3.2
Impact Factor
ISSN: 1449-1907
Int J Med Sci 2012; 9(9):801-807. doi:10.7150/ijms.4838 This issue Cite
Research Paper
Low-Magnitude High-Frequency Vibration Inhibits RANKL-Induced Osteoclast Differentiation of RAW264.7 Cells
Department of Orthopedic and Spinal Surgery, Nanfang Hospital, Southern Medical University, China.
* Song-Hui Wu and Zhao-Ming Zhong contributed equally to this work.
Received 2012-7-8; Accepted 2012-10-15; Published 2012-10-26
Abstract
Osteoclasts are the key participants in regulation of bone mass. Low-magnitude high-frequency vibration (LMHFV) has been found to be anabolic to bone in vivo. This study aimed to investigate the effect of LMHFV on osteoclast differentiation in vitro. Murine monocyte cell line RAW264.7 cells in the presence of receptor activator of nuclear factor-kappaB ligand (RANKL) were treated with or without LMHFV at 45 Hz (0.3 g) for 15 min day−1. Tartrate resistant acid phosphatase (TRAP)-positive multinucleated cells (MNCs) and actin ring formation were evaluated. Expression of the osteoclast-specific genes, such as cathepsin K, matrix metallopeptidase-9 (MMP-9) and TRAP, were analyzed using real time-PCR. c-Fos, an osteoclast-specific transcription factor, was determined using Western blot. We found that LMHFV significantly decreased the number of RANKL-induced TRAP-positive MNCs (P<0.01), and inhibited the actin ring formation. The mRNA expression of the cathepsin K, MMP-9 and TRAP were down-regulated by LMHFV intervention (all P<0.001). Furthermore, LMHFV also inhibited the expression of c-Fos protein in the RANKL-treated RAW264.7 cells (P<0.05). Our results suggest that LMHFV can inhibit the RANKL-induced osteoclast differentiation of RAW264.7 cells, which give some new insight into the anabolic effects of LMHFV on bone.
Keywords: Low-magnitude, high-frequency, vibration, receptor activator of nuclear factor-kappa B ligand, osteoclast differentiation.
Introduction
Osteoclasts differentiate from the monocyte/macrophage haematopoietic lineage (1). They are the principal resorptive cells of bone, playing an important role in the formation of the skeleton and the regulation of bone mass (2). Excess osteoclast number and activity, relative to bone-forming osteoblasts, dictate the development of osteoporosis, which is characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to bone fragility and the increased susceptibility to fracture (3).
Low-magnitude high-frequency vibration (LMHFV) is a form of non-invasive and biophysical intervention which provides cyclic loadings. LMHFV, when applied to the entire body of subjects, was found to be anabolic to bone in vivo , which may act through promoting bone formation (4,5), enhancing bone morphology (6), increasing bone strength (7), and attenuating bone resorption (5). LMHFV was clinically confirmed as a potential therapeutic approach to osteoporosis (4,5). Recently, it has been demonstrated that LMHFV did not enhance the osteogenic differentiation of bone marrow mesenchymal stromal cells (8). Whether LMHFV exerts its anabolic effects in vitro via regulating osteoclast differentiation remains largely unknown.
Receptor activator of nuclear factor- kappaB (RANK) ligand (RANKL), a member of the tumor necrosis factor family, can bind and activate RANK in the osteoclast precursor cells, which is required for osteoclast differentiation and activation (9,10). It was well-demonstrated that RANKL was able to induce osteoclast differentiation in the murine monocytic cell line RAW264.7 (10), an osteoclast precursor, which is widely used for the study of osteoclastogenesis in vitro (11-13).
In the present study, we hypothesized that LMHFV could inhibit osteoclast differentiation. To test this hypothesis, we developed an innovative device generating vertical vibration, and subjected RAW264.7 cells in the presence of RANKL to vibration at a magnitude of 0.3 g and a frequency of 45 Hz. We then evaluated the osteoclast differentiation by analyzing the tartrate resistant acid phosphatase (TRAP) positive cell formation, actin rings formation, the expression of osteoclast-specific genes and the osteoclast-specific transcription factor, c-Fos.
Materials and methods
Cell cultures and osteoclast differentiation
RAW264.7 cells were cultured in RPMI-1640 medium supplemented with 10% Fetal Bovine Serum (FBS) (Gibco, Montevideo, Uruguay), 2 mM glutamine, 100 IU/ml penicillin and 100 mg/ml streptomycin (Gibco, Grand Island, NY, USA) at 37 °C in 5% CO2 in humidified air. To induce differentiation of RAW264.7 cells, after cells were seeded overnight, the medium was shifted to phenol red-free α-MEM (Gibco, Grand Island, NY, USA) supplemented with 10% Foetal Bovine Serum Charcoal Stripped (FBS-CS) (BioInd, Kibbutz Beit Haemek, Israel). The medium was replaced once every 3 days.
The experimental regimens included 3 groups: (A) Control group, cells were incubated in phenol red-free α-MEM with 10% FBS-CS. (B) RANKL group, the differentiation medium consisted of phenol red-free α-MEM with 10% FBS-CS and 50 ng/ml recombinant mouse RANKL (rmRANKL) (R&D systems, Minneapolis, MN, USA). (C) LMHFV group, RAW264.7 cells cultured in the differentiation medium were subjected to LMHFV at 45 Hz, 0.3 g for 15 min per day, once a day. This vibration protocol was selected according to several previous studies (14-17).
Vibration apparatus
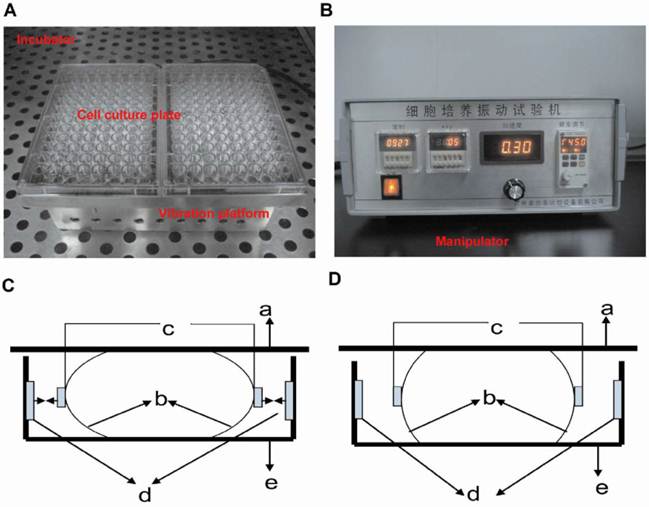
The vibration apparatus is customly made for generating vertical vibration to cells. The apparatus consists of a manipulator and a vibrating platform to hold the culture dishes (Figure 1). The platform is moved by an electromagnetic actuator (not visible) and placed inside a 37 °C, 5% CO2, humidified air incubator. The vibration magnitude, frequency and vibration direction are controlled and monitored by the manipulator out of the incubator, and the preset settings in the manipulator are applied to the platform. When the culture dishes in the LMHFV group are plated on the platform, RAW264.7 cells are subjected to vibration. The control group and RANKL group are plated on similar platform and kept in the same incubator, but not subjected to vibration.
Determination of osteoclastogenesis
To examine the effect of LMHFV on osteoclast formation, RAW264.7 cells were incubated in 96-well cell culture clusters at a concentration of 3×103 cells/well for 4 days. Cells were washed with phosphate buffered saline (PBS) and fixed in paraformaldehyde 4% in PBS for 10min, following by incubating for 20 min at 37 °C in the TRAP staining solution (Sigma Aldrich, St. Louis, MO, USA). TRAP-positive multinucleated (≧3 nuclei and ≧10 nuclei) cells (MNCs) were numbered under microscopic examination (17).
For F-actin ring staining, cells in 3.5 cm confocal dishes were washed and fixed. Then, cells were stained with Rhodamine-Phalloidin (Enzo, Farmingdale, NY, USA) in dark for 50 min at room temperature. Cell nucleies were counterstained with DAPI (KeyGEN, Nanjing, China) for 10 min. Specimens were examined with Olympus FV1000 confocal microscope (Olympus, Tokyo, Japan).
Total RNA extraction and real-time RT-PCR
RAW264.7 cells were treated with or without LMHFV for 3 days. Total RNA was extracted using RNAiso plus (Takara, Dalian, China) according to the manufacturer's instructions. The total RNA was reverse-transcribed into cDNA using an M-MLV Reverse Transcriptase kit (Invitrogen, Carlsbad, CA, USA), and the resultant cDNA mixture was diluted 10 fold in RNase Free dH2O. Real-time RT-PCR amplification was performed using SYBR® Premix Ex TaqTM kit (Takara, Dalian, China) in a 20 μl reaction containing 0.4 μl of each primer, 0.4 μl ROX Reference Dye II and 2 μl of cDNA. The PCR primers were shown as follows: cathepsin K sense 5′-CAG CAG AAC GGA GGC ATT GA-3′ and antisense 5′-CCT TTG CCG TGG CGT TAT AC-3′, MMP-9 sense 5′-GCC CTG GAA CTC ACA CGA CA-3′ and antisense 5′-TTG GAA ACT CAC ACG CCA GAA G-3′, TRAP sense 5′-CTA CCT GTG TGG ACA TGA CCA-3′ and antisense 5′-GCA CAT AGC CCA CAC CGT TC-3′, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sense 5′-AAA TGG TGA AGG TCG GTG TG-3′ and antisense 5′-TGA AGG GGT CGT TGA TGG-3′. cDNA amplification and detection were carried out in the ABI7500 (Applied Biosystems, Foster City, CA, USA) as follows: the reaction mixture was initially incubated at 95 °C for 30 sec to denature DNA. Amplification was performed for 40 cycles of 95 °C for 5 sec and 60 °C for 34 sec. The specificity of the PCR products was verified by melting curve analysis. Gene expression was calculated using the comparative Ct method. We calculated the Ct values of both the calibrator and the samples of interest by normalizing them to the endogenous housekeeping gene GAPDH.
Protein preparation and Western blot analysis
The c-Fos expression was assayed by Western Blot. Cells were harvested and the total protein was extracted. Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL, USA). Protein samples (20μg) were separated on 10% sodium dodecyl sulfate polyacrylamide gel and then transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). The membranes were blocked for 1 h at room temperature with 5% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 (TTBS), and then probed with the following antibodies, anti-mouse-c-Fos (62 kDa, 1:400) (Santa Cruz, California, USA), GAPDH (37 kDa, 1: 10000 ) (KangChen Bio-tech, Shanghai, China), in 5% nonfat milk in TTBS for 16 h at 4 °C. The membranes were then incubated by the goat anti-Rabbit IgG-Horseradish peroxidase (1:5000, SouthernBiotech, Birmingham, AL, USA) for 1 h at room temperature. The immunoreactive bands were visualized using an ECL Plus kit (Pierce, Rockford, IL, USA).
Vibration apparatus and its working principle. The vibration apparatus includes the vibration platform (A) and manipulator (B). Vertical vibration is generated by both left and right electromagnet working synchronously or stopping synchronously. So the elastic supporting component compresses or stretches and the vibration plate moves down (C) and up (D) correspondingly (a: vibration plate; b: elastic supporting component; c: iron; d: electromagnet; e: bedplate).
Statistical analysis
Results were represented as mean ± standard deviation (SD) for three separate experiments. Data was statistically analyzed by one-way analysis of variance (ANOVA) using SPSS13.0 software, Dunnett's T3 test was used to test significant difference between groups. Two-tailed P <0.05 was considered as statistical significant.
Results
LMHFV inhibited RANKL-induced increase of TRAP-positive MNCs in RAW 264.7 cells
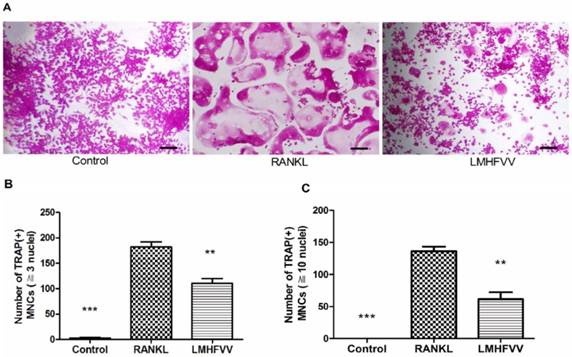
To determine whether LMHFV stimulation induced osteoclast differentiation, we firstly evaluated the formation of TRAP-positive MNCs by TRAP staining. Only few TRAP-positive MNCs (≧3 nuclei) was observed in control group, and the number of TRAP-positive MNCs was increased approximately 70-fold by RANKL treatment. However, RANKL-induced TRAP-positive MNCs were significantly inhibited by LMHFV (P<0.01) (Figure 2A, B). Furthermore, Figure 2C also showed that RANKL treatment increased the number of large TRAP-positive MNCs (≧10 nuclei), which were significantly inhibited by LMHFV challenge (P<0.01).
Effect of LMHFV on RANKL-induced actin rings formation in RAW264.7 cells
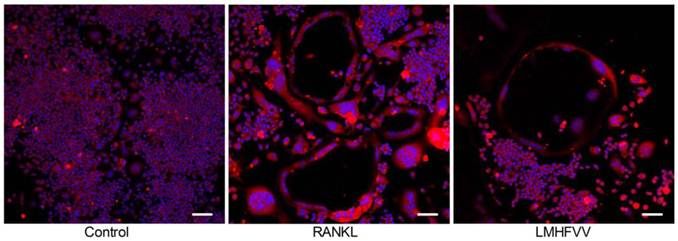
Actin ring is one of cell adhesion structures, which plays an essential role in osteoclast function (18). We found that the TRAP-positive MNCs formed actin rings in the RANKL group and LMHFV group. The number of the actin rings was decreased in the LMHFV group compared to that in the RANKL group. Moreover, the distance between the basolateral membrane facing the marrow space and the ruffled border (osteoclast height), an index of osteoclast polarization, was reduced in the LMHFV group (Figure 3). These results suggest that LMHFV disrupts actin cytoskeletal organization.
LMHFV down-regulated the expression of osteoclast-specific genes in RANKL-treated RAW264.7 cells
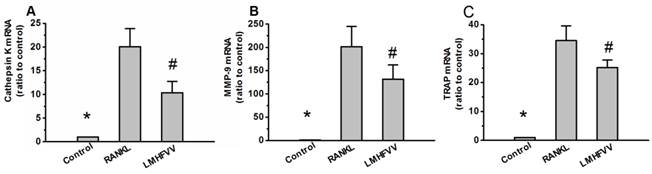
To investigate the effect of LMHFV on the expression of osteoclast-specific genes in the RANKL-treated RAW264.7 cells, we monitored the expression of osteoclast-specific genes including cathepsin K, matrix metallopeptidase 9 (MMP-9) and TRAP by real-time RT-PCR. As shown in Figure 4. The gene expressions of cathepsin K, MMP-9 and TRAP were low in control group, and were up-regulated in RANKL group. However, exposure of RANKL-treated RAW264.7 cells to LMHFV resulted in a significant down-regulation of cathepsin K, MMP-9 and TRAP expression (P<0.001).
LMHFV inhibited RANKL-induced osteoclast differentiation of RAW 264.7 cells. Cells in 96-well cell culture clusters were incubated for 4 days in control medium without RANKL (control group), differentiation medium (RANKL group), differentiation medium with LMHFV (LMHFV group), and then subjected to stain with TRAP. (A) TRAP-positive MNCs (bar =100 μm). (B) the number of TRAP-positive MNCs (≧3 nuclei). (C) the number of large TRAP-positive MNCs (≧10 nuclei). Data and pictures shown are representative of three independent experiments. All values are shown as mean ± SD. **P<0.01, ***P<0.001 vs the RANKL group.
LMHFV inhibited RANKL-induced the actin rings formation. RAW 264.7 cells in 3.5 cm confocal dishs were incubated for 4 days in control medium without RANKL (control group), differentiation medium (RANKL group), differentiation medium with LMHFV (LMHFV group), and then subjected to stain with Rhodamine-Phalloidin and DAPI. Red: Rhodamine-Phalloidin (for the actin cytoskeleton) and blue: DAPI (for nuclei). Pictures shown are representative of three independent experiments. (bar =100 μm).
LMHFV down-regulated the expression of osteoclast-specific genes. RAW 264.7 cells in 6-well cell culture clusters were incubated for 3 days in control medium without RANKL (control group), differentiation medium (RANKL group), differentiation medium with LMHFV (LMHFV group), and then real-time RT-PCR analysis to measure the gene expression of cathepsin K(A), MMP-9(B) and TRAP(C). Values are shown as mean ± SD of three independent experiments. * #P<0.001 vs the RANKL group.
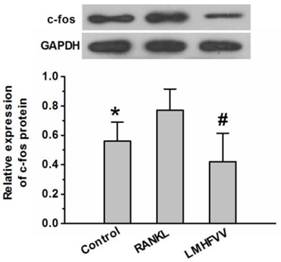
LMHFV down-regulated the expression of c-Fos protein
The c-Fos is considered as one of the most important osteoclast-specific transcription factors (19). We examined whether LMHFV modulated the transcription factors in RANKL-stimulated RAW264.7 cells using Western Blot analysis. As shown in Figure 5, RANKL treatment up-regulated expression of of c-Fos protein compared to that in the control group (P<0.05). However, LMHFV intervention significantly depressed the RANKL-induced up-regulation of c-Fos (P<0.05).
LMHFV prevented RANKL-induced expression of c-Fos in RAW264.7 cells. Cells in 6-well cell culture clusters were incubated for 3 days in control medium without RANKL (control group), differentiation medium (RANKL group), differentiation medium with LMHFV (LMHFV group), and then Western Blot to to measure the expression of c-fos protein. Values are shown as mean ± SD of three independent experiments. * #P<0.05 vs the RANKL group.
Discussion
Osteoporosis results from the rate of bone resorption by osteoclast outpacing the rate of bone formation by osteoblast. The effect of LMHFV on preventing bone loss and anti-osteoporosis was well-documented in many clinical studies (4,5), which highlight the importance of determining the effect of LMHFV on bone cell differentiation. Previous studies demonstrated that LMHFV (0.3g, 60Hz ) did not enhance the osteogenic differentiation of mesenchymal stromal cells in the presence of osteogenic induction medium( 8), but LMHFV (0.3g, 30, 60, or 90 Hz) induced osteocyte-like MLO-Y4 cells to produce soluble factors that inhibited osteoclast formation (20). In this study, we found that LMHFV directly inhibited the RANKL-induced osteoclast differentiation of RAW264.7 cells.
Increased bone resorption by osteoclast leads to an imbalance in bone remodeling and osteoporosis (21). Cathepsin K, MMP-9 and TRAP genes typify the osteoclast lineage, and are related to the development of mature osteoclasts (22,23). To investigate the effect of LMHFV on osteoclast differentiation, we measured the number of TRAP-positive MNCs and expression of cathepsin K, MMP-9 and TRAP. We found that LMHFV significantly inhibited the RANKL-induced an increase of TRAP-positive MNCs and up-regulation of osteoclast-specific genes. The current results were supported by other studies which demonstrated that mechanical signals were involved in the inhibition of osteoclastogenesis (24,25).
Bone resorption activation is a process that multinuclear cells are recruited, adhere to bone and differentiate into a mature osteoclast, and then secrete lytic enzymes into sealing zone to absorb bone. The number of nuclei per osteoclast is positively associated with the resorbing capability (26). Actin rings and ruffled borders are necessary for sealing zones and bone excavation, which is an essential process for osteoclast function (18,27). TRAP and cathepsin K are the two main enzymes responsible for the degradation of bone mineral and collagen matrices (22). Moreover, MMP-9 facilitates migration of osteoclast precursors toward bone surface and initiation of bone resorption during differentiation (23, 28). The present study showed that LMHFV stimulation significantly inhibited cathepsin K, MMP-9 and TRAP expression in RAW264.7 cells assessed by real time-PCR, which was consistent with a decrease in the number of large TRAP-positive MNCs. Furthermore, we also demonstrated that LMHFV prevented the formation of actin rings. These data suggest that LMHFV inhibits the osteoclast activation.
c-Fos, a critical component of the dimeric transcription factor activator protein-1 (Ap-1), appears to be a key regulator of osteoclast differentiation (19). c-Fos pathway, activated by RANKL, accelerates process of osteoclast differentiation by promoting NFATc1 expression (19). Our results showed that LMHFV treatment can down-regulate c-Fos expression of RAW264.7 cells in the presence of RANKL, which might be involved in the biological event that LMHFV inhibits the RANKL-induced osteoclast differentiation.
In conclusion, our data suggest that LMHFV inhibits the RANKL-induced osteoclast differentiation of RAW264.7 cells and down-regulates c-Fos expression. These findings give some new insight into the anabolic effects of LMHFV on bone. LMHFV provides the basis for a non-pharmacological approach to increase bone mass and reduce osteoporosis.
Acknowledgements
This work was supported in part by a National Natural Science Foundation of China (No.81000785) , a Science and Technology Planning Project of Guangdong Province, China (No.2008B030301343), and a President Grant of Nanfang Hospital, Southern Medical University, China (No.2009B023).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Udagawa N, Takahashi N, Akatsu T. et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260-4
2. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-42
3. Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504-8
4. Garman R, Gaudette G, Donahue LR. et al. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732-40
5. Xie L, Jacobson JM, Choi ES. et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059-66
6. Rubin C, Recker R, Cullen D. et al. Prevention of postmenopausal bone loss by a low-magnitude, high-frequency mechanical stimuli: a clinical trial assessing compliance, efficacy, and safety. J Bone Miner Res. 2004;19:343-51
7. Judex S, Boyd S, Qin YX. et al. Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng. 2003;31:12-20
8. Lau E, Lee WD, Li J. et al. Effect of low-magnitude, high-frequency vibration on osteogenic differentiation of rat mesenchymal stromal cells. J Orthop Res. 2011;29:1075-80
9. Lacey DL, Timms E, Tan HL. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165-76
10. Hsu H, Lacey DL, Dunstan CR. et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999;96:3540-5
11. Rahman MM, Bhattacharya A, Fernandes G. Docosahexaenoic acid is more potent inhibitor of osteoclast differentiation in RAW 264.7 cells than eicosapentaenoic acid. J Cell Physiol. 2008;214:201-9
12. Kitami S, Tanaka H, Kawato T. et al. IL-17A suppresses the expression of bone resorption-related proteinases and osteoclast differentiation via IL-17RA or IL-17RC receptors in RAW264.7 cells. Biochimie. 2010;92:398-404
13. Rahman MM, Bhattacharya A, Fernandes G. Conjugated linoleic acid inhibits osteoclast differentiation of RAW264.7 cells by modulating RANKL signaling. J Lipid Res. 2006;47:1739-48
14. Xie L, Jacobson JM, Choi ES. et al. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059-66
15. Xie LQ, Rubin C, Judex S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J Appl Physiol. 2008;104:1056-62
16. Chow DH, Leung KS, Qin L. et al. Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J Orthop Res. 2011;29:746-752
17. Garman R, Gaudette G, Donahue LR. et al. Low-level accelerations applied in the absence of weight bearing can enhance trabecular bone formation. J Orthop Res. 2007;25:732-40
18. Zhou Z, Immel D, Xi CX. et al. Regulation of osteoclast function and bone mass by RAGE. J Exp Med. 2006;203:1067-80
19. Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251-64
20. Lau E, Al-Dujaili S, Guenther A. et al. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508-15
21. Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508-14
22. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-42
23. Ishibashi O, Niwa S, Kadoyama K. et al. MMP-9 antisense oligodeoxynucleotide exerts an inhibitory effect on osteoclastic bone resorption by suppressing cell migration. Life Sci. 2006;79:1657-60
24. Suzuki N, Yoshimura Y, Deyama Y. et al. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int J Mol Med. 2008;21:291-6
25. Kim CHCY, You L, Yellowley CE. et al. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043-7
26. Piper K, Boyde A, Jones SJ. The relationship between the number of nuclei of an osteoclast and its resorptive capability in vitro. Anat Embryol (Berl). 1992;186:291-9
27. Rubin J, Greenfield EM. Osteoclast: Origin and Differentiation. In: (ed.) Bronner F, Farach-Carson MC, Rubin J. Bone Resorption, 1st ed. London: Springer-Verlage London Limited. 2005:1-23
28. Yu X, Huang Y, Collin-Osdoby P. et al. Stromal cell-derived factor-1 (SDF-1) recruits osteoclast precursors by inducing chemotaxis, matrix metalloproteinase-9 (MMP-9) activity, and collagen transmigration. J Bone Miner Res. 2003;18:1404-18
Author contact
![]() Corresponding author: Jian-Ting Chen, Department of Orthopedic and Spinal Surgery, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou 510515, China. Tel. +86-20-61641723, Fax. +86-20-62787195, E-mail. chenjt99com.
Corresponding author: Jian-Ting Chen, Department of Orthopedic and Spinal Surgery, Nanfang Hospital, Southern Medical University, 1838 North Guangzhou Avenue, Guangzhou 510515, China. Tel. +86-20-61641723, Fax. +86-20-62787195, E-mail. chenjt99com.

 Global reach, higher impact
Global reach, higher impact