CHB patients. (B) Results of GSTP1 mRNA expression in CHB and HCC patients.

Results of oxidant and antioxidant levels in CHB and HCC patients. (A) Difference of MDA level in plasma of CHB and HCC patients. (B) Difference of XOD level in plasma of CHB and HCC patients. (C) Difference of GSH level in plasma of CHB and HCC patients. (D) Difference of GST level in plasma of CHB and HCC patients.
(A) Correlation between GSTP1 expression level indicated by mean fluorescence intensity (MFI) and prothrombin activity (PTA) in HCC and CHB patients. (B) Correlation between GSTP1 expression level and malondialdehyde (MDA). (C) Correlation between GSTP1 expression level and xanthine oxidase (XOD). (D) Correlation between GSTP1 expression level and reduced glutathione hormone (GSH). (E) Correlation between GSTP1 expression level and glutathione-S-transferases (GST).
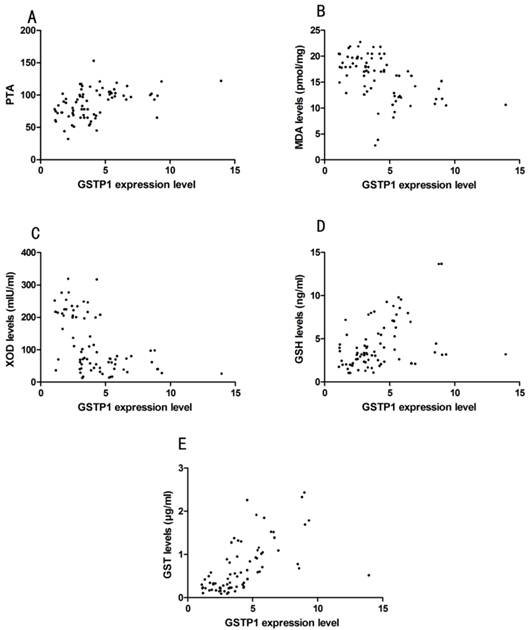
Correlation between GSTP1 level and laboratory parameters
Pearson correlation analyze showed that GSTP1 expression level indicated by MFI did not correlate with ALT, AST, TBIL, except for PTA (Pearson correlation: 0.29, P=0.01, Fig. 3). GSTP1 protein expression level was correlated with MDA (Pearson correlation: -0.42, P<0.01), XOD (Pearson correlation: -0.40, P=0.01), GSH (Pearson correlation: 0.42, P<0.01) and GST (Pearson correlation: 0.65, P<0.01), which is demonstrated in Fig. 3.
Discussion
Flow cytometry has been used in clinical study more and more widely, for it can assess cell surface markers and intracellular markers at single-cell level, especially in blood diseases [19-21]. As referring to liver diseases, researchers employed flow cytometry to study the intrahepatic immunological environment of chronic viral hepatitis [22]. Most of the studies paid attention to immune-related cells, inflammatory factors and their receptors, such as Th17 cells, IL-10 and IL-6 receptor [23, 24]. In the present study, flow cytometry was used to assay GSTP1 protein in peripheral blood of HCC patients and CHB patients. Our study implies that flow cytometry is a feasible way to detect GSTP1 protein at single-cell level. We found that GSTP1 protein expression in HCC patients was significantly decreased than in CHB patients. Moreover, we also demonstrated that GSTP1 mRNA expression in HCC patients was statistically lower compared to CHB patients. As well-known, GST family is involved in the detoxification of carcinogens. Therefore, our result suggests that decreased GSTP1 expression takes part in the development of HCC from CHB, which is the most studied member of GST family.
In agreement with our results, other researchers also found decreased GSTP1 expression in various cancers, such as prostate cancer and breast cancer [25,26]. Furthermore, a recent study on patients with HCC also demonstrated that GSTP1 mRNA expression was reduced due to the promoter methylation of GSTP1 [27]. Several studies have found that GSTP1 change could be a clue to detect prostate cancer [28-30]. As referring to HCC, previous studies revealed that silencing of GSTP1 expression caused by promoter methylation was involved in hepatocarcinogenesis and early stage of HCC [31, 32]. In addition, decreased GSTP1 expression could facilitate high level of alfatoxin B1-DNA adducts, which is an important dietary carcinogen of HCC [33]. Combined with the above findings, we conclude that reduced GSTP1 expression is associated with high risk of HCC development form CHB, which is worth further investigation. GSTP1 expression change may be a characterization in the development of HCC from CHB. Detection of GSTP1 expression by flow cytometry is feasible and it directs more targeted therapy for HCC patients with lower GSTP1 expression levels.
Considering that GSTP1 plays an important role in antioxidant defenses, we further assayed levels of other oxidants and antioxidants in patients with HCC and CHB. Significantly higher MDA and XOD plasma levels were found in HCC patients than in CHB patients. In contrast, statistically lower GSH and GST levels were detected in HCC patients compared with CHB patients. Our results indicate that HCC patients are under much more severer imbalance of oxidants and antioxidants CHB patients. The imbalance attributes to increased oxidants and decreased antioxidants such as GSH and GSTP1. Other isoforms of GST family may also contribute to the imbalance, such as GSTA1, the predominant isoforms, which needs further studies [34].
In accordance with our findings, a previous study reported that severe oxidative stress existed not only in patients with CHB but also in CHC patients, and it could lead to hepatic oxidative DNA damage [35]. Our study provides more evidence for the theory that oxidative stress is capable of damaging DNA and promoting the malignancy in development of HCC [36]. The mechanism involved may be that series transcription factors including NF-κB, AP-1, p53, HIF-1α, PPAR-γ could be activated by oxidative stress [34]. Those factors can induce over 500 genes' expression, some of which could contribute to carcinogenesis from chronic inflammation [34]. In fact, continuous inflammatory and regenerative stimuli during the long progression of HBV infection is also involve in the carcinogenesis of HCC. HBV protein X (HBx) has been documented to inhibit the production of certain anti-oncogenes such as p53 [37]. On the other hand, inflammatory cells activated by HBV infection can secrete reactive oxygen and nitrogen species, and eventually result in oxidative stress. Oxidative stress can damage hepatic DNA primarily by hydroxyl radicals [38]. Moreover, other oxidants such as superoxide radicals and hydrogen peroxides can enhance intracellular DNA damage and modify cell proliferation, apoptosis and cell motility in the development of HCC [39]. Based on the above findings, we conclude that oxidative stress and chronic inflammation take part in HCC initiation (an early event of HCC carcinogenesis may be integration of hepatitis B virus genome and hepatic inflammation induced by HBsAg favors this process), promotion (hepatic DNA could be damaged by oxidative stress along with inflammation), and progression ( malignant cells becomes more aggressive ). The above model of cancer development has been verified in mouse recently [40-43]. Therefore, it is reasonable to speculate that supplement of antioxidants such as GSH could be useful in the treatment of HCC patients and reduce the incidence of HCC development from CHB.
Our study also reveals that decreased GSTP1 expression is involved in the imbalance of oxidant and anti-oxidant, as it is correlated with MDA, XOD, GSH and GST analyzed by Pearson correlation. However, it should be noted that this study only examined 38 Chinese HCC patients, further investigations on more patients and other ethnicities should be carried out to verify our findings. Moreover, other members of GST family such as GSTM1 and GSTT1 have also been investigated in HCC. A meta analysis showed that GSTM1 and GSTT1 null genotype might increase the risk of HCC [44]. However, the clinical demographic characteristics of these protein from large population of HCC patients have not been studied yet, which will be detected in our further studies.
In summary, detection of GSTP1 protein by flow cytometry is feasible in clinical research. Reduced GSTP1 expression might contribute to the oxidant/antioxidant imbalance in HCC patients compared with CHB patients.
Abbreviations
AFP, alpha fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHB, chronic hepatitis B; ELISA, enzyme-linked-immunosorbent-assay; GSH, reduced glutathione hormone; GST, glutathione-S-transferases; GSTP1, glutathione-S-transferase P1; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; MDA, malondialdehyde; MFI, mean fluorescence intensity; QRT-PCR, quantitative reverse transcription polymerase chain reaction; PBMC, peripheral blood mononuclear cell; PBS, phosphate buffer saline; PTA, prothrombin activity; TBIL, total bilirubin; XOD, xanthine oxidase.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81171579, 81201287), Key Project of Chinese Ministry of Science and Technology (2012ZX10002007, 2013ZX10002001) and Natural Science Foundation of Shandong Province (ZR2010HM070, ZR2010HQ040).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Grimm D, Thimme R, Blum HE. HBV life cycle and novel drug targets. Hepatol Int. 2011;5:644-53
2. Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107
3. Bruix J & Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-36
4. Cougot D, Neuveut C, Buendia MA. HBV induced carcinogenesis. J Clin Virol. 2005;34:S75-8
5. Severi T, Vander Borght S, Libbrecht L. et al. HBx or HCV core gene expression in HepG2 human liver cells results in a survival benefit against oxidative stress with possible implications for HCC development. Chem Biol Interact. 2007;168:128-34
6. Esrefoglu M. Oxidative stress and benefits of antioxidant agents in acute and chronic hepatitis. Hepat Mon. 2012;12:160-7
7. Bolukbas C, Bolukbas FF, Horoz M, Aslan M, Celik H, Erel O. Increased oxidative stress associated with the severity of the liver disease in various forms of hepatitis B virus infection. BMC Infect Dis. 2005;5:95
8. Nishikawa T, Nakajima T, Katagishi T. et al. Oxidative stress may enhance the malignant potential of human hepatocellular carcinoma by telomerase activation. Liver Int. 2009;29:846-56
9. van Haaften RI, Haenen GR, Evelo CT, Bast A. Effect of vitamin E on glutathione-dependent enzymes. Drug Metab Rev. 2003;35:215-53
10. Katoh T, Yamano Y, Tsuji M, Watanabe M. Genetic polymorphisms of human cytosol glutathione S-transferases and prostate cancer. Pharmacogenomics. 2008;9:93-104
11. Lee JM, Wu MT, Lee YC. et al. Association of GSTP1 polymorphism and survival for esophageal cancer. Clin Cancer Res. 2005;11:4749-53
12. Kweekel DM, Koopman M, Antonini NF. et al. GSTP1 Ile105Val polymorphism correlates with progression-free survival in MCRC patients treated with or without irinotecan: a study of the Dutch Colorectal Cancer Group. Br J Cancer. 2008;99:1316-21
13. Chen YL, Tseng HS, Kuo WH, Yang SF, Chen DR, Tsai HT. Glutathione S-Transferase P1 (GSTP1) gene polymorphism increases age-related susceptibility to hepatocellular carcinoma. BMC Med Genet. 2010;11:46
14. Reszka E, Jabłonowski Z, Wieczorek E, Gromadzińska J, Sosnowski M, Wąsowicz W. GSTP1 mRNA expression in human circulating blood leukocytes is associated with GSTP1 genetic polymorphism. Clin Biochem. 2011;44:1153-5
15. Wang J, Qin Y, Li B, Sun Z, Yang B. Detection of aberrant promoter methylation of GSTP1 in the tumor and serum of Chinese human primary hepatocellular carcinoma patients. Clin Biochem. 2006;39:344-8
16. Niu D, Zhang J, Ren Y, Feng H, Chen WN. HBx genotype D represses GSTP1 expression and increases the oxidative level and apoptosis in HepG2 cells. Mol Oncol. 2009;3:67-76
17. Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61-70
18. Suriapranata IM, Sudania WM, Tjong WY. et al. Alpha-feto protein gene polymorphisms and risk of HCC and cirrhosis. Clin Chim Acta. 2010;411:351-8
19. Clutter MR, Heffner GC, Krutzik PO, Sachen KL, Nolan GP. Tyramide signal amplification for analysis of kinase activity by intracellular flow cytometry. Cytometry A. 2010;77:1020-31
20. Mancuso P, Antoniotti P, Quarna J. et al. Validation of a standardized method for enumerating circulating endothelial cells and progenitors: flow cytometry and molecular and ultrastructural analyses. Clin Cancer Res. 2009;15:267-73
21. Lee WM, Grindle K, Pappas T. et al. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626-34
22. Sprengers D, van der Molen RG, Kusters JG. et al. Flow cytometry of fine-needle-aspiration biopsies: a new method to monitor the intrahepatic immunological environment in chronic viral hepatitis. J Viral Hepat. 2005;12:507-12
23. Li J, Wu W, Peng G. et al. HBcAg induces interleukin-10 production, inhibiting HBcAg-specific Th17 responses in chronic hepatitis B patients. Immunol Cell Biol. 2010;88:834-41
24. Zhang F, Yao S, Yuan J. et al. Elevated IL-6 receptor expression on CD4+ T cells contributes to the increased Th17 responses in patients with chronic hepatitis B. Virol J. 2011;8:270
25. Singal R, van Wert J, Bashambu M. Cytosine methylation represses glutathione S-transferase P1 (GSTP1) gene expression in human prostate cancer cells. Cancer Res. 2001;61:4820-6
26. Lin X, Nelson WG. Methyl-CpG-binding domain protein-2 mediates transcriptional repression associated with hypermethylated GSTP1 CpG islands in MCF-7 breast cancer cells. Cancer Res. 2003;63:498-504
27. Li Z, Zhang H, Yang J, Hao T, Li S. Promoter hypermethylation of DNA damage response genes in hepatocellular carcinoma. Cell Biol Int. 2012;36:427-32
28. Bernardini S, Miano R, Iori R. et al. Hypermethylation of the CpG islands in the promoter region of the GSTP1 gene in prostate cancer: a useful diagnostic and prognostic marker? Clin Chim Acta. 2004;350:181-8
29. Rybicki BA, Neslund-Dudas C, Nock NL. et al. Prostate cancer risk from occupational exposure to polycyclic aromatic hydrocarbons interacting with the GSTP1 Ile105Val polymorphism. Cancer Detect Prev. 2006;30:412-22
30. Woodson K, O'Reilly KJ, Hanson JC. et al. The usefulness of the detection of GSTP1 methylation in urine as a biomarker in the diagnosis of prostate cancer. J Urol. 2008;179:508-11
31. Zhong S, Tang MW, Yeo W. et al. Silencing of GSTP1 gene by CpG island DNA hypermethylation in HBV-associated hepatocellular carcinomas. Clin Cancer Res. 2002;8:1087-92
32. Lee S, Lee HJ, Kim JH. et al. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol. 2003;163:1371-8
33. Zhang YJ, Chen Y, Ahsan H. et al. Silencing of glutathione S-transferaseP1 by promoter hypermethylation and its relationship to environmental chemical carcinogens in hepatocellular carcinoma. Cancer Lett. 2005;221:135-43
34. Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603-16
35. Fujita N, Sugimoto R, Ma N. et al. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. Viral Hepat. 2008;15:498-507
36. Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;40:1-11
37. Feitelson MA. Hepatitis B virus in hepatocarcinogenesis. J Cell Physiol. 1999;181:188-202
38. Calvisi DF, Conner EA, Ladu S, Lemmer ER, Factor VM, Thorgeirsson SS. Activation of the canonical Wnt/beta-catenin pathway confers growth advantages in c-Myc/E2F1 transgenic mouse model of liver cancer. J Hepatol. 2005;42:842-9
39. Marra M, Sordelli IM, Lombardi A. et al. Molecular targets and oxidative stress biomarkers in hepatocellular carcinoma: an overview. J Transl Med. 2011;10:171
40. Chan HL, Sung JJ. Hepatocellular carcinoma and hepatitis B virus. Semin Liver Dis. 2006;26:153-61
41. Zhou L, Yang Y, Tian D, Wang Y. Oxidative stress-induced 1, N6-ethenodeoxyadenosine adduct formation contributes to hepatocarcinogenesis. Oncol Rep. 2013;29:875-84
42. Sun J, Wiklund F, Hsu FC. et al. Interactions of sequence variants in interleukin-1 receptor-associated kinase 4 and the toll-like receptor 6-1-10 gene cluster increase prostate cancer risk. Cancer Epidemiol. Biomarkers Prev. 2006;15:480-5
43. Staib F, Robles AI, Varticovski L. et al. The p53 tumor suppressor network is a key responder to microenvironmental components of chronic inflammatory stress. Cancer Res. 2005;65:10255-64
44. Wang B, Huang G, Wang D. et al. Null genotypes of GSTM1 and GSTT1 contribute to hepatocellular carcinoma risk: evidence from an updated meta-analysis. J Hepatol. 2010;53:508-18
Author contact
![]() Corresponding author: Professor Kai Wang, MD, PhD. Department of Hepatology, Qilu Hospital of Shandong University and Institute of Hepatology, Shandong University, Wenhuaxi Road 107#, Jinan 250012, China. Tel: +86-531-86630809 Fax: +86-531-86927544 E-mail: wangdoc876com; wangdoc876com.cn.
Corresponding author: Professor Kai Wang, MD, PhD. Department of Hepatology, Qilu Hospital of Shandong University and Institute of Hepatology, Shandong University, Wenhuaxi Road 107#, Jinan 250012, China. Tel: +86-531-86630809 Fax: +86-531-86927544 E-mail: wangdoc876com; wangdoc876com.cn.

 Global reach, higher impact
Global reach, higher impact