Impact Factor
ISSN: 1449-1907
Int J Med Sci 2013; 10(4):377-381. doi:10.7150/ijms.5224 This issue Cite
Research Paper
Curcumin Attenuates Diabetic Neuropathic Pain by Downregulating TNF-α in a Rat Model
1. Department of air logistics, the 463 Hospital of PLA, Shenyang 110042, PR China.
2. Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, USA.
3. Department of Anatomy, Histology and Embryology, the Fourth Military Medical University, Xi'an, 710032, PR China.
4. Department of Anesthesiology, Xijing Hospital the Fourth Military Medical University, Xi'an, 710032, PR China.
5. Department of neurosurgery, the 463 Hospital of PLA, Shenyang 110042, PR China.
* These authors contributed equally to this work.
Received 2012-9-15; Accepted 2013-2-10; Published 2013-2-20
Abstract
The mechanisms involved in diabetic neuropathic pain are complex and involve peripheral and central pathophysiological phenomena. Proinflammatory tumour necrosis factor α (TNF-α) and TNF-α receptor 1, which are markers of inflammation, contribute to neuropathic pain. The purpose of this experimental study was to evaluate the effect of curcumin on diabetic pain in rats. We tested 24 rats with diabetes induced by a single intraperitoneal injection of streptozotocin and 24 healthy control rats. Twelve rats in each group received 60 mg/kg oral curcumin daily for 28 days, and the other 12 received vehicle. On days 7, 14, 21, and 28, we tested mechanical allodynia with von Frey hairs and thermal hyperalgesia with radiant heat. Markers of inflammation in the spinal cord dorsal horn on day 28 were estimated with a commercial assay and Western blot analysis. Compared to control rats, diabetic rats exhibited increased mean plasma glucose concentration, decreased mean body weight, and significant pain hypersensitivity, as evidenced by decreased paw withdrawal threshold to von Frey hairs and decreased paw withdrawal latency to heat. Curcumin significantly attenuated the diabetes-induced allodynia and hyperalgesia and reduced the expression of both TNF-α and TNF-α receptor 1. Curcumin seems to relieve diabetic hyperalgesia, possibly through an inhibitory action on TNF-α and TNF-α receptor 1.
Keywords: diabetic neuropathic pain, hyperalgesia, curcumin, tumour necrosis factor α, tumour necrosis factor α receptor 1.
Introduction
Diabetes mellitus is a chronic metabolic disorder related to insulin deficiency and can involve many organs. Diabetic neuropathy is a common complication that affects sensory neurons, motor neurons, and the autonomic nervous system. Diabetic pain is one of the most common symptoms of diabetic neuropathy and is characterised by spontaneous pain, hyperalgesia, and paraesthesias [1, 2]. The mechanism that underlies diabetic pain is complicated. The pathogenesis of hyperglycaemia-related diabetic neuropathy involves multiple mechanisms, including oxidative and nitrosative stress, activation of nuclear factor-κB (NF-κB), and mitochondrial dysfunction [3-5]. Several lines of clinical and experimental evidence indicate, however, that neuroinflammation is an important factor in the process of central sensitization, which has been associated with the activation of glial cells [6-8]. Various studies have shown that glial cells, particularly microglia, are activated in uncontrolled hyperglycaemic conditions in the spinal cord [9-10]. After undergoing phenotypic changes, activated microglia release various proinflammatory cytokines, including interleukin 1β (IL-1β) and tumour necrosis factor α (TNF-α), which have been implicated directly in the induction of neuropathic pain [9]. Although the role of microglia and their pharmacological modulation are poorly understood, the expression of proinflammatory cytokines in the dorsal horn of the spinal cord is thought to contribute to the pathogenesis of diabetic neuropathy.
Counteracting the proinflammatory effects of microglia-produced cytokines may be one means of treating diabetic neuropathy. Curcumin is a naturally occurring polyphenolic pigment extracted from the Curcuma longa, which has antitumor, antioxidant, and anti-inflammatory properties [11]. What's more, curcumin is well absorbed and has good tissue penetration, including through the blood-brain-barrier [12]. We investigated whether the anti-inflammatory actions of this agent would attenuate hyperalgesia in a diabetic rat model and explored the mechanism or mechanisms involved.
Materials and methods
Animals
We obtained 48 male Sprague-Dawley rats (200-250 g) from the Experimental Animal Centre of the Fourth Military Medical University. The animals were housed under standard laboratory conditions, were maintained on a 12-h light-dark cycle, and had free access to food and water. The experimental protocols were approved by the Institutional Animal Ethics Committee of the Fourth Military Medical University.
Experimental diabetic model
We induced diabetes in 24 rats by administering one dose of streptozotocin prepared in citrate buffer (pH 4.4, 0.1 M). The streptozotocin was injected intraperitoneally at a dose of 65 mg/kg. Twenty-four age-matched control rats were administered an equal volume of citrate buffer vehicle. Forty-eight hours after the injections, we confirmed diabetes by collecting blood samples through the tail vein. Plasma glucose levels were estimated with a commercial blood glucose analyser (Accusoft, Roche Diagnostics, Laval, QC, Canada). Rats with fasting plasma glucose levels higher than 300mg/dL were deemed to be diabetic and were included in the study. Body weight and plasma glucose levels were recorded twice weekly for the duration of the study.
Treatment
Diabetic and control rats were randomly selected and divided into four groups of 12 animals each. Twelve diabetic rats and 12 control rats received oral curcumin treatment (60 mg/kg) daily from day 3 to day 28. The remaining 12 diabetic rats and 12 control rats were treated with curcumin vehicle (0.5% w/v sodium carboxymethylcellulose) over the same time period. Drug and vehicle were prepared immediately before administration and were delivered in a constant volume of 5 mL/kg body weight.
Assessment of mechanical allodynia
Paw withdrawal thresholds were measured with the up-down testing paradigm 1 day before injection of streptozotocin or vehicle and on days 7, 14, 21, and 28 after injections. Rats were placed in cages with mesh floors and covered with transparent plastic boxes. They were allowed to acclimatize to their surroundings for a minimum of 20 min in a temperature-controlled room (25ºC) before being tested. Von Frey hairs in log increments of force (0.38, 0.57, 1.23, 1.83, 3.66, 5.93, 9.13, and 13.1 g) were applied for a duration of 4-6 s to the region between the foot pads in the plantar aspect of the hind paw. Abrupt paw withdrawal, licking, and shaking were taken to be positive responses.
Assessment of thermal hyperalgesia
To assess nociceptive responses to thermal stimuli, we placed rats in animal holders with gentle restraint and applied radiant heat to the plantar surface of the test paw; a cut-off time of 20 s was used to prevent tissue damage. The thermal withdrawal latency from the radiant heat was recorded with a plantar test (Hargreaves' method) analgesia metre (IITC Life Science, Woodland Hills, CA, USA). Abrupt paw withdrawal, licking, and shaking were taken to be positive responses.
Estimation of TNF-α level
Rats were sacrificed with an overdose of inhalational isoflurane on day 28. The spinal cords were separated quickly and stored at −70ºC until they were ready to be processed for biochemical estimations. The spinal cord lumbosacral enlargement (L5) was homogenized in lysis buffer that contained 50 mM tris(hydroxymethyl)aminomethane (pH 8.0), 150 mM sodium chloride, and 1% 4-nonylphenyl-polyethylene glycol (Nonidet P-40, Sigma-Aldrich, USA). The homogenate was centrifuged at 5000 x g for 15 min at 4ºC for isolation of total supernatant protein. Protein concentration was determined with Pierce BCA Protein Assay Kit (Thermo Scientific Pierce Protein Biology Products, Rockford, IL, USA) according to the instructions of the manufacturer. TNF-α concentration was measured with a rat TNF-α kit (R&D Systems, Minneapolis, MN, USA), and the results were plotted on a standard curve.
Expression of TNF-α receptor 1
We used Western blot analysis to measure expression of TNF-α receptor 1 in the spinal cord dorsal horn samples. The protein extracts were separated on 7.5% polyacrylamide gels by electrophoresis and transferred onto polyvinylidene difluoride membrane. After the membranes were incubated in blocking buffer (5% nonfat milk in Tris-buffered saline with Tween 20 [TBST]) for 1 h at room temperature, they were incubated overnight at 4ºC in the presence of antibody to TNF-α receptor 1 (1:500 in 5% nonfat milk in TBST). Protein weights were measured against Precision Plus protein standards (Bio-Rad, Hercules, CA, USA). After being washed in phosphate-buffered saline with Tween 20, the membrane was incubated with a secondary antibody coupled to horseradish peroxidase (1:2000) for 2 h at room temperature. Proteins were visualized by chemiluminescence with ECL detection reagent (GE Healthcare-Amersham, Pittsburgh, PA, USA). The membranes were reprobed with antibody to β-actin for use as an internal loading control.
Statistical analyses
All data are presented as mean and standard error of the mean (SEM). Differences between and within groups were compared with one-way analysis of variance (ANOVA). We also used pair-wise comparisons between groups, performed with the Student-Newman-Keuls post hoc test. Statistical significance was set at p < 0.05. All analyses were carried out with SPSS version 13.0 (SPSS Inc., New York, NY, USA).
Results
Body weight and blood glucose levels
At 28 days after streptozotocin injection, diabetic rats exhibited significantly increased blood glucose levels and reduced body weight compared with control rats. Hyperglycaemia and body weight improved significantly in diabetic rats that were treated with curcumin compared with diabetic rats that were treated with vehicle (Table 1).
Effect of curcumin on mean body weight and blood glucose levels.
| Group | Body weight (g) | Blood glucose (mg/dL) | ||
|---|---|---|---|---|
| Day 0 | Day 28 | Day 0 | Day 28 | |
| Control + Vehicle | 220±1.31 | 293±2.18 | 116±3.81 | 121±2.73 |
| Control + Curcumin | 238±3.02 | 285±3.63 | 102±3.85 | 108±3.24 |
| DM + Vehicle | 240±1.57 | 195±2.42* | 114±4.87 | 573±8.86** |
| DM + Curcumin | 235±2.06 | 249±2.75 | 109±5.28 | 348±6.47# |
DM = diabetes mellitus. * p = 0.02, ** p = 0.004 vs. Controls; # p = 0.01 vs. DM + Vehicle.
Mechanical allodynia and thermal hyperalgesia
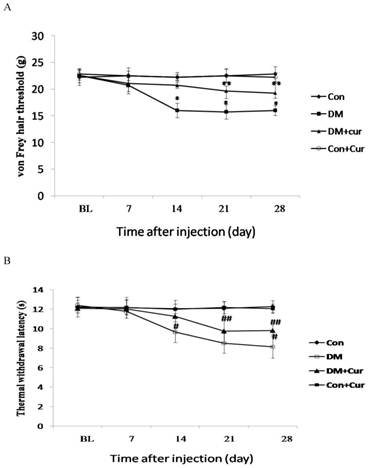
By day 28, paw withdrawal thresholds to von Frey hair stimulation were significantly lower in diabetic rats than in control rats, indicating the presence of mechanical allodynia. However, allodynia was less in diabetic rats treated with curcumin than in those treated with vehicle. Paw withdrawal thresholds did not differ significantly between the control groups during the entire observation period, irrespective of curcumin treatment (Fig. 1A).
The threshold for thermal hyperalgesia was significantly decreased by day 14 after streptozotocin injection compared to that of control rats and continued to develop up to day 28 in diabetic rats. Hyperalgesia was notably less in diabetic rats treated with curcumin than in diabetic rats treated with vehicle. Chronic administration of curcumin had no effect on thermal withdrawal latency in the control rats (Fig. 1B).
TNF-α and TNF-α receptor 1
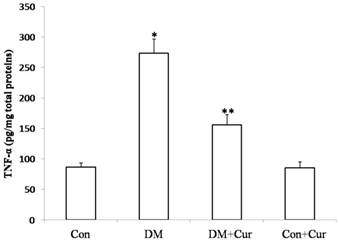
TNF-α level in spinal cord dorsal horn was markedly higher in diabetic rats than in controls (274.36± 18.97 pg/mg vs. 91.87 ± 6.83pg/mg). Diabetic rats that received curcumin had significantly lower levels of TNF-α than did those that received vehicle (158.33 ± 12.18 pg/mg vs. 274.36± 18.97 pg/mg). Curcumin treatment had no effect on spinal cord TNF-α level in control rats (92.14 ± 6.59 pg/mg vs. 91.87 ± 6.83pg/mg; Fig. 2).
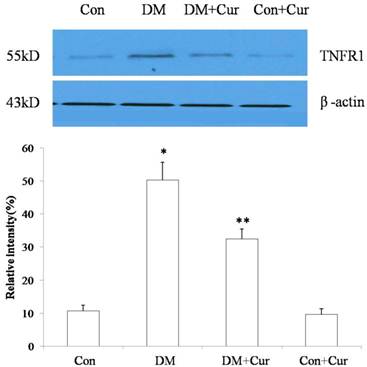
The concentration of TNF-α receptor 1 in spinal cord dorsal horn was increased in diabetic rats compared with that in control rats. Treatment with curcumin reduced expression of TNF-α receptor 1 in diabetic rats compared to expression in rats that received vehicle (Fig. 3). Curcumin had no effect on expression of TNF-α receptor 1 in control rats.
Effect of curcumin (Cur) treatment on the pain threshold in diabetic rats. Diabetes mellitus (DM) was associated with decreases in mechanical paw withdrawal threshold (A) and thermal paw withdrawal latency (B), but the decreases were significantly less in the curcumin-treated diabetic group than in the vehicle-treated diabetic group. Data are expressed as mean ± S.E.M. (n = 12 per group). BL, baseline; Con, control *p = 0.01 vs. control group; ** p = 0.03 vs. DM group; # p = 0.01 vs. control group; ## p = 0.04 vs. DM group.
Mean ± SEM TNF-α concentrations in spinal cord dorsal horn. Analyses were carried out with enzyme-linked immunosorbent assay; n = 6 rats for TNF-α ELISA. *p = 0.02 vs. control groups; Con, control, DM, diabetes mellitus; Cur, curcumin; **p = 0.006 vs. DM group.
Western blot of TNF-α receptor 1 (TNFR1) in spinal cord dorsal horn on day 28 after rats were injected with streptozotocin (DM) or citrate buffer (Control [Con]). Results are mean ± SEM of three independent experiments. Immunoblotting of β-actin confirmed equal loading. *p = 0.01 vs. control group; **p = 0.02 vs. DM group (n = 6 per group).
Discussion
Evidence indicates that hyperglycaemia has toxic effects on neurons because of increased intracellular glucose oxidation, which leads to an increase in production of reactive species [13,14]. TNF-α concentration in spinal cord dorsal horn are elevated in several neuropathological disorders associated with hyperalgesia [15, 16]. In our study, rats with streptocotozin-induced diabetes had significantly elevated blood glucose levels, decreased body weights, and reduced pain threshold compared with control rats. In diabetic rats treated with curcumin pain, the decrease in pain threshold was substantially less than that in diabetic rats treated with vehicle.
Previous preclinical studies have demonstrated an association between elevated TNF-α levels and altered pain behaviour [7, 17]. In the family of proinflammatory cytokines, TNF-α is regarded as a trigger for a cytokine signalling cascade [18]. Interaction between TNF-α and TNF-α receptor 1 leads to the activation of NF-κB, which in turn induces transcription of genes that encode inflammatory and other pain-related mediators, such as TNF-α, interleukin 6, and cyclooxygenase 2[19].
It is well known that TNF-α generates pathological pain via peripheral actions. Increasing evidence suggests that TNF-α has central actions in pain sensitization. The levels of TNF-α and TNF-α receptor 1 in dorsal root ganglia and spinal cord dorsal horn increase after peripheral nerve injury and in other neuropathic pain models. Intrathecal delivery of recombinant TNF-α (r TNF-α) can induce mechanical allodynia and thermal hyperalgesia in rats and intrathecal administration of the TNF-α inhibitor etanercept can attenuate inflammatory pain. Furthermore, TNF-α combined with transient receptor potential subtype V1 (TRPV1) can powerfully modulate spinal synaptic plasticity [20].
Pretreatment with the NF-κB inhibitor pyrrolidine dithiocarbamate can relieve mechanical allodynia and downregulate expression of TNF-α and TNF-α receptor 1 [19]. Studies have revealed that curcumin can attenuate thermal hyperalgesia in a diabetic mouse model by inhibiting serum TNF-α [21,22]. In the present study, we found that increased pain hypersensitivity behaviour in diabetic rats coincided with an increased expression of TNF-α and TNF-α receptor 1. Curcumin can inhibit NF-κB activity and expression of inflammatory cytokines [23]. The expression of TNF-α and TNF-α receptor 1 increased markedly in spinal cord dorsal horn of experimental diabetic rats, but treatment with curcumin reduced the expression of both compared with vehicle treatment.
Conclusions
The results of this study indicate that the expression of TNF-α and TNF-α receptor 1 in spinal cord dorsal horn increases in a diabetic rat model. Treatment with curcumin seems to lessen mechanical allodynia and thermal hyperalgesia through downregulation of TNF-α and TNF-α receptor 1 expression. Further investigation is warranted to explore the effects of curcumin on diabetic pain.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grants No. 81100816; 81000563).
Competing Interests
The authors declare that there are no conflicts of interest.
References
1. Yagihashi S, Yamagishi S, Wada R. Pathology and pathogenetic mechanisms of diabetic neuropathy: correlation with clinical signs and symptoms. Diabetes Res Clin Pract. 2007;77(Suppl 1):S184-S189
2. Al-Nimer MS, Al-Ani FS, Ali FS. Role of nitrosative and oxidative stress in neuropathy in patients with type 2 diabetes mellitus. J Neurosci Rural Pract. 2012;3:41-44
3. Green CJ, Pedersen M. et al. Elevated NF-kappaB activation is conserved in human myocytes cultured from obese type 2 diabetic patients and attenuated by AMP-activated protein kinase. Diabetes. 2011;60:2810-2819
4. Newsholme P, Gaudel C, Krause M. Mitochondria and diabetes. An intriguing pathogenetic role. Adv Exp Med Biol. 2012;942:235-247
5. Rodrigues AM, Bergamaschi CT, Araujo RC. et al. Effects of training and nitric oxide on diabetic nephropathy progression in type I diabetic rats. Exp Biol Med (Maywood). 2011;236:1180-1187
6. Zhang YL, Xu JM, Zhou P. et al. Distinct activation of tumor necrosis factor-alpha and interleukin-6 in the spinal cord after surgical incision in rats. Mol Med Report. 2012;5:1423-1427
7. Tumati S, Largent-Milnes TM, Keresztes A. et al. Repeated morphine treatment-mediated hyperalgesia, allodynia and spinal glial activation are blocked by co-administration of a selective cannabinoid receptor type-2 agonist. J Neuroimmunol. 2012;244:23-31
8. Wen YR, Tan PH, Cheng JK. et al. Microglia: a promising target for treating neuropathic and postoperative pain, and morphine tolerance. J Formos Med Assoc. 2011;110:487-494
9. Jung WW, Kim HS, Shon JR. et al. Intervertebral disc degeneration-induced expression of pain-related molecules: glial cell-derived neurotropic factor as a key factor. J Neurosurg Anesthesiol. 2011;23:329-334
10. Gao X, Kuo J, Jiang H. et al. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol. 2004;68:51-61
11. Adeghate E, Parvez SH. Nitric oxide and neuronal and pancreatic beta cell death. Toxicology. 2000;153:143-156
12. Mythri RB, Bharath MM. Curcumin: a potential neuroprotective agent in Parkinson's disease. Curr Pharm Des. 2012;18:91-99
13. Pittenger GL, Malik RA, Burcus N. et al. Specific fiber deficits in sensorimotor diabetic polyneuropathy correspond to cytotoxicity against neuroblastoma cells of sera from patients with diabetes. Diabetes Care. 1999;22:1839-1844
14. Andrade P, Visser-Vandewalle V, Del RJ. et al. The thalidomide analgesic effect is associated with differential TNF-alpha receptor expression in the dorsal horn of the spinal cord as studied in a rat model of neuropathic pain. Brain Res. 2012;1450:24-32
15. Choi JI, Svensson CI, Koehrn FJ. et al. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243-253
16. Spicarova D, Nerandzic V, Palecek J. Modulation of spinal cord synaptic activity by tumor necrosis factor alpha in a model of peripheral neuropathy. J Neuroinflammation. 2011;8:177
17. Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J Neuroinflammation. 2010;7:27
18. Zhang L, Berta T, Xu ZZ. et al. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. Pain. 2011;152:419-427
19. Nishida M, Nishiumi S, Mizushina Y. et al. Monoacetylcurcumin strongly regulates inflammatory responses through inhibition of NF-kappaB activation. Int J Mol Med. 2010;25:761-767
20. Park CK, Lu N, Xu ZZ. et al. Resolving TRPV1- and TNF-alpha-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. 2011;31:15072-15085
21. Sharma S, Kulkarni SK, Agrewala JN. et al. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J Pharmacol. 2006;536:256-261
22. Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha. Phytother Res. 2007;21:278-283
23. Huang G, Yang Y, Xu Z. et al. Downregulation of B lymphocyte stimulator expression by curcumin in B lymphocyte via suppressing nuclear translocation of NF-kappaB. Eur J Pharmacol. 2011;650:451-457
Author contact
![]() Corresponding author: Yu-shu Dong, Department of neurosurgery, the 463 Hospital of PLA, NO. 46, Xiaoheyan Road, Shenyang 110042, PR China . Tel: +86 24-28845374 Fax +86 24-24833149 Email: dongyushu463com; Wu-gang Hou, Department of Anesthesiology, Xijing Hospital the Fourth Military Medical University, No. 15 Changle West Road, Xi'an, 710032, PR China Tel: +86 29-84775344 Fax +86 29-84775337 Email:houwgmzcom.
Corresponding author: Yu-shu Dong, Department of neurosurgery, the 463 Hospital of PLA, NO. 46, Xiaoheyan Road, Shenyang 110042, PR China . Tel: +86 24-28845374 Fax +86 24-24833149 Email: dongyushu463com; Wu-gang Hou, Department of Anesthesiology, Xijing Hospital the Fourth Military Medical University, No. 15 Changle West Road, Xi'an, 710032, PR China Tel: +86 29-84775344 Fax +86 29-84775337 Email:houwgmzcom.

 Global reach, higher impact
Global reach, higher impact