3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(4):392-398. doi:10.7150/ijms.5770 This issue Cite
Research Paper
Glutathione S-transferase P1 Ile105Val Polymorphism and Oral Cancer Risk: A Meta-Analysis
1. Laboratory Medicine, Zhejiang Provincial People's Hospital, Hangzhou, Zhejiang, 310014, China;
2. Medical school of Taizhou University, Taizhou, Zhejiang 318000, China;
3. Department of Clinical Laboratory, Taizhou Municipal Hospital, Taizhou, Zhejiang, 318000, China.
* Weixing Li and Jiayu Chen contributed equally to this work.
Received 2012-12-27; Accepted 2013-1-25; Published 2013-2-21
Abstract
Objective The glutathione S-transferase P1 (GSTP1) gene has been suggested to play an important role in the pathogenesis of oral cancer. However, the results have been inconsistent. In this study, we performed a meta-analysis to clarify the association of GSTP1 Ile105Val polymorphisms with oral cancer risk.
Methods Published literature from PubMed and EMBASE were retrieved. Pooled odds ratio (OR) with 95% confidence interval (CI) was calculated using fixed- or random-effects model.
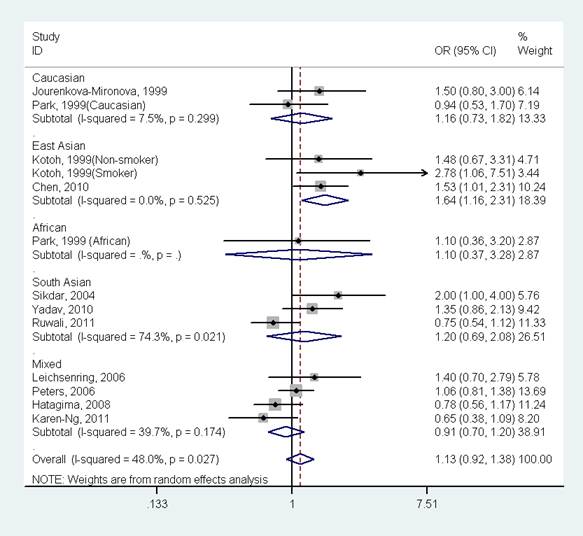
Results 13 studies (1803 oral cancer cases and 2998 controls) for GSTP1 Ile105Val polymorphism were included in the meta-analysis. The results indicated that there was no significant association between GSTP1 Ile105Val polymorphism and oral cancer in the overall population (OR=1.30, 95%CI=0.92-1.38, I2=48.0%, p for heterogeneity=0.027). Further subgroup analysis by ethnicity suggested that GSTP1 Ile105Val polymorphism was significantly associated with oral cancer only in East Asians (OR=1.64, 95%CI=1.16-2.31, I2=0.0%, p for heterogeneity=0.525), but not in Caucasians (OR=1.16, 95%CI=0.73-1.82, I2=7.5%, p for heterogeneity=0.299), Africans (OR=1.10, 95%CI=0.37-3.28), South Asians (OR=1.20, 95%CI=0.69-2.08, I2=74.3%, p for heterogeneity=0.021) and mixed population (OR=0.91, 95%CI=0.70-1.20, I2=39.7%, p for heterogeneity=0.174).
Conclusions The present meta-analysis has limited evidence to support the association of GSTP1 Ile105Val polymorphism with HCC risk in the overall population. However, GSTP1 Ile105Val polymorphism might be associated with risk of oral cancer in East Asians.
Keywords: GSTP1, Polymorphism, Oral cancer, Meta-analysis.
Introduction
Oral cancer is a serious public health problem worldwide [1]. It is believed that oral cancer is a complex disease caused by both genetic and environmental factors, as well as their interactions. Epidemiology studies have indicated that environmental factors including tobacco smoking, alcohol consumption and betel-quid chewing contribute to the development of oral cancer [2]. In addition, genetic factors also play important roles in the pathogenesis of oral cancer.
Most tobacco carcinogens are metabolized by enzymatic complex mechanisms involving both activation (phase I) and detoxification (phase II) reactions [3]. The detoxification efficiency of GST enzymes is determined by the presence, amount, and nature of the isoenzymes coded by GSTT1, GSTM1, and GSTP1 genes [4]. The deleted variants of the GSTM1 and GSTT1 loci result in loss of functional activity [5]. The polymorphism at codon 105 of GSTP1 gene has been reported to cause differences in catalytic activity. In other words, electrophilic compounds are reported to be detoxified less efficiently in individuals with variant genotypes of GSTP1 (Ile/Val and Val/Val) when compared with those with wild-type genotype [6]. Recently, a meta-analysis has indicated that the GSTM1 null genotype may be associated with a higher risk of oral cancer in Asians but not in Caucasians; the GSTT1 null genotype may not be associated with oral cancer [7]. However, no meta-analysis has examined the association between GSTP1 Ile105Val polymorphism and risk of oral cancer, although many individual studies have been published with inconsistent results [8-18]. Thus, in this study, we performed a meta-analysis to clarify the association between GSTP1 Ile105Val polymorphism and risk of oral cancer.
Materials and methods
Literature and search strategy
Literature databases including PubMed and Embase were searched. The search strategy to identify all possible studies involved the use of the following key words: (GSTP1 or glutathione S-transferase P1) and (variant or variation or polymorphism) and oral cancer. The publication language was restricted to English. The reference lists of retrieved articles were manually searched. If more than one article were published using the same data, only the study with largest sample size was included. The literature search was updated on December 12, 2012.
Inclusion criteria and data extraction
The studies included in the meta-analysis must meet all the following inclusion criteria: (1) evaluating the association between GSTP1 Ile105Val polymorphism and oral cancer; (2) using case-control or cohort design; (3) providing sufficient data for calculation of odds ratio (OR) with 95% confidence interval (CI). The following information was extracted from each study: (1) name of the first author; (2) year of publication; (3) country; (4) ethnicity; (5) sample size of cases and controls; (6) source of controls; (7) covariates' adjusted OR with 95%CI under a dominant model; and (8) whether or not the genotypes in Hardy-Weinberg equilibrium (HWE) in controls. The two authors independently assessed the articles for compliance with the inclusion/exclusion criteria, resolved disagreements and reached a consistent decision.
Statistical analysis
The association between GSTP1 Ile105Val polymorphism and oral cancer was estimated by calculating pooled adjusted OR with 95% CI under a dominant genetic model. The significance of pooled OR was determined by Z test (p<0.05 was considered statistically significant). Q test was performed to evaluate to the between-study heterogeneity. A random- (DerSimonian-Laird method [19]) or fixed- (Mantel-Haenszel method [20]) effects model was used to calculate pooled OR in the presence (p<=0.10) or absence (p>0.10) of heterogeneity, respectively. Subgroup analysis by ethnicity, whether adjustment for smoking status (no vs. yes), and number of cases (n<150 vs n≥150) was performed. Publication bias was assessed by Begg's test [21] and Egger's test [22] (p<0.05 was considered statistically significant). Data analysis was performed using STATA version 11 (StataCorp LP, College Station, Texas, USA).
Results
Characteristics of the studies
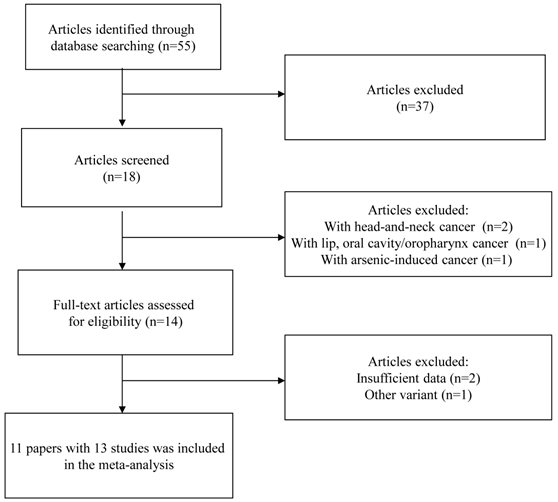
A flow chart of inclusion/exclusion of the individual studies was presented as Figure 1. The literature search identified a total of 55 potentially relevant papers. Of these, 37 papers were excluded because of obvious irrelevance by reading the titles and abstracts. In addition, two papers were excluded because they examined the association between GSTP1 Ile105Val polymorphism and head-and-neck cancer [23, 24]; one paper was excluded because it investigated the association between GSTP1 Ile105Val polymorphism and the combined cancer including lip, oral cavity/oropharynx cancer [25]; one paper was excluded because it examined the association between GSTP1 Ile105Val polymorphism and arsenic-induced cancer [26]. Then, 14 papers met the primary inclusion criteria [8-18, 27-29]. In addition, two papers were excluded because they did not provide sufficient data for calculation of OR with 95%CI [27,28]; one paper was excluded because it examined the association between GSTP1 mitochondrial polymorphisms and oral cancer [29]. It should be noted that since the paper by Kotoh, et al. [9] provided the data stratified for smoking status and the paper by Park, et al. [10] provided the data stratified for ethnicity, they were considered as separate studies in the following data analysis. At last, 13 studies (1803 oral cancer cases and 2998 controls) were included in the final meta-analysis. The characteristics of the included studies are listed in Table 1.
Flow chart of inclusion/exclusion of the individual studies
Characteristics of the studies included in the meta-analysis.
| Study | Country | Ethnicity | No. of cases | No. of controls | Dominant model | Source of controls | Adjustment * | In HWE | |
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | ||||||||
| Jourenkova- Mironova, 1999 [8] | France | Caucasian | 67 | 172 | 1.50 | 0.80-3.00 | Hospital-based | 1, 2, 3, 4, 5 | Yes |
| Kotoh,1999 (Non-smoker) [9] | Japan | East Asian | 30 | 122 | 1.48 | 0.67-3.31 | Hospital-based | 1, 2 | Yes |
| Kotoh,1999 (Smoker) [9] | Japan | East Asian | 53 | 122 | 2.78 | 1.06-7.51 | Hospital-based | 1, 2 | Yes |
| Park,1999 (Caucasian) [10] | USA | Caucasian | 104 | 175 | 0.94 | 0.53-1.70 | Hospital-based | 3, 5 | Yes |
| Park,1999 (African) [10] | USA | African | 53 | 85 | 1.10 | 0.36-3.20 | Hospital-based | 3, 5 | Yes |
| Sikdar, 2004 [11] | India | South Asian | 256 | 259 | 2.00 | 1.00-4.00 | Hospital-based | 1,2,3 | Yes |
| Leichsenring, 2006 [12] | Brazil | Mixed | 72 | 60 | 1.40 | 0.70-2.79 | Hospital-based | None | Yes |
| Peters, 2006 [13] | USA | Mixed | 352 | 753 | 1.06 | 0.81-1.38 | Population-based | 1, 2, 3, 5, 6 | Yes |
| Hatagima, 2008 [14] | Brazil | Mixed | 231 | 212 | 0.78 | 0.56-1.17 | Hospital-based | 1, 2, 3, 5, 6 | Yes |
| Chen, 2010 [15] | China | East Asian | 164 | 274 | 1.53 | 1.01-2.31 | Hospital-based | 2 | Yes |
| Yadav, 2010 [16] | India | South Asian | 136 | 270 | 1.35 | 0.86-2.13 | Hospital-based | 3, 5, 7 | Yes |
| Karen-Ng, 2011 [17] | Malaysia | Mixed | 115 | 116 | 0.65 | 0.39-1.09 | Hospital-based | 1, 2, 4, 5, 6,,7 | Yes |
| Ruwali, 2011 [18] | India | South Asian | 170 | 500 | 0.75 | 0.54-1.12 | Hospital-based | 2, 3, 4,5 | Yes |
* 1, sex; 2; age; 3, tobacco consumption; 4, cigarette consumption; 5, alcohol consumption; 6, race; 7, betel quid chewing OR, odds ratio; CI, confidence interval; HWE, Hardy-Weinberg equilibrium.
Meta-analysis results
The results indicated that there was no significant association between GSTP1 Ile105Val polymorphism and oral cancer in the overall population (OR=1.13, 95%CI=0.92-1.38, I2=48.0%, p for heterogeneity=0.027, Fig 2 and Table 2). Further subgroup analysis by ethnicity suggested that GSTP1 Ile105Val polymorphism was significantly associated with oral cancer only in East Asians (OR=1.64, 95%CI=1.16-2.31, I2=0.0%, p for heterogeneity=0.525), but not in Caucasians (OR=1.16, 95%CI=0.73-1.82, I2=7.5%, p for heterogeneity=0.299), Africans (OR=1.10, 95%CI=0.37-3.28), South Asians (OR=1.20, 95%CI=0.69-2.08, I2=74.3%, p for heterogeneity=0.021) and mixed populations (OR=0.91, 95%CI=0.70-1.20, I2=39.7%, p for heterogeneity=0.174) (Fig 3 and Table 2). In the stratified analysis by whether adjustment for smoking status, the effect size was significant in studies not adjusting for smoking status (OR=1.61, 95%CI=1.15-2.24, I2=0.0%, p for heterogeneity=0.494), but was not significant in studies controlling for smoking status (OR=1.02, 95%CI=0.83-1.25, I2=39.7%, p for heterogeneity=0.093) (Table 2). However, in studies with number of cases more than 150 or less than 150, the effect size was both not significant (Table 2).
Potential publication bias
No publication bias was detected for the association between GSTP1 Ile105Val polymorphism and oral cancer (Begg's test: p=0.127; Egger's test: p=0.114).
Discussion
To our knowledge, this is the first meta-analysis investigating the association between GSTP1 Ile105Val polymorphism and oral cancer risk. The present meta-analysis including 1803 oral cancer cases and 2998 controls did not support the significant association between GSTP1 Ile105Val polymorphism and oral cancer risk in the overall population. However, GSTP1 Ile105Val polymorphism was significantly associated with oral cancer in East Asians.
Forest plot of the meta-analysis of the association between GSTP1 Ile105Val variant and oral cancer under a dominant model.
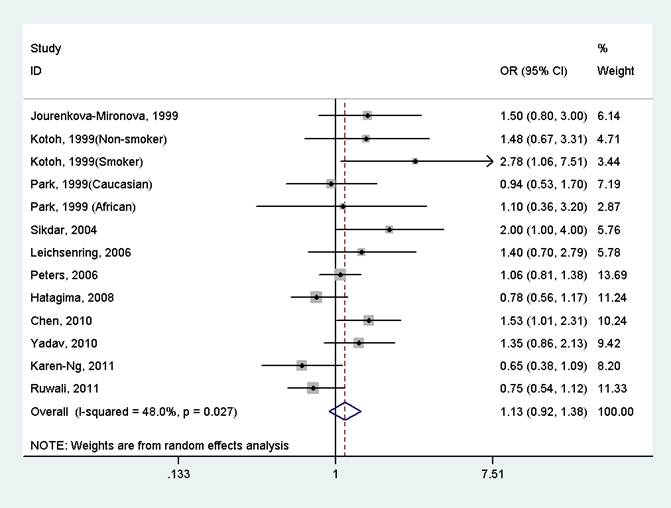
Meta-analysis of the association between GSTP1 Ile105Val variant and oral cancer under a dominant model.
| No. of studies | OR | 95 %CI | P z | Statistical model | I2 (%) | P H | |
|---|---|---|---|---|---|---|---|
| All | 13 | 1.13 | 0.92-1.38 | 0.243 | Random | 48.0 | 0.027 |
| Ethnicity | |||||||
| Caucasian | 2 | 1.15 | 0.74-1.79 | 0.523 | Fixed | 7.5 | 0.299 |
| East Asian | 3 | 1.64 | 1.16-2.31 | 0.005 | Fixed | 0.0 | 0.525 |
| South Asian | 3 | 1.20 | 0.69-2.08 | 0.524 | Random | 74.3 | 0.021 |
| Mixed | 4 | 0.93 | 0.77-1.13 | 0.703 | Fixed | 39.7 | 0.174 |
| African | 1 | 1.10 | 0.37-3.28 | 0.475 | - | - | - |
| Adjustment for smoking | |||||||
| No | 3 | 1.61 | 1.15-2.24 | 0.005 | Fixed | 0.0 | 0.494 |
| Yes | 10 | 1.02 | 0.83-1.25 | 0.864 | Random | 39.7 | 0.093 |
| No. of cases | |||||||
| <150 | 8 | 1.17 | 0.93-1.47 | 0.171 | Fixed | 29.9 | 0.189 |
| ≥150 | 5 | 1.07 | 0.79-1.45 | 0.669 | Random | 67.1 | 0.016 |
Abbreviations: OR, odds ratio; CI, confidence interval. P z, P value for Z test. P H, P value based on Q test for between-study heterogeneity.
Forest plot of the meta-analysis of the association between GSTP1 Ile105Val variant and oral cancer stratified by ethnicity under a dominant model.
Recently, many meta-analyses have been performed to investigate the association between GSTP1 Ile105Val polymorphism and many types of cancer risk (e.g., prostate cancer [30], gastric cancer [31], esophageal cancer [32], head and neck cancer [33], lung cancer [34], breast cancer [35], ovarian cancer [36] and thyroid cancer [37]). The results indicated that GSTP1 polymorphism was not significantly associated with prostate cancer [30], esophageal cancer [32], head and neck cancer [33], lung cancer [34], ovarian cancer [36] and thyroid cancer [37]; this polymorphism might contribute to the development of gastric cancer [31] or breast cancer [35] in East Asians, but not in other ethnic populations. However, besides our study, these meta-analyses did not consider the effect of gene-gene and gene-environment interactions in the development of cancer. One polymorphism with modest effect may be not associated with cancer susceptibility, but the synthesis of many genes or gene gene-environment interactions might increase cancer risk.
In this meta-analysis, we used covariate's adjusted OR with 95% CI to calculate the pooled estimate, thus, more precise effect was obtained. However, some potential limitations in our study should be considered. First, gene-gene and gene-environment interactions were not addressed in our meta-analysis. However, we further performed subgroup analysis based on whether adjustment for smoking status. The result indicated that smoking strengthened the effect of the Ile105Val polymorphism on oral cancer. Second, most individual studies only provided adjusted OR with 95% CI under a dominant model. Thus, we are unable to estimate the effect of Ile105Val polymorphism under other genetic models, such as co-dominant model, recessive model and additive model. Third, there was significant between-study heterogeneity for Ile105Val polymorphism. We performed subgroup analysis based on ethnicity and the heterogeneity only existed in South Asians, suggesting this subgroup population is the source of between-study heterogeneity.
In summary, the present meta-analysis has limited evidence to support the association of GSTP1 Ile105Val polymorphism with risk of oral cancer. However, GSTP1 Ile105Val polymorphism might be associated with risk of oral cancer in East Asians. Further large-scale studies with the consideration for gene-gene/gene-environment interactions should be conducted to investigate the association.
Acknowledgements
The work was funded by Zhejiang Medicine, Health, and Science (no.2010KYB127), Zhejiang Province Chinese Medicine Research Program (2012ZA130) and Zhejiang Gong Yi Xing Technology Application Project (2012C33025).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Walker DM, Boey G, McDonald L. The pathology of oral cancer. Pathology. 2003;35(5):376-383
2. Cheong SC, Chandramouli GV, Saleh A. et al. Gene expression in human oral squamous cell carcinoma is influenced by risk factor exposure. Oral Oncol. 2009;45(8):712-719
3. Lazarus P, Park JY. Metabolizing enzyme genotype and risk for upper aerodigestive tract cancer. Oral Oncol. 2000;36(5):421-431
4. Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30(6):445-600
5. Wiencke JK, Kelsey KT, Lamela RA. et al. Human glutathione Stransferase deficiency as a marker of susceptibility to epoxide-induced cytogenetic damage. Cancer Res. 1990;50(5):1585-1590
6. Bolt HM, Thier R. Relevance of the deletion polymorphisms of the glutathione S-transferases GSTT1 and GSTM1 in pharmacology and toxicology. Curr Drug Metab. 2006;7(6):613-628
7. Zhang ZJ, Hao K, Shi R. et al. Glutathione S-transferase M1 (GSTM1) and glutathione S-transferase T1 (GSTT1) null polymorphisms, smoking, and their interaction in oral cancer: a HuGE review and meta-analysis. Am J Epidemiol. 2011;173(8):847-857
8. Jourenkova-Mironova N, Voho A, Bouchardy C. et al. Glutathione S-transferase GSTM1, GSTM3, GSTP1 and GSTT1 genotypes and the risk of smoking-related oral and pharyngeal cancers. Int J Cancer. 1999;81(1):44-48
9. Katoh T, Kaneko S, Takasawa S. et al. Human glutathione S-transferase P1 polymorphism and susceptibility to smoking related epithelial cancer; oral, lung, gastric, colorectal and urothelial cancer. Pharmacogenetics. 1999;9(2):165-169
10. Park JY, Schantz SP, Stern JC. et al. Association between glutathione S-transferase pi genetic polymorphisms and oral cancer risk. Pharmacogenetics. 1999;9(4):497-504
11. Sikdar N, Paul RR, Roy B. Glutathione S-transferase M3 (A/A) genotype as a risk factor for oral cancer and leukoplakia among Indian tobacco smokers. Int J Cancer. 2004;109(1):95-101
12. Leichsenring A, Losi-Guembarovski R, Maciel ME. et al. CYP1A1 and GSTP1 polymorphisms in an oral cancer case-control study. Braz J Med Biol Res. 2006;39(12):1569-1574
13. Peters ES, McClean MD, Marsit CJ. et al. Glutathione S-transferase polymorphisms and the synergy of alcohol and tobacco in oral, pharyngeal, and laryngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2196-2202
14. Hatagima A, Costa EC, Marques CF. et al. Glutathione S-transferase polymorphisms and oral cancer: a case-control study in Rio de Janeiro, Brazil. Oral Oncol. 2008;44(2):200-207
15. Chen MK, Tsai HT, Chung TT. et al. Glutathione S-transferase P1 and alpha gene variants; role in susceptibility and tumor size development of oral cancer. Head Neck. 2010;32(8):1079-1087
16. Yadav DS, Devi TR, Ihsan R. et al. Polymorphisms of glutathione-S-transferase genes and the risk of aerodigestive tract cancers in the Northeast Indian population. Genet Test Mol Biomarkers. 2010;14(5):715-723
17. Karen-Ng LP, Marhazlinda J, Rahman ZA. et al. Combined effects of isothiocyanate intake, glutathione S-transferase polymorphisms and risk habits for age of oral squamous cell carcinoma development. Asian Pac J Cancer Prev. 2011;12(5):1161-1166
18. Ruwali M, Singh M, Pant MC. et al. Polymorphism in glutathione S-transferases: susceptibility and treatment outcome for head and neck cancer. Xenobiotica. 2011;41(12):1122-1130
19. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188
20. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719-748
21. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101
22. Egger M, Davey Smith G, Schneider M. et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634
23. Morita S, Yano M, Tsujinaka T. et al. Genetic polymorphisms of drug-metabolizing enzymes and susceptibility to head-and-neck squamous-cell carcinoma. Int J Cancer. 1999;80(5):685-688
24. Olshan AF, Weissler MC, Watson MA. et al. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(2):185-191
25. Buch SC, Nazar-Stewart V, Weissfeld JL. et al. Case-control study of oral and oropharyngeal cancer in whites and genetic variation in eight metabolic enzymes. Head Neck. 2008;30(9):1139-1147
26. Ghosh P, Basu A, Mahata J. et al. Cytogenetic damage and genetic variants in the individuals susceptible to arsenic-induced cancer through drinking water. Int J Cancer. 2006;118(10):2470-2478
27. Jourenkova-Mironova N, Mitrunen K, Bouchardy C. et al. High-activity microsomal epoxide hydrolase genotypes and the risk of oral, pharynx, and larynx cancers. Cancer Res. 2000;60(3):534-536
28. Masood N, Kayani MA, Malik FA. et al. Genetic variation in carcinogen metabolizing genes associated with oral cancer in pakistani population. Asian Pac J Cancer Prev. 2011;12(2):491-495
29. Datta S, Majumder M, Biswas NK. et al. Increased risk of oral cancer in relation to common Indian mitochondrial polymorphisms and Autosomal GSTP1 locus. Cancer. 2007;110(9):1991-1999
30. Gong M, Dong W, Shi Z. et al. Genetic Polymorphisms of GSTM1, GSTT1, and GSTP1 with Prostate Cancer Risk: A Meta-Analysis of 57 Studies. PLoS One. 2012;7(11):e50587
31. Bao LD, Niu JX, Song H. et al. Association between the GSTP1 codon 105 polymorphism and gastric cancer risk: an updated meta-analysis. Asian Pac J Cancer Prev. 2012;13(8):3687-3693
32. Zhao Y, Wang F, Shan S. et al. Genetic polymorphism of p53, but not GSTP1, is association with susceptibility to esophageal cancer risk - a meta-analysis. Int J Med Sci. 2010;7(5):300-308
33. Lang J, Song X, Cheng J. et al. Association of GSTP1 Ile105Val Polymorphism and Risk of Head and Neck Cancers: A Meta-Analysis of 28 Case-Control Studies. PLoS One. 2012;7(11):e48132
34. Feng X, Zheng BS, Shi JJ. et al. Association of glutathione S-transferase P1 gene polymorphism with the susceptibility of lung cancer. Mol Biol Rep. 2012;39(12):10313-10323
35. Lu S, Wang Z, Cui D. et al. Glutathione S-transferase P1 Ile105Val polymorphism and breast cancer risk: a meta-analysis involving 34,658 subjects. Breast Cancer Res Treat. 2011;125(1):253-259
36. Economopoulos KP, Sergentanis TN, Vlahos NF. Glutathione S-transferase M1, T1, and P1 polymorphisms and ovarian cancer risk: a meta-analysis. Int J Gynecol Cancer. 2010;20(5):732-737
37. Li J, Long J, Hu Y. et al. Glutathione S-transferase M1, T1, and P1 polymorphisms and thyroid cancer risk: A meta-analysis. Cancer Epidemiol. 2012;36(6):e333-340
Author contact
![]() Corresponding author: Chibo Liu, Department of Clinical Laboratory, Taizhou Municipal Hospital, Taizhou, Zhejiang,318000, China, Tel.: 86-576-8885-8213, Fax: 86-576-8885-8024, E-mail: chibo_liucom Jiayu Chen, Medical school of Taizhou University, Taizhou, Zhejiang 318000, China. E-mail: chenyujia10com.
Corresponding author: Chibo Liu, Department of Clinical Laboratory, Taizhou Municipal Hospital, Taizhou, Zhejiang,318000, China, Tel.: 86-576-8885-8213, Fax: 86-576-8885-8024, E-mail: chibo_liucom Jiayu Chen, Medical school of Taizhou University, Taizhou, Zhejiang 318000, China. E-mail: chenyujia10com.

 Global reach, higher impact
Global reach, higher impact